This concise review reports the current knowledge about resident or exogenous stem/progenitor populations and their derived bioproducts demonstrating therapeutic effects in kidney regeneration upon injury. In addition, possible approaches to nephrogenesis and organ generation using organoids, decellularized kidneys, and blastocyst complementation are surveyed.
Keywords: Kidney, Marrow stromal stem cells, Renal, Progenitor cells
Abstract
The kidney is a specialized low-regenerative organ with several different types of cellular lineages; however, the identity of renal stem/progenitor cells with nephrogenic potential and their preferred niche(s) are largely unknown and debated. Most of the therapeutic approaches to kidney regeneration are based on administration of cells proven to enhance intrinsic reparative capabilities of the kidney. Endogenous or exogenous cells of different sources were tested in rodent models of ischemia-reperfusion, acute kidney injury, or chronic disease. The translation to clinics is at the moment focused on the role of mesenchymal stem cells. In addition, bioproducts from stem/progenitor cells, such as extracellular vesicles, are likely a new promising approach for reprogramming resident cells. This concise review reports the current knowledge about resident or exogenous stem/progenitor populations and their derived bioproducts demonstrating therapeutic effects in kidney regeneration upon injury. In addition, possible approaches to nephrogenesis and organ generation using organoids, decellularized kidneys, and blastocyst complementation are surveyed.
Introduction
The intrinsic capacity for tissue repair and regeneration necessary to reacquire functionality after ischemic, toxic, or inflammatory insults is limited in the mammalian kidney. Acute kidney injury remains a major cause of in-hospital morbidity and mortality, despite the supply of supportive care. In addition, partial/altered remodeling of the damaged tissue, as a consequence of repeated acute injuries, may lead to tissue fibrosis and possibly to organ failure. Regeneration of injured portions of the tubules may occur through mechanisms that rely on the intrinsic ability of resident progenitors or dedifferentiated resident cells to proliferate and substitute the damaged tissue. Approaches based on stem/progenitor cell administration or pharmacological modulation can be viewed as a promising option able to foster this intrinsic renal regeneration (Fig. 1). At variance with this, in chronic renal failure, in which most of the functioning tissue is lost, a stem cell-based therapy would need to regenerate whole new nephrons. Although in lower species, such as in fish and amphibians, nephrogenesis may occur, this mechanism is lost in mammals. Attempts to generate new nephrons are currently under investigation using organ culture and decellularized renal tissue (Fig. 1).
Figure 1.
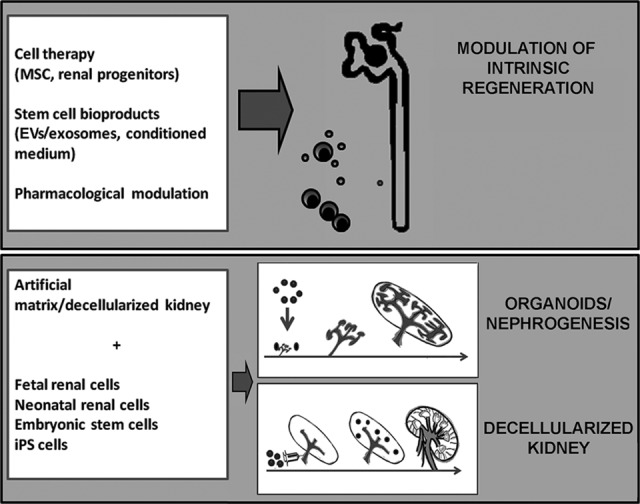
Schematic illustration depicting the different approaches to renal repair and regeneration. Therapeutic approaches using stem/progenitor cells, bioproducts, or drugs can be viewed as a promising option able to foster an intrinsic renal regeneration. The possibility of regenerating whole new nephrons (i.e., nephrogenesis) is required when the functioning renal tissue is lost and renal progenitors are exhausted. Renal organoids or bioengineered kidneys could generate a critical mass of functioning renal tissue, possibly exploiting artificial devices and decellularized kidneys. Abbreviations: EV, extracellular vesicle; iPS, induced pluripotent stem; MSC, mesenchymal stem cell.
