In two mouse models of retinitis pigmentosa, intravitreal transplantations of adherently cultivated neural stem cells that had been modified to secrete ciliary neurotrophic factor resulted in a sustained delivery of functionally relevant quantities of the cytokine to the dystrophic retina. These transplantations may thus represent a useful method for preclinical studies aimed at evaluating the therapeutic potential of a cell-based administration of neurotrophic factors in mouse models of photoreceptor degeneration.
Keywords: Neural stem cell, Stem cell transplantation, Retinal photoreceptors, Lentiviral vector, Ciliary neurotrophic factor
Abstract
A continuous intraocular delivery of neurotrophic factors (NFs) is being explored as a strategy to rescue photoreceptor cells and visual functions in degenerative retinal disorders that are currently untreatable. To establish a cell-based intraocular delivery system for a sustained administration of NFs to the dystrophic mouse retina, we used a polycistronic lentiviral vector to genetically modify adherently cultivated murine neural stem (NS) cells. The vector concurrently encoded a gene of interest, a reporter gene, and a resistance gene and thus facilitated the selection, cloning, and in vivo tracking of the modified cells. To evaluate whether modified NS cells permit delivery of functionally relevant quantities of NFs to the dystrophic mouse retina, we expressed a secretable variant of ciliary neurotrophic factor (CNTF) in NS cells and grafted the cells into the vitreous space of Pde6brd1 and Pde6brd10 mice, two animal models of retinitis pigmentosa. In both mouse lines, grafted cells attached to the retina and lens, where they differentiated into astrocytes and some neurons. Adverse effects of the transplanted cells on the morphology of host retinas were not observed. Importantly, the CNTF-secreting NS cells significantly attenuated photoreceptor degeneration in both mutant mouse lines. The neuroprotective effect was significantly more pronounced when clonally derived NS cell lines selected for high expression levels of CNTF were grafted into Pde6brd1 mice. Intravitreal transplantations of modified NS cells may thus represent a useful method for preclinical studies aimed at evaluating the therapeutic potential of a cell-based intraocular delivery of NFs in mouse models of photoreceptor degeneration.
Introduction
Progressive dysfunction and degeneration of photoreceptors, as it occurs in retinitis pigmentosa or age-related macular degeneration, results in currently incurable visual impairment or blindness [1]. Corrective gene therapy, optogenetic therapy, implantations of electronic retinal prostheses, cell replacement strategies and neuroprotective strategies are among the approaches aimed at establishing treatments for such conditions [2–6]. All these therapeutic strategies have achieved remarkable results in animal models, and some have entered clinical trials [4, 7–9].
Neuroprotective strategies do not target the specific cause of a disease but instead attempt to limit the consequences (i.e., degeneration of photoreceptors and deterioration of visual function) and may thus be applicable across a broad range of degenerative retinal disorders. During the last two decades, a number of neurotrophic factors (NFs) have been shown to attenuate photoreceptor degeneration and to partly preserve retinal function in a variety of animal models of hereditary retinal degeneration and other diseases involving photoreceptor loss [3, 10]. Because NFs normally have short half-life times, do not ordinarily cross the blood-retina barrier, and bear the risk of unacceptable side effects when administered systemically, strategies are being developed that permit a local and sustained delivery of these factors to the retina. These include intravitreal implantations of biodegradable factor-loaded delivery devices, forced expression of NFs in endogenous retinal cells using viral or nonviral vectors, and intraocular transplantations of cells genetically modified to secrete such factors [11–16].
Intraocular implantations of encapsulated and genetically modified cells provide a straightforward strategy to use cells as vectors to deliver NFs to dystrophic retinas, as the encapsulation protects not only the grafted cells from the immune system of the host but also the host retina from potential adverse effects of the grafted cells. Studies with encapsulated human retinal pigment epithelium (RPE) cells modified to secrete ciliary neurotrophic factor (CNTF) have indeed demonstrated the feasibility of this approach in large animal models [17, 18] and human patients with degenerative retinal disorders [19–22]. However, the size of the encapsulated cell implants precludes their use in preclinical studies aimed at evaluating and optimizing this therapeutic approach in small species, such as the mouse with its numerous genetic or acutely induced models of degenerative retinal disorders.
Because stem cells are highly expandable and amenable to genetic modifications, they not only hold promise for cell replacement strategies but also may serve as cellular vectors to deliver therapeutic gene products to diseased retinas. Neurally committed stem and progenitor cells are of particular interest in this context, as they have been shown to survive for extended periods of time in host eyes after transplantation [23–27] and to confer some neuroprotective activity to photoreceptors even when grafted without prior genetic modifications [13, 23, 28, 29]. Neural stem/progenitor cells are usually expanded as free-floating cellular aggregates, called neurospheres [30], which contain only a few stem cells but instead are mainly composed of committed neural progenitor cells [31]. When neurosphere cells are cultured under adherent conditions, however, they give rise to pure populations of continuously self-renewing clonogenic neural stem cells, which can be extensively expanded in culture [32]. These cells, which in analogy to continuously self-renewing embryonic stem (ES) cells have been termed neural stem (NS) cells, differentiate into neurons, astrocytes, and oligodendrocytes in vitro and after transplantation into the brain and spinal cord [32, 33] and are thus among the candidate cell types to deliver therapeutic gene products to the diseased nervous system.
CNTF is a member of the interleucin-6 cytokine family and has neuroprotective effects on various nerve cell types of the central and peripheral nervous system [34]. In the retina, CNTF protects photoreceptors and ganglion cells from degeneration, and it is probably the most extensively studied NF in the context of degenerative retinal disorders [3, 7]. Although CNTF potently attenuates photoreceptor loss in various animal models of inherited and acquired retinal disorders, it negatively affects retinal function in a dose-dependent and reversible manner [35–38]. Of note, CNTF is currently being evaluated in clinical trials for the treatment of inherited retinal degenerations and geographic atrophy, and some positive effects have been reported [19–22].
To establish a cell-based intraocular delivery system for a sustained administration of NFs to the dystrophic mouse retina, we took advantage of the strong protective effect of CNTF on retinal structure and expressed this cytokine in NS cells. The neuroprotective effect of the CNTF-secreting NS cells on photoreceptor cells was evaluated in Pde6brd1 and Pde6brd10 mutant mice, two animal models of autosomal recessive retinitis pigmentosa [39, 40].
Materials and Methods
Animals
Neural stem cells were isolated from the cerebral cortex of 14-day-old C57BL/6J wild-type mouse embryos. Pde6brd1 and Pde6brd10 mutant mice were maintained on a C57BL/6J background and genotyped by polymerase chain reaction (PCR) [40, 41]. All animal experiments were approved by the local ethics committee and were in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Isolation, Cultivation, and Differentiation of NS Cells
To establish NS cell cultures [32] from the cerebral cortex of mouse embryos, we first generated neurosphere cultures according to standard protocols [24, 42]. After two or three passages, neurospheres were enzymatically dissociated, and cells were further cultivated under adherent conditions in tissue culture flasks coated with 0.1% Matrigel (BD Biosciences, Heidelberg, Germany, http://www.bd.com) in NS-A medium (Euroclone, Pero, Italy, http://www.euroclonegroup.it) supplemented with 10 ng/ml fibroblast growth factor-2 (FGF-2) and 10 ng/ml epidermal growth factor (EGF; both from TEBU, Offenbach, Germany, http://www.tebu-bio.com), 1% modified N2 [32], and 1% B27 (Life Technologies, Darmstadt, Germany, http://www.lifetech.com). Astrocytic differentiation of NS cells was induced by maintaining cultures for 5 days in NS-A medium containing 1% fetal calf serum (Life Technologies) and 2% B27. Neuronal differentiation was induced by cultivating NS cells for 5 days in NS-A medium supplemented with 5 ng/ml FGF-2, 1% N2, and 2% B27, followed by a further cultivation period of 5 days in a 1:1 mixture of NS-A and Neurobasal medium (Life Technologies) containing 0.25% N2 and 2% B27.
