Abstract
Active DNA helicase I (Mr 180,000) can be isolated from Escherichia coli F+ strains but not F- strains. The transfer of the F sex factor to F- strains by conjugation permits the purification of the enzyme from the transconjugant strains. We conclude from this that helicase I is coded for by a portion of the F factor. Results also obtained by using recombinant plasmids carrying different DNA fragments of the F factor transfer region suggest that DNA helicase I is identical to the product of traI, one of the transfer genes of the F factor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Monem M., Dürwald H., Hoffmann-Berling H. DNA unwinding enzyme II of Escherichia coli. 2. Characterization of the DNA unwinding activity. Eur J Biochem. 1977 Sep 15;79(1):39–45. doi: 10.1111/j.1432-1033.1977.tb11781.x. [DOI] [PubMed] [Google Scholar]
- Abdel-Monem M., Dürwald H., Hoffmann-Berling H. Enzymic unwinding of DNA. 2. Chain separation by an ATP-dependent DNA unwinding enzyme. Eur J Biochem. 1976 Jun 1;65(2):441–449. doi: 10.1111/j.1432-1033.1976.tb10359.x. [DOI] [PubMed] [Google Scholar]
- Abdel-Monem M., Hoffmann-Berling H. Enzymic unwinding of DNA. 1. Purification and characterization of a DNA-dependent ATPase from Escherichia coli. Eur J Biochem. 1976 Jun 1;65(2):431–440. doi: 10.1111/j.1432-1033.1976.tb10358.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R., Willetts N. Characterisation of an in vivo system for nicking at the origin of conjugal DNA transfer of the sex factor F. J Mol Biol. 1980 Jan 15;136(2):129–150. doi: 10.1016/0022-2836(80)90309-5. [DOI] [PubMed] [Google Scholar]
- Geider K., Hoffmann-Berling H. Proteins controlling the helical structure of DNA. Annu Rev Biochem. 1981;50:233–260. doi: 10.1146/annurev.bi.50.070181.001313. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Willetts N. S. Construction and characterization of multicopy plasmids containing the entire F transfer region. Plasmid. 1980 Nov;4(3):292–304. doi: 10.1016/0147-619x(80)90068-2. [DOI] [PubMed] [Google Scholar]
- Kingsman A., Willetts N. The requirements for conjugal DNA synthesis in the donor strain during flac transfer. J Mol Biol. 1978 Jul 5;122(3):287–300. doi: 10.1016/0022-2836(78)90191-2. [DOI] [PubMed] [Google Scholar]
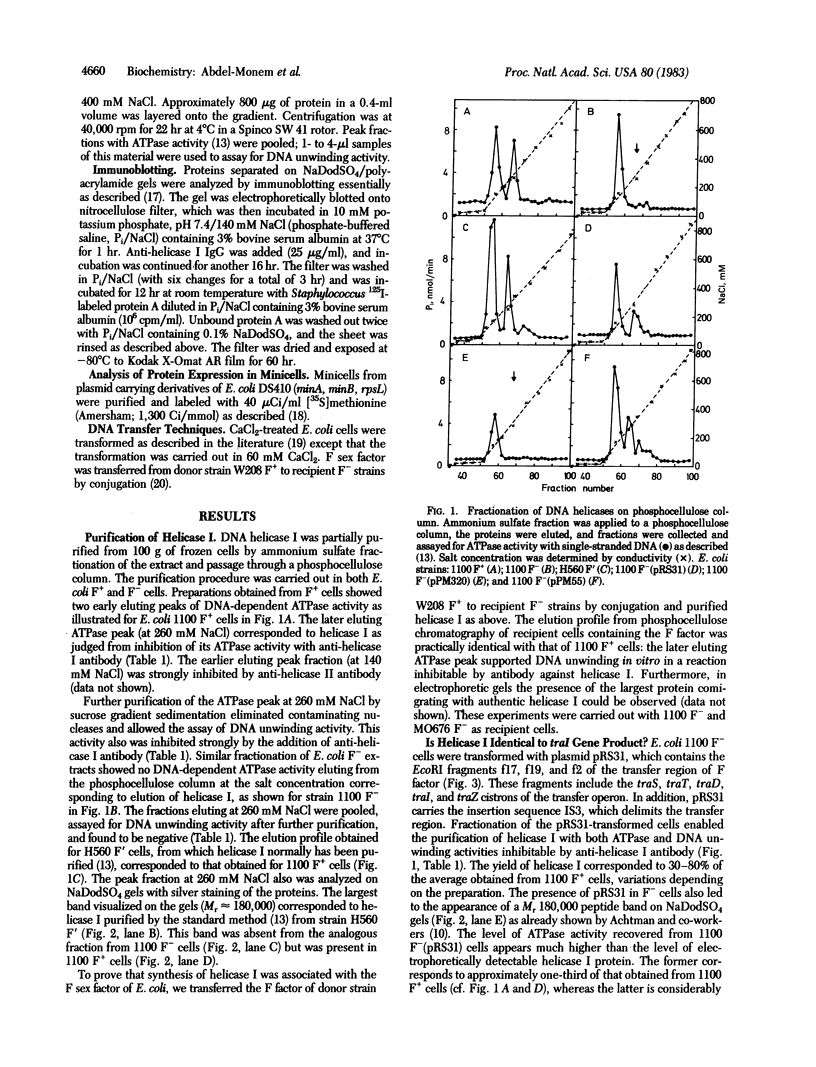
- Klinkert M. Q., Klein A., Abdel-Monem M. Studies on the functions of DNA helicase I and DNA helicase II of Escherichia coli. J Biol Chem. 1980 Oct 25;255(20):9746–9752. [PubMed] [Google Scholar]
- Kuhn B., Abdel-Monem M., Hoffmann-Berling H. DNA helicases. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):63–67. doi: 10.1101/sqb.1979.043.01.011. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Kusecek B., Morelli G., Fisseau C., Achtman M. Analysis of the promoter-distal region of the tra operon of the F sex factor of Escherichia coli K-12 encoded by EcoRI restriction fragments f17, f19, and f2. J Bacteriol. 1982 Apr;150(1):76–88. doi: 10.1128/jb.150.1.76-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer S., Blohm D., Geider K. Expression of Ti-plasmid DNA in E. coli: comparison of homologous fragments cloned from Ti plasmids of Agrobacterium strains C58 and Ach5. Plasmid. 1980 Sep;4(2):196–204. doi: 10.1016/0147-619x(80)90009-8. [DOI] [PubMed] [Google Scholar]
- Scott J. F., Eisenberg S., Bertsch L. L., Kornberg A. A mechanism of duplex DNA replication revealed by enzymatic studies of phage phi X174: catalytic strand separation in advance of replication. Proc Natl Acad Sci U S A. 1977 Jan;74(1):193–197. doi: 10.1073/pnas.74.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurray R. A., Nagaishi H., Clark A. J. Molecular cloning of DNA from F sex factor of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1976 Jan;73(1):64–68. doi: 10.1073/pnas.73.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Yarranton G. T., Das R. H., Gefter M. L. Enzyme-catalyzed DNA unwinding. Mechanism of action of helicase III. J Biol Chem. 1979 Dec 10;254(23):12002–12006. [PubMed] [Google Scholar]