In this review, we present data on the role of progenitor/stem cells in renal regeneration and on the perspectives of their clinical application in short to medium term, discussing the advantages, limitations, and future challenges related to this rapidly evolving field. Three areas of interest are covered: (a) the possible use of mesenchymal stem cells (MSCs) of different origins and their possible substitution with MSC-derived bioproducts, such as microvesicles and exosomes; (b) the involvement of resident progenitors cells in renal regeneration; and (c) the recent advances in nephrogenesis and kidney organ reconstruction using organoids, decellularized kidney, and blastocyst complementation. These different approaches are shown in Table 1.
Table 1.
Different approaches for renal tissue repair using stem/progenitor cells and organ generation
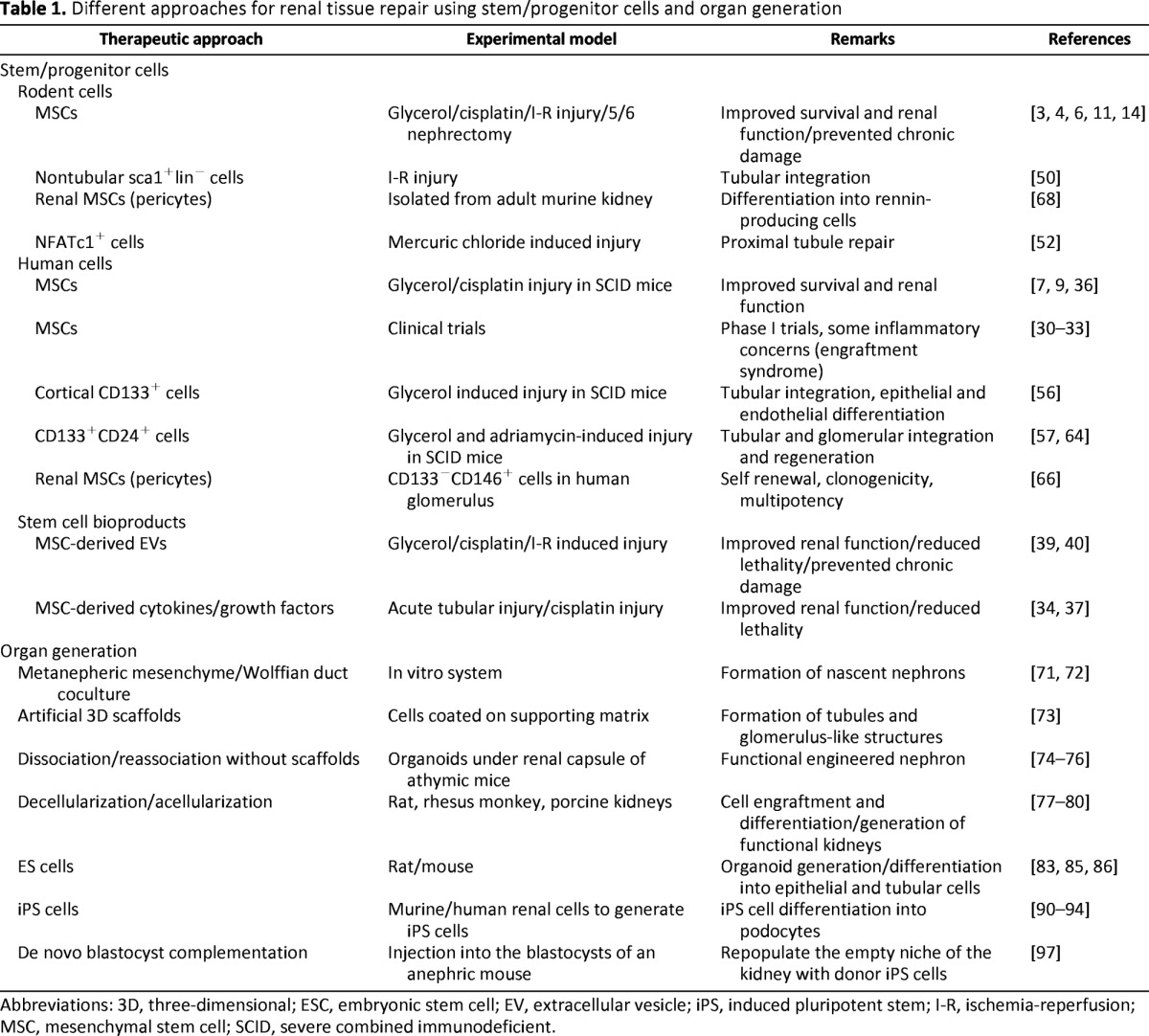
Abbreviations: 3D, three-dimensional; ESC, embryonic stem cell; EV, extracellular vesicle; iPS, induced pluripotent stem; I-R, ischemia-reperfusion; MSC, mesenchymal stem cell; SCID, severe combined immunodeficient.