Lentiviral Vectors and NS Cell Transduction
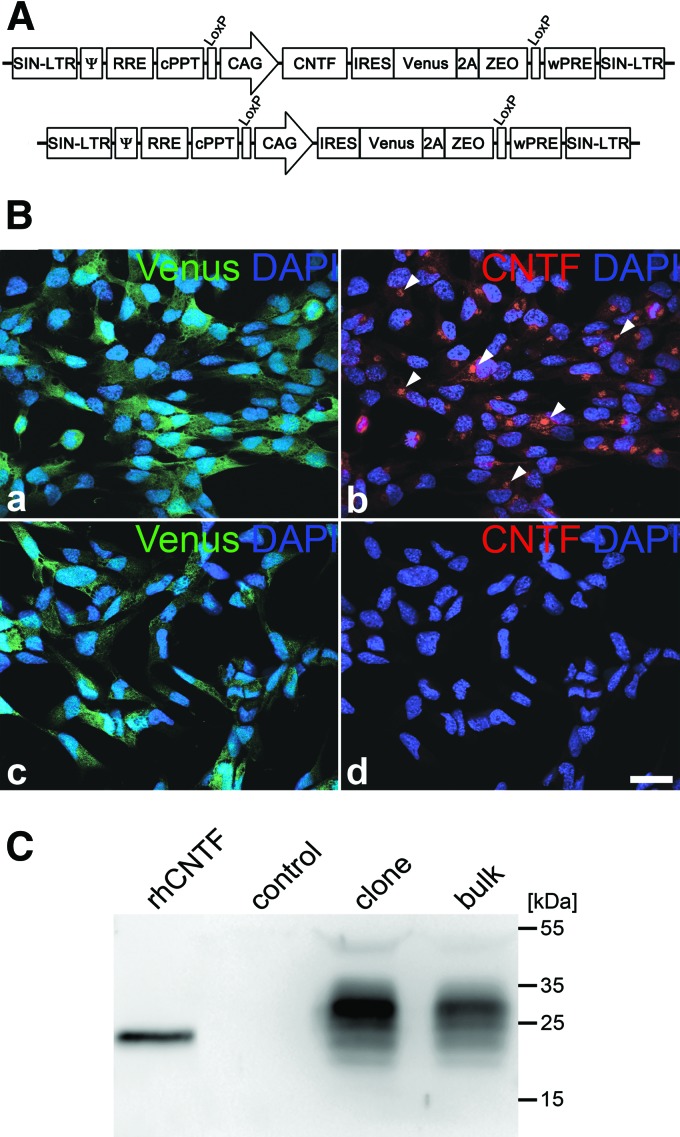
The open reading frame of mouse CNTF was PCR amplified from mouse brain cDNA and ligated in-frame with the Ig κ-chain leader sequence of pSecTag2 B (Life Technologies). The secretable variant of CNTF was then cloned into pCAG-IRES-Venus-2A-ZEO, giving rise to pCAG-CNTF-IRES-Venus-2A-ZEO. The vector is based on the lentiviral “gene ontology” (LeGO) vectors [43, 44] and contains the internal ribosome entry site (IRES) of the encephalomyocarditis virus and a Venus reporter gene separated from a zeocin (ZEO) resistance gene by a P2A sequence of porcine teschovirus-1 under regulatory control of the cytomegalovirus enhancer/chicken β-actin (CAG) promoter (Fig. 1A). Lentiviral particles, pseudotyped with the envelope G protein of the vesicular stomatitis virus, were produced as described (http://www.lentigo-vectors.de).
Figure 1.
Generation of CNTF-secreting neural stem (NS) cell cultures. (A): The lentiviral vector used in this study encoded a secretable variant of mouse CNTF under regulatory control of the human CAG promoter. The vector additionally encoded a Venus reporter gene and a zeocin resistance gene, both being located downstream of an internal ribosome entry site of the encephalomyocarditis virus and separated from each other by a P2A sequence (top). The same construct, but lacking the CNTF cDNA, served as a control vector (bottom). (B): NS cells were transduced with pCAG-CNTF-IRES-Venus-2A-ZEO. Cells with high expression levels of the reporter gene were clonally expanded and immunostained with anti-CNTF antibodies (Ba, Bb). Note that all cells in the CNTF-NS clone were positive for Venus (Ba) and showed strong CNTF immunoreactivity in a perinuclear location (Bb). A clonal NS cell line derived from cultures transduced with the control vector pCAG-IRES-Venus-2A-ZEO, in comparison, expressed Venus (Bc) but no detectable levels of CNTF (Bd). Scale bar = 20 μm. (C): CNTF was detected in the culture supernatants from CNTF-NS cell bulk cultures (bulk) and clonally derived CNTF-NS cell lines (clone), but not in supernatants from control-NS cell cultures (control). Abbreviations: Ψ, packaging signal; CAG, cytomegalovirus enhancer/chicken β-actin; CNTF, ciliary neurotrophic factor; cPPT, central polypurine tract; DAPI, 4′,6-diamidino-2-phenylindole; IRES, internal ribosome entry site; LoxP, recognition site of Cre recombinase; rhCNTF, recombinant human ciliary neurotrophic factor; RRE, rev-responsive element; SIN-LTR, self-inactivating long-terminal repeat; wPRE, woodchuck hepatitis virus posttranscriptional regulatory element; ZEO, zeocin.
NS cells were spinoculated with pCAG-CNTF-IRES-Venus-2A-ZEO to derive CNTF-secreting NS cells (CNTF-NS cells) or with pCAG-IRES-Venus-2A-ZEO to derive NS cells for control experiments (control-NS cells), and further expanded in the presence of 200 μg/ml zeocin (InvivoGen, San Diego, CA, http://www.invivogen.com/) to select for positive cells. To establish clonally derived control-NS and CNTF-NS cell lines, single cells with the highest expression levels of the reporter gene were plated into 96-well plates by fluorescence activated cell sorting (FACS; FACSAria; BD Biosciences, San Diego, CA, http://www.bdbiosciences.com).
Immunocytochemistry, Immunoblot Analysis, and Enzyme-Linked Immunosorbent Assay
Cells were fixed in 4% paraformaldehyde (PA), blocked, and stained with polyclonal rabbit anti-CNTF antibodies (kind gift of Dr. M. Sendtner). Astrocytes and neurons in differentiated NS cell cultures were identified with monoclonal mouse anti-glial fibrillary acidic protein (GFAP) and anti-microtubule associated protein 2 antibodies (both from Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), respectively. Primary antibodies were detected with Cy3- or Cy5-conjugated secondary antibodies (Jackson Immunoresearch Laboratories, West Grove, PA, http://www.jacksonimmuno.com).
To evaluate secretion of CNTF, culture supernatants were concentrated with Amicon Ultra Centrifugal Filter devices (Millipore, Bedford, MA, http://www.millipore.com) and analyzed in immunoblots using polyclonal rabbit anti-CNTF antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com) and horseradish peroxidase-conjugated secondary antibodies (Jackson Immunoresearch Laboratories). Immunoreactive bands were visualized using SuperSignal West Dura Extended Duration Substrate (ThermoFisher Scientific, Rockford, IL, http://www.thermofisher.com/global/en/home.asp). Secretion of CNTF was quantified with an enzyme-linked immunosorbent assay (ELISA) kit for mouse CNTF (USCN Life Science Inc., Wuhan, China, http://www.uscnk.com). ELISA plates were analyzed with a Sunrise microplate reader and Magellan software (Tecan, Männedorf, Switzerland, http://www.tecan.com).
Intravitreal Cell Transplantations and Immunohistochemistry
NS cells were intravitreally grafted into 7-day-old Pde6brd1 and 14-day-old Pde6brd10 mice. Animals were deeply anesthetized, a glass micropipette was inserted into the vitreous space at the junction between sclera and cornea, and 1 and 2 μl of vitreous fluid were removed from the eyes of Pde6brd1 and Pde6brd10 mice, respectively. Pde6brd1 mice received injections of 1 μl of phosphate-buffered saline (PBS) containing 3.8 × 105 CNTF-NS cells or no cells into one eye, and 3.8 × 105 control-NS cells in 1 μl of PBS into the contralateral eye. Pde6brd10 mice received injections of 2 μl of PBS containing 7.6 × 105 CNTF-NS cells or no cells into one eye, and 7.6 × 105 control-NS cells in 1 μl of PBS into the contralateral eye. Care was taken not to damage the lens during the removal of vitreous fluid or the injection of cells or vehicle solution. Pde6brd1 mutants were analyzed at postnatal day 15 (P15) and P25. Pde6brd10 mice were kept in a 12-hour-light/12-hour-dark cycle with illumination levels of ∼200 lux and were analyzed at P30.