Cell Therapy With MSCs
Experimental Models, Sources, and Administration
MSCs are the most established type of stem cells to support renal repair and the most advanced in clinical development. They can typically be isolated from bone marrow (BM), but other sources of cells sharing similar phenotype and properties have been reported, such as adipose tissue and neonatal birth-associated tissues (umbilical cord, placenta, and amniotic fluid) [1]. Although MSCs are able to differentiate in multiple cell lines, including the renal one, it is now accepted that their behavior in vivo after therapeutic administration is mainly restricted to mesodermal tissues [2]. Despite the lack of direct nephrogenic differentiation potential, BM-MSCs from rodents and humans have been shown to enhance the intrinsic recovery of kidney injury in experimental models of acute renal injury induced by ischemic or toxic stimuli by paracrine mechanisms [3–6]. In these studies, treatment with BM-MSCs promoted proximal tubular cell proliferation, counteracted apoptosis, and, preserving microvascular integrity, contributed to ameliorate renal tissue oxygenation (Fig. 2).
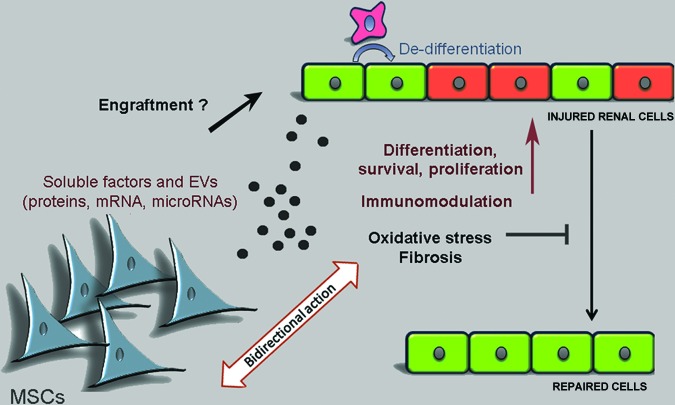
Figure 2.
Mechanisms of MSCs in renal repair. Administered MSCs exert a protective action in renal repair mainly through paracrine factors, including proteins and EV-released genetic material. Mechanisms involved in tissue repair include dedifferentiation and proliferation of normal resident cell, immunomodulation, inhibition of fibrosis, and scavenger effect of oxidative stress. In addition, a bidirectional action of paracrine factors/EVs (from MSCs to injured cells and back) may contribute to the modulation of tissue repair. Engraftment in the injured tissue could also participate in tissue repair. Abbreviations: EV, extracellular vesicle; MSC, mesenchymal stem cell.
The beneficial effect of BM-MSCs was subsequently confirmed using MSCs from other sources, with some differences and peculiarities related to the source of origin. Morigi et al., comparing BM- and cord blood-derived MSCs, showed a higher efficacy of cord blood-derived MSCs in preventing lethality in an acute model of renal injury induced by cisplatin [7]. This finding was ascribed to a higher expression of genes involved in matrix remodeling and in angiogenesis in cord blood-derived MSCs than in BM-MSCs [8]. Similarly, Hauser et al. reported an increased persistence of amniotic fluid-derived MSCs in the kidney with respect to BM-MSCs, with cells detectable 2 weeks after the injection [9]. Differences could also be ascribed to species, as reported for porcine adipose-tissue MSCs, which showed a reduced immune-modulating activity compared with those from mice or humans, resulting in limited protection in an acute kidney injury model [10].
Regarding chronic renal failure, some conflicting results were reported on the effect of MSCs in models of renal fibrosis. MSCs were shown to exert a beneficial effect in rat models of fibrosis induced by 5/6 nephrectomy or by unilateral ureteral obstruction [11, 12]. Furthermore, MSCs reduced fibrosis in a porcine model of renal artery stenosis [13]. The effect of MSCs in preventing the renal fibrotic processes may be mainly related to protection from injury development, rather than to regeneration of the damaged tissue, as shown in a rat model of chronic allograft nephropathy [14]. In contrast, no clinical response was observed when cryopreserved, allogeneic adipose MSCs were tested in cats with spontaneous kidney failure [15].