Eyes from Pde6brd1, Pde6brd10, or age-matched untreated wild-type mice were fixed in 4% PA, cryoprotected, frozen, serially sectioned at a thickness of 25 μm, and stained with polyclonal rabbit anti-recoverin antibodies (Millipore). Lenses with attached donor cells were stained with polyclonal rabbit anti-CNTF and anti-GFAP (Dako, Glostrup, Denmark, http://www.dako.com) or anti-β-tubulin III (Sigma-Aldrich) antibodies. Lenses from Pde6brd10 mice with grafted CNTF-NS or control-NS cell bulk cultures, or from Pde6brd1 mice with grafted CNTF-NS or control-NS cell clones (n = 3 for each genotype and cell population) were stained with polyclonal rabbit anti-Ki67 antibodies (Abcam, Cambridge, U.K., http://www.abcam.com) to identify proliferating donor cells. Between 150 and 400 donor cells were analyzed per lens to determine the percentage of Ki-67-positive donor cells. Sections and lenses were analyzed with an Olympus FV 1000 confocal microscope (Olympus, Hamburg, Germany, http://www.olympus-global.com).
Photoreceptor Counts
Merged images of the entire nasal halves of central (i.e., in the plane of the optic disc) retinal sections were prepared using Photoshop CS3 software (Adobe Systems Inc., San Jose, CA, http://www.adobe.com). Photoreceptors were counted in three areas located at defined distances from the optic disc, corresponding to 25%, 50%, and 75% of the length of the nasal retina. Each area covered the outer nuclear layer over a length of 220 μm. Photoreceptor numbers were determined in Pde6brd1 and Pde6brd10 mice with intravitreally injected NS cells or PBS, and in 15- and 30-day-old untreated wild-type mice. Six animals from three independent experiments were analyzed for each experimental group. Statistical analysis of data was performed with the two-way analysis of variance test.
Results
Lentiviral Expression of CNTF in NS Cells
To establish NS cell cultures from the cerebral cortex of embryonic mice, we converted neurosphere cultures into adherently growing cultures and further expanded the cells in the presence of EGF, FGF-2, and N2. Under these conditions, cultures consisted of a pure population of symmetrically dividing clonogenic NS cells with molecular features reminiscent of neurogenic radial glia [32, 33]. To genetically modify these cells, we generated a polycistronic lentiviral vector encoding a secretable variant of mouse CNTF, a Venus reporter gene, and a zeocin resistance gene under control of the CAG promoter (pCAG-CNTF-IRES-Venus-2A-ZEO; Fig. 1A). The same construct but lacking the CNTF cDNA served as a control vector (pCAG-IRES-Venus-2A-ZEO; Fig. 1A). NS cells were spinoculated with pCAG-CNTF-IRES-Venus-2A-ZEO and pCAG-IRES-Venus-2A-ZEO to derive CNTF-NS and control-NS cells, respectively. Further cultivation of cells in the presence of zeocin gave rise to CNTF-NS and control-NS cell cultures exclusively composed of Venus-positive cells. Immunocytochemical analysis of these bulk cultures revealed expression of CNTF in virtually every CNTF-NS cell, whereas control-NS cells lacked detectable levels of CNTF immunoreactivity (not shown, but clonal cell lines are given in Fig. 1B). Immunoblot analysis of culture supernatants confirmed secretion of CNTF from CNTF-NS cell bulk cultures, whereas supernatants from control-NS cell bulk cultures lacked detectable levels of the cytokine (Fig. 1C). Because NS cells differentiated into astrocytes and neurons after intravitreal transplantations, we additionally analyzed expression of CNTF in differentiated NS cell cultures. CNTF immunoreactivity was detectable in virtually all astrocytes and neurons derived from CNTF-NS cells, but not in astrocytes and neurons derived from control-NS cells (Fig. 2).
Figure 2.
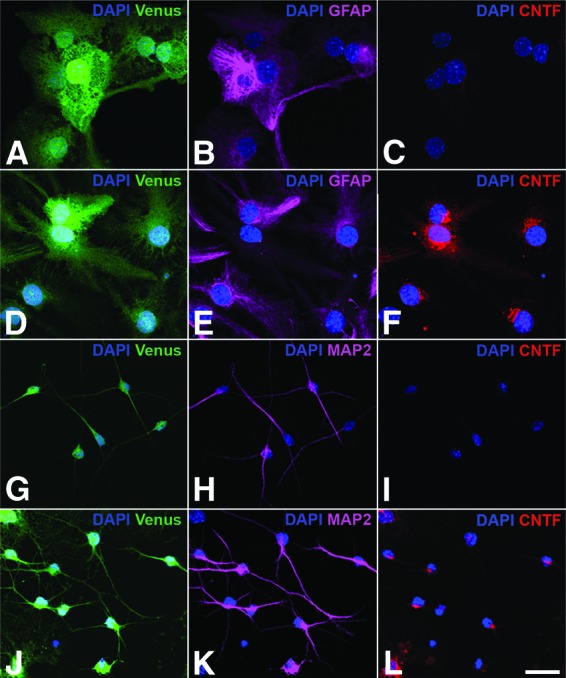
Analysis of CNTF expression in differentiated neural stem (NS) cell cultures. NS cells were transduced with pCAG-CNTF-IRES-Venus-2A-ZEO (D–F, J–L) or the control vector pCAG-IRES-Venus-2A-ZEO (A–C, G–I), and positive cells were selected with zeocin and differentiated into astrocytes (A–F) or neurons (G–L). Note the coexpression of Venus and CNTF in virtually all GFAP-positive astrocytes (D–F) and MAP2-positive neurons (J–L) derived from CNTF-NS cells. Astrocytes (A–C) and neurons (G–I) derived from control-NS cells, in comparison, expressed Venus but no detectable levels of CNTF. All cultures were counterstained with DAPI to visualize cell nuclei. Scale bar = 20 μm. Abbreviations: CNTF, ciliary neurotrophic factor; DAPI, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein; MAP2, microtubule-associated protein 2.
CNTF-Secreting NS Cell Clones
To establish NS cell cultures with elevated expression levels of CNTF, we next generated clonally derived CNTF-NS cell lines. Assuming that expression levels of CNTF and the reporter gene from the polycistronic lentiviral vector are proportional to each other, we isolated individual NS cells with the highest expression levels of Venus using FACS to establish clonal cell lines with high levels of CNTF expression. Immunocytochemistry (Fig. 1B) and immunoblot analysis of culture supernatants (Fig. 1C) demonstrated expression of CNTF in all CNTF-NS cell clones established, whereas control-NS cell clones lacked expression of the cytokine. ELISA analysis of culture supernatants from a CNTF-NS cell clone that showed high expression levels of CNTF in immunoblots revealed secretion of 5.4 ng of CNTF per 1 × 106 cells in 24 hours. The original CNTF-NS cell bulk culture, in comparison, produced 4.0 ng of CNTF per 1 × 106 cells in 24 hours. Expression of CNTF in the clonal cell line was thus increased by ∼35% when compared with the original bulk culture, and the clone was therefore selected for transplantation experiments. Expression of CNTF in the CNTF-NS cell clone remained detectable by immunocytochemistry and immunoblot analysis for at least 40 passages, and ELISA analysis of culture supernatants revealed secretion of similar quantities of CNTF at passages 33 and 46, demonstrating stable expression of the cytokine.
Characterization of Intravitreally Grafted NS Cells In Vivo
Analysis of eyes from Pde6brd10 and Pde6brd1 mice with grafted CNTF-NS or control-NS cells revealed the presence of numerous Venus-positive donor cells that were attached to the posterior pole of the lens (Fig. 3; supplemental online Fig. 1) and the vitreal side of the retina (Fig. 4). Integration of donor cells into the host retinas was not observed (Fig. 4). Immunohistochemical analysis of lenses with attached donor cells revealed that grafted CNTF-NS and control-NS cell bulk cultures (Fig. 3) or clonal cell lines (supplemental online Fig. 1) were differentiated into GFAP-positive astrocytes, and some β-tubulin III-positive nerve cells. In addition, we found expression of Ki-67 in 2.6% and 1.8% of the grafted CNTF-NS cells and in 3.7% and 1% of the grafted control-NS cells in Pde6brd1 and Pde6brd10 mice, respectively. Furthermore, CNTF immunoreactivity was detectable only in donor cells derived from grafted CNTF-NS cell bulk cultures or clones, but not in donor cells derived from grafted control-NS cell bulk cultures or clones (Fig. 3; supplemental online Fig. 1). Finally, we observed no adverse effects of the grafted NS cells on the morphology of either Pde6brd10 (Fig. 5) or Pde6brd1 retinas (Fig. 4; supplemental online Figs. 2, 3).
Figure 3.
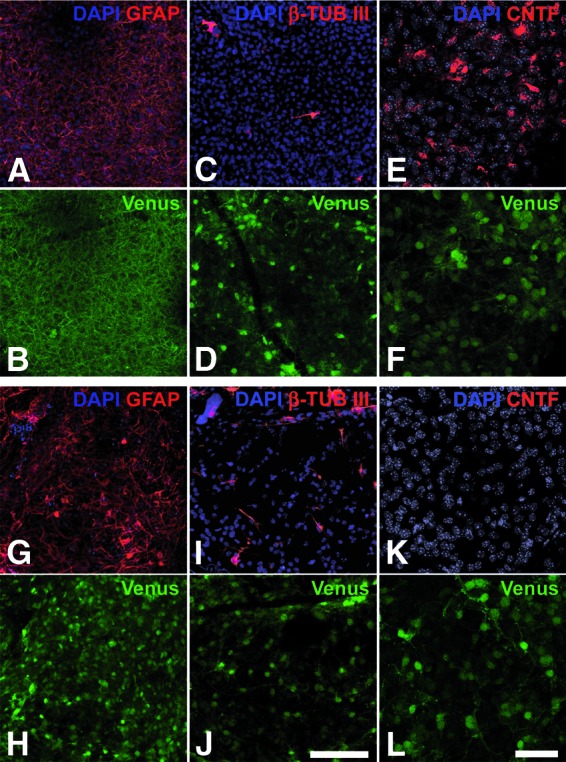
Characterization of intravitreally grafted CNTF-neural stem (NS) and control-NS cell bulk cultures in Pde6brd10 mice. Analysis of eyes from Pde6brd10 mutant mice with intravitreally grafted CNTF-NS (A–F) and control-NS cell bulk cultures (G–L) revealed the presence of dense layers of Venus-positive cells (B, D, F, H, J, L) that were attached to the posterior poles of the lenses. The majority of Venus-positive donor cells were differentiated into GFAP-positive astrocytes (compare [A] with [B], and [G] with [H]), and some into β-tubulin III-positive neurons (compare [C] with [D], and [I] with [J]). Expression of CNTF was detectable in donor cells derived from CNTF-NS cells (E) but not in donor cells derived from control-NS cells (K). Scale bars = 100 μm (bar in [J] applies to [A–D, G–J]) and 50 μm (bar in [L] applies to [E, F, K, L]). Abbreviations: β-TUB III, β-tubulin III; CNTF, ciliary neurotrophic factor; DAPI, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein.
Figure 4.
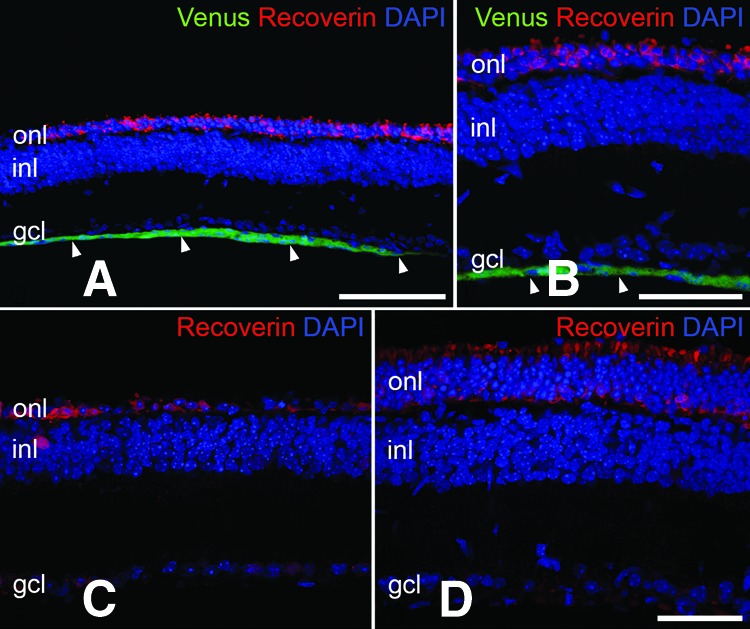
Attenuation of photoreceptor loss in Pde6brd1 mice at advanced stages of retinal degeneration. The clonal ciliary neurotrophic factor (CNTF)-neural stem (NS) and control-NS cell lines were intravitreally grafted into 7-day-old Pde6brd1 mice, and animals were analyzed at postnatal day 25. Note that the transplanted cells had formed a layer of Venus-positive cells that was attached to the vitreal side of the retina (arrowheads in [A, B]). Integration of donor cells into the host retinas was not detectable (A, B). The outer nuclear layer of eyes with the grafted control-NS cell line consisted of only one row of photoreceptor nuclei at this developmental age (C). The outer nuclear layer of the CNTF-treated contralateral retina, in comparison, still consisted of several rows of photoreceptor nuclei (D), demonstrating significant attenuation of photoreceptor loss by the grafted CNTF-NS cell clone. Scale bars = 100 μm (A), 50 μm (B–D). Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; gcl, ganglion cell layer; inl, inner nuclear layer; onl, outer nuclear layer.
Figure 5.
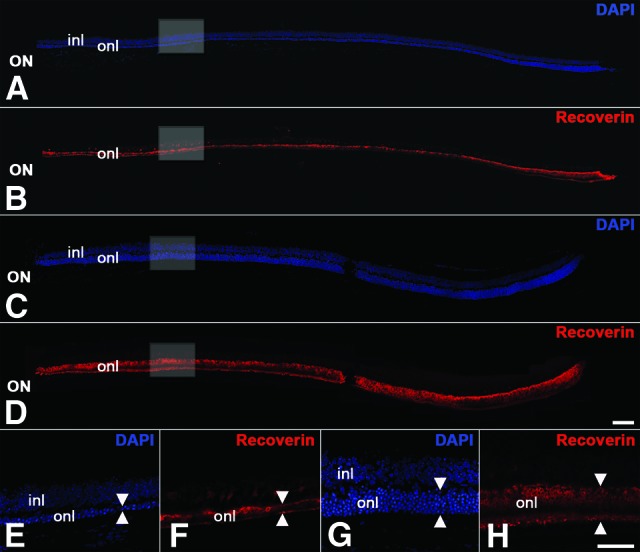
Intravitreally grafted ciliary neurotrophic factor (CNTF)-neural stem (NS) bulk cultures attenuate photoreceptor degeneration in Pde6brd10 mice. Control-NS cell bulk cultures were grafted into one eye (A, B, E, F) and CNTF-NS cell bulk cultures into the contralateral eye (C, D, G, H) of 14-day-old Pde6brd10 mice, and animals were analyzed 16 days later. Analysis of central retinal sections stained with DAPI and anti-recoverin antibodies revealed a significantly thicker outer nuclear layer in eyes that had received CNTF-NS cell grafts ([C, D], arrowheads in [G, H]) than in the contralateral eyes that had received control-NS cell grafts ([A, B], arrowheads in [E, F]). Note that the rescue effect of the grafted cells was evident along the entire length of the retinal section (C, D). Adverse effects of the grafted cells on the morphology of the host retinas were not detectable (A–H). (E, F) and (G, H) are higher magnifications of the boxed areas in (A, B) and (C, D), respectively. Scale bars = 100 μm (bar in [D] applies to [A–D]) and 50 μm (bar in [H] applies to [E–H]). Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; inl, inner nuclear layer; ON, optic nerve; onl, outer nuclear layer.