The possible use of BM-MSCs from patients with end-stage kidney disease may also present some limitation. Uremia was reported to affect the cell functional properties, thus possibly preventing the possible use of autologous BM-MSCs [16, 17]. At variance with this, Reinders et al. [18] showed that BM-MSCs from patients with end-stage renal disease were suitable for autologous therapy. This is of particular importance as these cells are the only ones, at present, used in clinical trials. Interestingly, it was recently shown by the same group that MSCs derived from adipose tissue were not affected by uremia [19] and provided renal protection in a model of chronic renal failure and fibrosis [20].
MSCs isolated from adipose tissue also offer advantages compared with BM-MSCs in terms of collection, tissue processing, and clinical complications. In fact, the collection of bone marrow aspirate is a painful and invasive procedure, can be accompanied by the risk of infection, and sometimes yields low numbers of MSCs after processing [1].
The method of administration of MSCs may influence their localization into damaged tissues, as MSCs are prone to being trapped in the tissue capillaries of lung, spleen, and liver [21]. The administration route through the distal thoracic aorta (from the left carotid or femoral artery) may provide benefits in comparison with intravenous administration, as it avoids the pulmonary circulation obstruction. However, the intravascular administration of MSCs may lead to prothrombotic events [22]. For these reasons, different routes of MSC administration have been verified in animal models of kidney diseases. Direct MSC implantation into the renal parenchyma [23] and injection into the renal subcapsular region [24] have been proven to be efficient. In a recent work, Zhuo et al. [25] compared the biodistribution of MSCs by bioluminescence imaging tracing after their injection in the tail vein or in the renal artery in a rat model of kidney injury. In this study, even though MSCs were entrapped in lung, liver, and spleen, both administrations showed improvement of the damage, suggesting that the paracrine effect exerted by MSCs localized in distant sites could be responsible for their beneficial effects [25]. Interestingly, a meta-analysis of experimental models of renal failure in mice and rats (21 studies analyzed) showed that arterial delivery of MSCs caused greater reduction in elevated creatinine compared with intrarenal delivery and intravenous injection [26].
MSCs: Clinical Trials
Based on their anti-inflammatory and tissue regenerating properties [27, 28], BM-MSCs are currently being used in clinical trials for the regeneration and repair of different tissues (kidney, liver, lung, and heart) (see ClinicalTrials.gov, http://www.clinicaltrials.gov). In nephrology, the first phase I clinical trial evaluated the effect of allogeneic MSC administration to open-heart surgery patients at high risk of acute renal failure [29]. The results demonstrated the safety and efficacy of MSC therapy, as no treated patient required hemodialysis. Two subsequent trials, performed to evaluate the effect of MSCs in renal transplant patients, have recently been published [30, 31]. Although the protollerogenic effects of MSCs in organ transplants are not discussed here, as they are beyond the scope of this review, these clinical trials have shown that MSCs were safe and provided an early recovery of graft function. However, early post-transplant MSC infusion was found to be possibly associated with acute graft dysfunction (one patient out of two in the trial) because of MSC localization into the graft, called engraftment syndrome [30]. This negative effect, accompanied by neutrophil recruitment and complement C3 deposition, was possibly attributable to the inflammatory milieu occurring after kidney transplantation, leading to altered MSC functions [32].
Recently, a phase I study of autologous BM-MSCs was set up for the treatment of allograft rejection after renal transplantation from living donors [33]. In this work, MSCs were given intravenously, at intervals of 7 days, to patients when a protocol renal biopsy at 1 or 6 months showed signs of rejection and/or an increase in interstitial fibrosis or tubular atrophy. Despite the low number of patients treated (n = 6), two renal biopsies after MSC treatment showed resolution of interstitial fibrosis and tubular atrophy and reduction of cell infiltrates, suggesting a regenerative and anti-inflammatory effect of the cell therapy on the renal tissue [33]. The negative short-term effect of MSC administration referred to the engraftment syndrome was not observed in this trial. This could have been due to a late timing of MSC administration, beyond 6 months, after transplantation [33].