CNTF-NS Cells Protect Photoreceptors in Pde6brd10 and Pde6brd1 Mice
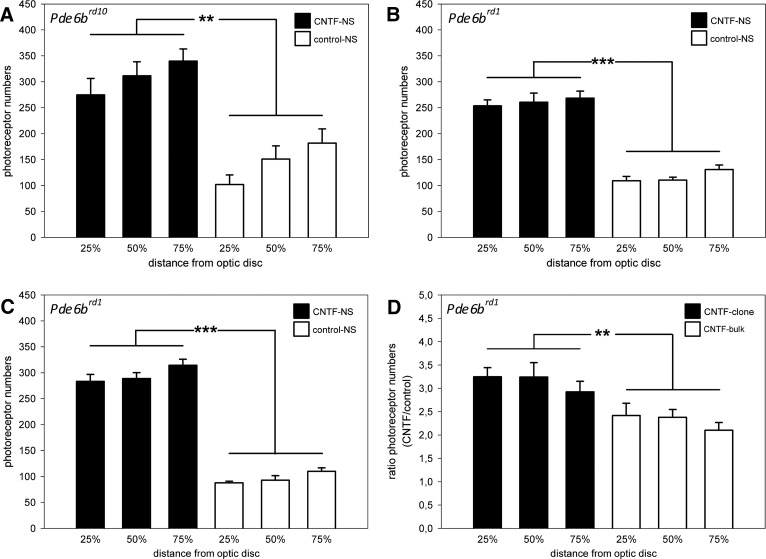
The neuroprotective effect of CNTF-NS cell bulk cultures on photoreceptors was analyzed in 30-day-old Pde6brd10 mice (Fig. 5). A comparison of eyes with grafted CNTF-NS cell bulk cultures and contralateral eyes with grafted control-NS cell bulk cultures consistently revealed a significantly thicker outer nuclear layer in the CNTF-treated eyes (Fig. 5). Importantly, the photoreceptor rescue in CNTF-treated eyes was evident over the entire length of all retinal sections analyzed. To quantify the neuroprotective effect of the CNTF-NS cells, photoreceptor numbers were determined in the nasal halves of retinal sections at three positions, corresponding to 25%, 50%, and 75% of the distance between optic disc and periphery of the retina. In CNTF-treated retinas, 274.8 ± 31.6 (mean ± SEM), 311.5 ± 26.9, and 339.8 ± 23.5 photoreceptors were present at the 25%, 50%, and 75% positions, respectively. In the contralateral control retinas, in comparison, we counted 101.7 ± 18.9, 151.0 ± 25.3, and 181.7 ± 27.3 photoreceptors at the 25%, 50%, and 75% positions, respectively (Fig. 6A). CNTF-treated retinas of Pde6brd10 mice thus contained 1.9- to 2.7-fold more photoreceptors than the contralateral control retinas (p < .01), depending on the retinal region analyzed. We additionally evaluated whether control-NS cells also protected photoreceptors in Pde6brd10 mice but found no significant differences between photoreceptor numbers in eyes with grafted control-NS cell bulk cultures (132.7 ± 8.8, 134.3 ± 10.5, and 154.0 ± 15.3 cells at the 25%, 50%, and 75% positions, respectively) and contralateral eyes that had received injections of PBS only (129.0 ± 26.8, 128.7 ± 30.0, and 139.0 ± 29.9 cells at the 25%, 50%, and 75% positions, respectively; supplemental online Fig. 4). Retinas from untreated 30-day-old wild-type mice (supplemental online Fig. 4) analyzed for comparison contained 526.2 ± 11.8 (25% position), 531.2 ± 19.8 (50% position), and 503.9 ± 8.6 (75% position) photoreceptors.
Figure 6.
Photoreceptor numbers in Pde6brd1 and Pde6brd10 mice with intravitreally grafted CNTF-NS cells and control-NS cells. Eyes from Pde6brd10 (A) and Pde6brd1 (B, C) mice that received intravitreal injections of either CNTF-NS cell bulk cultures (A, B) or a clonally derived CNTF-NS cell line (C) contained significantly more photoreceptors than the contralateral eyes that received injections of the corresponding control cells. Note that the clonally derived CNTF-NS cell line selected for high levels of CNTF expression had a significantly more pronounced neuroprotective effect on photoreceptors in Pde6brd1 mice than the original CNTF-NS cell bulk culture (D). Each bar in (A–C) represents the photoreceptor number (mean ± SEM) from six retinas at the indicated retinal positions. Each bar in (D) represents the quotient (mean ± SEM) of photoreceptor numbers in eyes with the grafted CNTF-NS cell clone and the contralateral eyes with the grafted control-NS cell clone (n = 6; filled bars), or in eyes with the grafted CNTF-NS cell bulk culture and the contralateral eyes with the grafted control-NS cell bulk culture (n = 6; open bars) at the indicated retinal positions. **, p < .01; ***, p < .001, according to the two-way analysis of variance test. Abbreviations: CNTF, ciliary neurotrophic factor; NS, neural stem.
CNTF-NS cell bulk cultures also potently protected photoreceptors in the Pde6brd1 mutant mouse. In 15-day-old Pde6brd1 mice, the outer nuclear layer was significantly thicker in eyes with grafted CNTF-NS cells than in the contralateral eyes with grafted control-NS cells (supplemental online Fig. 2). As in Pde6brd10 mice, the rescue effect was widespread and evident in all retinal regions analyzed (supplemental online Fig. 2). Quantitative analysis confirmed a significant protective effect of the CNTF-NS cell bulk culture also in this mutant, with 253.7 ± 11.4, 260.5 ± 17.7, and 268.3 ± 13.7 photoreceptors at the 25%, 50%, and 75% positions, respectively, as compared with 109.2 ± 8.6, 110.5 ± 5.6, and 130.7 ± 8.9 photoreceptors at the 25%, 50%, and 75% positions in control retinas, respectively (Fig. 6B). CNTF-treated retinas thus contained 2.1- to 2.4-fold more photoreceptors than the contralateral control eyes (p < .001). Retinas from untreated 15-day-old wild-type mice (supplemental online Fig. 4) analyzed for comparison contained 527.8 ± 9.8, 513.0 ± 12.6, and 487.3 ± 16.3 photoreceptors at the 25%, 50%, and 75% positions, respectively.
Neuroprotective Effect of a CNTF-NS Cell Clone in Pde6brd1 Mice
Intravitreal transplantations of a clonal cell line with a ∼35% increase in CNTF expression into Pde6brd1 (compare supplemental online Figs. 2 and 3) or Pde6brd10 mice (not shown) resulted in a photoreceptor rescue that appeared more pronounced than that observed with the original CNTF-NS cell bulk culture. Quantitative analysis of Pde6brd1 retinas confirmed a profound protection of photoreceptors by the grafted CNTF-NS cell clone, with 283.5 ± 13.3 (25% position), 288.7 ± 11.4 (50% position), and 314.5 ± 11.5 (75% position) photoreceptors in CNTF-treated retinas, as compared with 87.8 ± 3.0, 92.7 ± 8.9, and 110.0 ± 6.7 photoreceptors at the 25%, 50%, and 75% positions in control retinas, respectively (Fig. 6C). CNTF-treated Pde6brd1 eyes thus contained 2.9- to 3.2-fold more photoreceptors than the contralateral eyes with the grafted control-NS cell clone (p < .001). Transplantations of three other CNTF-NS cell clones also resulted in significant protection of photoreceptors in this mutant (data not shown). A significant neuroprotective effect of the CNTF-NS cell clone was still detectable in 25-day-old Pde6brd1 mice (Fig. 4). At this advanced stage of retinal degeneration, CNTF-treated retinas contained 134.0 ± 15.5, 154.5 ± 13.7, and 177.2 ± 18.3 photoreceptors, whereas the contralateral control retinas contained 41.0 ± 5.3, 40.2 ± 4.4, and 44.2 ± 6.3 photoreceptors at the 25%, 50%, and 75% positions, respectively. CNTF-treated retinas thus contained up to 4.0-fold more photoreceptor cells than the control retinas (p < .001). We also analyzed whether the control-NS cell clone attenuate photoreceptor loss in Pde6brd1 mice but found similar photoreceptor numbers in eyes with grafted control-NS cells (80.5 ± 4.9, 88.8 ± 4.3, and 126.2 ± 10.5 photoreceptors at the 25%, 50%, and 75% positions, respectively) and contralateral eyes injected with PBS only (97.0 ± 8.3, 97.2 ± 12.3, and 117.2 ± 12.3 photoreceptors at the 25%, 50%, and 75% positions, respectively; supplemental online Fig. 4).
To compare the neuroprotective activity of the CNTF-NS cell bulk culture and the CNTF-NS cell clone in Pde6brd1 mice, we calculated the quotients of photoreceptor numbers in retinas with grafted CNTF-NS cells and contralateral retinas with grafted control-NS cells for both experimental groups at the three retinal positions. Values for animals that had received injections of NS cell bulk cultures and animals that had received injections of clonal NS cell lines were 2.42 ± 0.26 (mean ± SEM) and 3.25 ± 0.2 (25% position), 2.38 ± 0.17 and 3.24 ± 0.31 (50% position), and 2.1 ± 0.17 and 2.92 ± 0.23 (75% position), respectively (Fig. 6D). The CNTF-NS cell clone thus protected photoreceptors more effectively than the original CNTF-NS cell bulk culture (p < .01; Fig. 6D), in line with the elevated expression level of CNTF in the clonal cell line.
Discussion
The intraocular implantation of genetically modified and encapsulated cells is among the strategies to achieve a local and sustained administration of NFs to the retina, and it has been successfully used in large animal species [17, 18] and human patients with advanced retinitis pigmentosa or geographic atrophy [19–22]. However, the implantation of encapsulated cells is not easily applicable to the numerous genetic or acutely induced mouse models of degenerative retinal disorders because of the small eyes of this species. To establish a cell-based intraocular delivery system for preclinical studies aimed at evaluating and optimizing this therapeutic approach in mouse models of photoreceptor degeneration, we therefore searched for a cell type that allows a sustained administration of NFs to the dystrophic mouse retina without the need of prior encapsulation.
Neural stem/progenitor cells, isolated from the developing or adult brain or derived from ES cells, survive for extended periods of time after intraocular transplantation [23–27]. Furthermore, these cells have been shown to attenuate photoreceptor degeneration in the Royal College of Surgeons (RCS) rat [13, 28, 29] and a mouse model of neuronal ceroid lipofuscinoses [23], even when grafted without prior genetic modifications. We therefore argued that neural stem cells isolated from the embryonic mouse brain and cultivated under adherent conditions in the presence of EGF and FGF-2 may serve as vehicles to deliver NFs to the dystrophic mouse retina. Cells maintained under such conditions give rise to pure populations of symmetrically dividing clonogenic neural stem cells and have therefore been termed NS cells, in analogy to continuously self-renewing ES cells [32]. NS cells can be extensively expanded in vitro and give rise to neurons, astrocytes, and oligodendrocytes in culture and after transplantation into the brain [32] or spinal cord [33]. NS cell cultures have also been established from the adult mouse brain, fetal human brain, ES cells, and induced pluripotent stem cells [32, 45–47].
To analyze whether a sustained delivery of functionally relevant quantities of NFs to the dystrophic mouse retina can be achieved by intraocular transplantations of modified NS cells, we expressed a secretable variant of this cytokine in NS cells. CNTF was selected because of its profound neuroprotective effect on photoreceptors, which we used as a measure for the efficacy of our approach. Initially demonstrated to attenuate light-induced degeneration of photoreceptors [48], CNTF has subsequently been shown to protect photoreceptors in a variety of animal models of inherited and acquired photoreceptor loss [3]. Of note, the therapeutic potential of CNTF for the treatment of inherited retinal degenerations and geographic atrophy is currently being evaluated in clinical studies using encapsulated human RPE cells modified to secrete this cytokine, and some positive results have been reported [19–22]. However, although CNTF potently preserves retinal structure, it negatively affects retinal function in a reversible and dose-dependent manner [35–38, 49]. These negative effects of CNTF are accompanied by a dysregulated expression of various genes, including some that encode components of the phototransduction cascade [50, 51].
To express CNTF in NS cells, we used a polycistronic lentiviral vector additionally encoding a resistance and a reporter gene. This construct facilitated the selection of modified NS cells, the characterization of the cells after transplantation, and the generation of clonal NS cell lines with elevated expression levels of CNTF. Analyses of modified NS cell cultures indeed revealed expression of the reporter gene in virtually every cell, and immunocytochemistry and immunoblot analysis of CNTF-NS cells demonstrated expression of the cytokine for at least 40 passages, corresponding to a culture period of more than 6 months. Robust expression of CNTF was also observed in astrocytes and neurons derived from CNTF-NS cells in vitro.
Expression of the Venus reporter gene allowed the identification and characterization of the modified NS cells after transplantation into Pde6brd1 and Pde6brd10 mice. In both mouse lines, grafted NS cells survived in the vitreous, where they formed dense layers of Venus-positive cells that were attached to the retina and lens. The vast majority of donor cells were identified as astrocytes, and some were identified as neurons. The detection of only a few proliferating donor cells in Pde6brd1 and Pde6brd10 mice is in line with the rapid neural differentiation of the grafted cells. Furthermore, we found sustained expression of CNTF in donor cells derived from CNTF-NS cells, although donor cells derived from control-NS cell lacked detectable levels of the cytokine. Robust survival and sustained expression of transgenes in NS cell-derived donor cells were also observed in a mouse model of glaucoma, where astrocytes and neurons with an ectopic expression of NFs survived in the vitreous for at least 4 months after transplantations (Kai Flachsbarth and U.B., unpublished results). Despite the presence of numerous Venus-positive donor cells in the vitreous cavity, integration of grafted cells into the host retinas was not apparent. This observation differs from results of other studies that have shown extensive integration of intravitreally grafted neural stem/progenitor cells into developing or dystrophic retinas [26, 52]. Intravitreal transplantations of NS cells into Pde6brd1 or Pde6brd10 mice before the onset of retinal degeneration and/or intrinsic differences between the neural stem/progenitor cells used in the different studies might account for these discrepant results. In fact, although we have transplanted cell populations highly enriched in neural stem cells, the other studies have grafted murine neural progenitor cells expanded in neurosphere cultures [52] or neural progenitor cells from the hippocampus of adult rats [26, 52].
The neuroprotective potential of the CNTF-secreting NS cells was evaluated in Pde6brd1 and Pde6brd10 mice, two animal models of retinitis pigmentosa with an early onset and rapid degeneration of photoreceptor cells [39, 40]. Intravitreal injections of CNTF-NS cells from a bulk culture that produced 4.0 ng of CNTF per 1 × 106 cells in 24 hours resulted in significant protection of photoreceptors in Pde6brd1 mice. Depending on the retinal region analyzed, CNTF-treated retinas contained up to 2.4-fold more photoreceptors than the contralateral retinas with grafted control-NS cells. Intravitreally grafted CNTF-secreting NS cells from the same bulk culture significantly attenuated photoreceptor degeneration also in Pde6brd10 mice, with up to 2.7-fold more photoreceptors in CNTF-treated eyes than in control eyes. Of note, the neuroprotective effect was widespread in both mouse lines and evident in all retinal regions analyzed. Furthermore, the grafted NS cells had no adverse effects on the morphology of host retinas in either mouse strain, in contrast to other cell types that we tested in intravitreal transplantation experiments. Mesenchymal stem cells, for instance, attenuate photoreceptor degeneration without prior genetic modification [53] and thus represent another candidate cell type to deliver NFs to the dystrophic retina. In our hands, however, these cells caused local retinal detachments resulting in an aggravated loss of photoreceptors. Furthermore, the immortalized neural progenitor cell line C17.2 [54] continued to proliferate after intravitreal transplantations, and grafted retinal stem cells isolated from the developing neuroretina [55] survived only poorly in the vitreous.
Because nonmodified neural stem/progenitors have been shown to attenuate photoreceptor degeneration [13, 23, 28, 29], we additionally evaluated the neuroprotective potential of control-NS cells in Pde6brd1 and Pde6brd10 mutant mice. Photoreceptor numbers in eyes that had received injections of either control-NS cells or the vehicle only, however, were not significantly different from each other in either mouse line. These apparently discrepant observations might be related to particular properties of the cell populations used for transplantation, to the particular animal model analyzed, or both. For instance, nonmodified human neural progenitor cells attenuate photoreceptor degeneration in the RCS rat in which the inability of RPE cells to phagocytose shed photoreceptor outer segments leads to progressive retinal degeneration. This neuroprotective activity of the human neural progenitor cells has been attributed, at least in part, to the endogenous expression of the neurotrophic factors brain-derived neurotrophic factor, insulin-like growth factor 1, and FGF-2 in this cell population and/or to the capability of subretinally located donor cells to phagocytose shed photoreceptor outer segments [13, 28, 29].
Expression levels of transgenes in modified bulk cultures may differ between different cultures and between different passages of the same culture, impeding the delivery of defined quantities of transgenes and the analysis of dose-dependent effects of secreted gene products using a cell-based delivery system. We therefore took advantage of the fact that NS cell cultures consist of homogeneous populations of clonogenic stem cells, and we established CNTF-secreting clonal NS cell lines from NS cells with high expression levels of the reporter gene to derive NS cell clones with high expression levels of the cytokine. The clonal NS cell lines indeed stably expressed elevated levels of CNTF compared with the original bulk cultures, and they effectively protected photoreceptors in both mutant mouse lines. Furthermore, we found that intravitreal transplantations of a clonal NS cell line with an ∼35% increase in CNTF expression resulted in a significantly more pronounced photoreceptor protection in Pde6brd1 mice than intravitreal transplantations of the original NS cell bulk culture. Attenuation of photoreceptor degeneration by the clonal cell line was still apparent in Pde6brd1 mice at developmental ages when the outer nuclear layer in this mutant is normally almost completely degenerated [56].
Conclusion
Intravitreal transplantations of adherently cultivated neural stem cells modified to secrete CNTF resulted in a sustained delivery of functionally relevant quantities of the cytokine to the dystrophic mouse retina, as indicated by the significant attenuation of photoreceptor loss in Pde6brd1 and Pde6brd10 mutant mice. Furthermore, a coexpressed reporter gene greatly facilitated the characterization of the modified stem cells after transplantation and the derivation of clonal NS cell lines with high expression levels of CNTF and profound neuroprotective effects on photoreceptors. Adverse effects of the grafted cells on the morphology of host retinas were not observed. The combined results demonstrate that intravitreal transplantations of genetically modified NS cells represent a useful method for preclinical studies aimed at evaluating the therapeutic potential of a cell-based administration of NFs in mouse models of photoreceptor degeneration.
Supplementary Material
Acknowledgments
We are grateful to Elke Becker, Sabine Helbing, Stephen Peters, Stefanie Schlichting, and the FACS-Core Unit of the University Medical Center (UMC) Hamburg-Eppendorf for excellent technical support; Dr. Michael Sendtner (University of Würzburg) for CNTF antibodies; Dr. Fabio Morellini for help with the statistics; Kai Flachsbarth for help with the ELISA; Dr. Susanne Bartsch for comments on the manuscript; and Ali Derin for animal care. This work was supported by grants from the Pro Retina foundation and Ernst-und-Claere-Jung foundation (to U.B.) and the Deutsche Forschungsgemeinschaft (SFB841/Z2; to B.F.). K.R. was supported by a young investigator grant within the Forschungsförderungsfonds der Medizinischen Fakultät (FFM) of the UMC Hamburg-Eppendorf (NWF-12/09). J.S. is currently affiliated with the Tianjin Medical University Eye Hospital, Tianjin, China.
Author Contributions
G.J.: collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; J.S., B.P., K.R., K.K., and W.J.: collection and assembly of data, data analysis and interpretation, final approval of manuscript; F.K. and C.S.: collection and assembly of data; G.R.: financial support, final approval of manuscript; B.F.: provision of study material, data analysis and interpretation, final approval of manuscript; U.B.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Wright AF, Chakarova CF, Abd El-Aziz MM, et al. Photoreceptor degeneration: Genetic and mechanistic dissection of a complex trait. Nat Rev Genet. 2010;11:273–284. doi: 10.1038/nrg2717. [DOI] [PubMed] [Google Scholar]
- 2.West EL, Pearson RA, MacLaren RE, et al. Cell transplantation strategies for retinal repair. Prog Brain Res. 2009;175:3–21. doi: 10.1016/S0079-6123(09)17501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenzel A, Grimm C, Samardzija M, et al. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24:275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Dagnelie G. Retinal implants: Emergence of a multidisciplinary field. Curr Opin Neurol. 2012;25:67–75. doi: 10.1097/WCO.0b013e32834f02c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busskamp V, Picaud S, Sahel JA, et al. Optogenetic therapy for retinitis pigmentosa. Gene Ther. 2012;19:169–175. doi: 10.1038/gt.2011.155. [DOI] [PubMed] [Google Scholar]
- 6.Smith AJ, Bainbridge JW, Ali RR. Prospects for retinal gene replacement therapy. Trends Genet. 2009;25:156–165. doi: 10.1016/j.tig.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Wen R, Tao W, Li Y, et al. CNTF and retina. Prog Retin Eye Res. 2012;31:136–151. doi: 10.1016/j.preteyeres.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cideciyan AV. Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retin Eye Res. 2010;29:398–427. doi: 10.1016/j.preteyeres.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 10.Buch PK, MacLaren RE, Ali RR. Neuroprotective gene therapy for the treatment of inherited retinal degeneration. Curr Gene Ther. 2007;7:434–445. doi: 10.2174/156652307782793531. [DOI] [PubMed] [Google Scholar]
- 11.Andrieu-Soler C, Aubert-Pouessel A, Doat M, et al. Intravitreous injection of PLGA microspheres encapsulating GDNF promotes the survival of photoreceptors in the rd1/rd1 mouse. Mol Vis. 2005;11:1002–1011. [PubMed] [Google Scholar]
- 12.Cayouette M, Gravel C. Adenovirus-mediated gene transfer of ciliary neurotrophic factor can prevent photoreceptor degeneration in the retinal degeneration (rd) mouse. Hum Gene Ther. 1997;8:423–430. doi: 10.1089/hum.1997.8.4-423. [DOI] [PubMed] [Google Scholar]
- 13.Gamm DM, Wang S, Lu B, et al. Protection of visual functions by human neural progenitors in a rat model of retinal disease. PLoS One. 2007;2:e338. doi: 10.1371/journal.pone.0000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGee Sanftner LH, Abel H, Hauswirth WW, et al. Glial cell line derived neurotrophic factor delays photoreceptor degeneration in a transgenic rat model of retinitis pigmentosa. Mol Ther. 2001;4:622–629. doi: 10.1006/mthe.2001.0498. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki M, Ikeda Y, Yonemitsu Y, et al. Simian lentiviral vector-mediated retinal gene transfer of pigment epithelium-derived factor protects retinal degeneration and electrical defect in Royal College of Surgeons rats. Gene Ther. 2003;10:1503–1511. doi: 10.1038/sj.gt.3302028. [DOI] [PubMed] [Google Scholar]
- 16.Read SP, Cashman SM, Kumar-Singh R. POD nanoparticles expressing GDNF provide structural and functional rescue of light-induced retinal degeneration in an adult mouse. Mol Ther. 2010;18:1917–1926. doi: 10.1038/mt.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tao W, Wen R, Goddard MB, et al. Encapsulated cell-based delivery of CNTF reduces photoreceptor degeneration in animal models of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2002;43:3292–3298. [PubMed] [Google Scholar]
- 18.Thanos CG, Bell WJ, O'Rourke P, et al. Sustained secretion of ciliary neurotrophic factor to the vitreous, using the encapsulated cell therapy-based NT-501 intraocular device. Tissue Eng. 2004;10:1617–1622. doi: 10.1089/ten.2004.10.1617. [DOI] [PubMed] [Google Scholar]
- 19.Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: Phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci USA. 2006;103:3896–3901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talcott KE, Ratnam K, Sundquist SM, et al. Longitudinal study of cone photoreceptors during retinal degeneration and in response to ciliary neurotrophic factor treatment. Invest Ophthalmol Vis Sci. 2011;52:2219–2226. doi: 10.1167/iovs.10-6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang K, Hopkins JJ, Heier JS, et al. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc Natl Acad Sci USA. 2011;108:6241–6245. doi: 10.1073/pnas.1018987108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kauper K, McGovern C, Sherman S, et al. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases. Invest Ophthalmol Vis Sci. 2012;53:7484–7491. doi: 10.1167/iovs.12-9970. [DOI] [PubMed] [Google Scholar]
- 23.Meyer JS, Katz ML, Maruniak JA, et al. Embryonic stem cell-derived neural progenitors incorporate into degenerating retina and enhance survival of host photoreceptors. Stem Cells. 2006;24:274–283. doi: 10.1634/stemcells.2005-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pressmar S, Ader M, Richard G, et al. The fate of heterotopically grafted neural precursor cells in the normal and dystrophic adult mouse retina. Invest Ophthalmol Vis Sci. 2001;42:3311–3319. [PubMed] [Google Scholar]
- 25.Takahashi M, Palmer TD, Takahashi J, et al. Widespread integration and survival of adult-derived neural progenitor cells in the developing optic retina. Mol Cell Neurosci. 1998;12:340–348. doi: 10.1006/mcne.1998.0721. [DOI] [PubMed] [Google Scholar]
- 26.Young MJ, Ray J, Whiteley SJ, et al. Neuronal differentiation and morphological integration of hippocampal progenitor cells transplanted to the retina of immature and mature dystrophic rats. Mol Cell Neurosci. 2000;16:197–205. doi: 10.1006/mcne.2000.0869. [DOI] [PubMed] [Google Scholar]
- 27.Banin E, Obolensky A, Idelson M, et al. Retinal incorporation and differentiation of neural precursors derived from human embryonic stem cells. Stem Cells. 2006;24:246–257. doi: 10.1634/stemcells.2005-0009. [DOI] [PubMed] [Google Scholar]
- 28.McGill TJ, Cottam B, Lu B, et al. Transplantation of human central nervous system stem cells: Neuroprotection in retinal degeneration. Eur J Neurosci. 2012;35:468–477. doi: 10.1111/j.1460-9568.2011.07970.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Girman S, Lu B, et al. Long-term vision rescue by human neural progenitors in a rat model of photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2008;49:3201–3206. doi: 10.1167/iovs.08-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 31.Tropepe V, Sibilia M, Ciruna BG, et al. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol. 1999;208:166–188. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- 32.Conti L, Pollard SM, Gorba T, et al. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005;3:e283. doi: 10.1371/journal.pbio.0030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glaser T, Pollard SM, Smith A, et al. Tripotential differentiation of adherently expandable neural stem (NS) cells. PLoS One. 2007;2:e298. doi: 10.1371/journal.pone.0000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sendtner M, Carroll P, Holtmann B, et al. Ciliary neurotrophic factor. J Neurobiol. 1994;25:1436–1453. doi: 10.1002/neu.480251110. [DOI] [PubMed] [Google Scholar]
- 35.Liang FQ, Aleman TS, Dejneka NS, et al. Long-term protection of retinal structure but not function using RAAV.CNTF in animal models of retinitis pigmentosa. Mol Ther. 2001;4:461–472. doi: 10.1006/mthe.2001.0473. [DOI] [PubMed] [Google Scholar]
- 36.McGill TJ, Prusky GT, Douglas RM, et al. Intraocular CNTF reduces vision in normal rats in a dose-dependent manner. Invest Ophthalmol Vis Sci. 2007;48:5756–5766. doi: 10.1167/iovs.07-0054. [DOI] [PubMed] [Google Scholar]
- 37.Schlichtenbrede FC, MacNeil A, Bainbridge JW, et al. Intraocular gene delivery of ciliary neurotrophic factor results in significant loss of retinal function in normal mice and in the Prph2Rd2/Rd2 model of retinal degeneration. Gene Ther. 2003;10:523–527. doi: 10.1038/sj.gt.3301929. [DOI] [PubMed] [Google Scholar]
- 38.Wen R, Song Y, Kjellstrom S, et al. Regulation of rod phototransduction machinery by ciliary neurotrophic factor. J Neurosci. 2006;26:13523–13530. doi: 10.1523/JNEUROSCI.4021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaVail MM, Sidman RL. C57BL-6J mice with inherited retinal degeneration. Arch Ophthalmol. 1974;91:394–400. doi: 10.1001/archopht.1974.03900060406015. [DOI] [PubMed] [Google Scholar]
- 40.Chang B, Hawes NL, Pardue MT, et al. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vision Res. 2007;47:624–633. doi: 10.1016/j.visres.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giménez E, Montoliu L. A simple polymerase chain reaction assay for genotyping the retinal degeneration mutation (Pdeb(rd1)) in FVB/N-derived transgenic mice. Lab Anim. 2001;35:153–156. doi: 10.1258/0023677011911525. [DOI] [PubMed] [Google Scholar]
- 42.Ader M, Schachner M, Bartsch U. Integration and differentiation of neural stem cells after transplantation into the dysmyelinated central nervous system of adult mice. Eur J Neurosci. 2004;20:1205–1210. doi: 10.1111/j.1460-9568.2004.03577.x. [DOI] [PubMed] [Google Scholar]
- 43.Weber K, Bartsch U, Stocking C, et al. A multicolor panel of novel lentiviral “gene ontology” (LeGO) vectors for functional gene analysis. Mol Ther. 2008;16:698–706. doi: 10.1038/mt.2008.6. [DOI] [PubMed] [Google Scholar]
- 44.Weber K, Mock U, Petrowitz B, et al. Lentiviral gene ontology (LeGO) vectors equipped with novel drug-selectable fluorescent proteins: New building blocks for cell marking and multi-gene analysis. Gene Ther. 2010;17:511–520. doi: 10.1038/gt.2009.149. [DOI] [PubMed] [Google Scholar]
- 45.Pollard SM, Conti L, Sun Y, et al. Adherent neural stem (NS) cells from fetal and adult forebrain. Cereb Cortex. 2006;16(suppl 1):i112–i120. doi: 10.1093/cercor/bhj167. [DOI] [PubMed] [Google Scholar]
- 46.Onorati M, Camnasio S, Binetti M, et al. Neuropotent self-renewing neural stem (NS) cells derived from mouse induced pluripotent stem (iPS) cells. Mol Cell Neurosci. 2010;43:287–295. doi: 10.1016/j.mcn.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Sun Y, Pollard S, Conti L, et al. Long-term tripotent differentiation capacity of human neural stem (NS) cells in adherent culture. Mol Cell Neurosci. 2008;38:245–258. doi: 10.1016/j.mcn.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 48.LaVail MM, Unoki K, Yasumura D, et al. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci USA. 1992;89:11249–11253. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bush RA, Lei B, Tao W, et al. Encapsulated cell-based intraocular delivery of ciliary neurotrophic factor in normal rabbit: Dose-dependent effects on ERG and retinal histology. Invest Ophthalmol Vis Sci. 2004;45:2420–2430. doi: 10.1167/iovs.03-1342. [DOI] [PubMed] [Google Scholar]
- 50.Rhee KD, Ruiz A, Duncan JL, et al. Molecular and cellular alterations induced by sustained expression of ciliary neurotrophic factor in a mouse model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2007;48:1389–1400. doi: 10.1167/iovs.06-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wen R, Song Y, Liu Y, et al. CNTF negatively regulates the phototransduction machinery in rod photoreceptors: Implication for light-induced photostasis plasticity. Adv Exp Med Biol. 2008;613:407–413. doi: 10.1007/978-0-387-74904-4_48. [DOI] [PubMed] [Google Scholar]
- 52.Sakaguchi DS, Van Hoffelen SJ, Theusch E, et al. Transplantation of neural progenitor cells into the developing retina of the Brazilian opossum: An in vivo system for studying stem/progenitor cell plasticity. Dev Neurosci. 2004;26:336–345. doi: 10.1159/000082275. [DOI] [PubMed] [Google Scholar]
- 53.Inoue Y, Iriyama A, Ueno S, et al. Subretinal transplantation of bone marrow mesenchymal stem cells delays retinal degeneration in the RCS rat model of retinal degeneration. Exp Eye Res. 2007;85:234–241. doi: 10.1016/j.exer.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 54.Snyder EY, Deitcher DL, Walsh C, et al. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. 1992;68:33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- 55.Merhi-Soussi F, Angenieux B, Canola K, et al. High yield of cells committed to the photoreceptor fate from expanded mouse retinal stem cells. Stem Cells. 2006;24:2060–2070. doi: 10.1634/stemcells.2005-0311. [DOI] [PubMed] [Google Scholar]
- 56.Carter-Dawson LD, LaVail MM, Sidman RL. Differential effect of the rd mutation on rods and cones in the mouse retina. Invest Ophthalmol Vis Sci. 1978;17:489–498. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.