Therapeutic Effect of MSC-Derived Growth Factors and Extracellular Vesicles
It has been established that MSCs interact with resident cells in endocrine and paracrine ways, releasing growth factors, cytokines, prostaglandins, enzymes, or extracellular vesicles [27, 34, 35] (Fig. 2). Exploiting the mechanisms of the paracrine effect of MSCs, MSC-derived products themselves can be explored as a therapeutic tool. MSC-derived conditioned medium containing growth and anti-inflammatory factors, such as hepatocyte growth factor, vascular endothelial growth factor, and insulin-like growth factor (IGF), was shown to allow kidney recovery after injury, in analogy with cell injection [34, 36, 37].
The cell activity of MSCs was also compared with that of MSC-derived extracellular vesicles (EVs). These membrane-covered particles released by cells can be subdivided on the basis of size, origin, and protein markers [38]. Exosomes have a mean size of 100 nm and are released from intracytoplasmatic multivesicular bodies. Microvesicles are instead released by membrane budding and have a larger size, ranging from 100 to 1,000 nm. Regardless of the differences in origin and size, EVs are characterized by their content in proteins, receptors, mRNAs, and microRNAs, which interact with the target cells, causing a biological effect [38].
MSC-derived EVs injected in veins have been shown to display a therapeutic role, improving the renal function in a glycerol-induced acute injury model and preventing lethality in a cisplatin-induced acute renal damage, as reported for MSCs themselves [39–41]. In particular, the effect of EVs collected from an overnight MSC culture was comparable to that obtained by injecting the same number of cells [39]. The effect of EVs is considered the result of EV uptake by damaged human tubular epithelial cells, as shown in vivo a few hours after administration using labeled EVs [39], and was shown to be related to the transfer of mRNA and microRNAs [39–41]. In fact, when conditioned medium or EVs from MSCs were treated with RNase to degrade RNA, the therapeutic effects were blunted. Indeed, human proteins were shown in the renal tissue of mice injected with human MSC-derived vesicles [39]. Moreover, a study by Tomasoni et al. [42] proved that MSC-derived EVs induced proliferation of cisplatin-damaged tubular cells by transferring the mRNA for IGF-1 receptor. In addition, repeated injections of MSC-derived EVs prevented renal fibrosis from occurring after ischemia-reperfusion injury [43].
All these preclinical data open new perspectives for the possible use of EVs or conditioned medium in clinical trials as in the case of MSCs. Although further studies regarding the safety, effectiveness, and biodistribution of MSC-derived EVs are required, they appear to be an alternative therapeutic approach to cells with several advantages. The potential risks associated with a stem cell therapy, such as maldifferentiation or tumor development, could be avoided. Cryopreserved EVs can be injected directly, thus appearing to be a ready-to-use drug. However, information on the required dosage of EVs is still insufficient, and comparison among experiments is difficult. EV administration dosage was mainly based on EV protein content [39–41], which may suffer from extravesicle protein contamination. Standardization of the number of EVs or of EV-donating cells could be useful.
Moreover, a relevant limit of the EV-based therapy is the absence of cell modulation by the local microenvironment encountered in vivo in the site of injury. Indeed, MSCs have been shown to modulate their therapeutic properties if exposed to a conducive inflammatory environment, so that a bidirectional influence from MSCs to the tissue and back may enhance and promote their effects [44] (Fig. 2). To mimic this aspect, MSCs could be conditioned in vitro before EV collection, using, for instance, a hypoxic treatment [45].
Modulation of Resident Progenitor Cells
Nephron development in mammals requires the differentiation of a renal progenitor population of mesenchymal cells into epithelial cells. Following the outgrowth of the ureteric bud, mesenchymal cells aggregate near the tips of newly formed branches, undergo mesenchymal to epithelial differentiation, and establish the renal vesicle, the precursor for the glomerular and renal tubule compartment [46]. The distal aspects of the branched duct therefore represent the niche for renal progenitors undergoing mesenchymal to epithelial transition [47]. In the adult kidney, this nephrogenic mesenchymal progenitor population disappears, possibly because of the loss of its niche [48]. Several studies, however, identified the presence of resident mesenchymal/epithelial progenitors in rodents and humans. We discuss here the possible role of these populations in renal repair and their potential therapeutic applications.
Adult Renal Progenitors
The presence of resident cells with the capability to differentiate into cells of the nephron and to contribute to renal repair has been shown in several murine studies [49–53]. Although these cells were identified with different markers and were localized in different parts of the nephron, they all shared the ability to resist apoptotic damage and to proliferate during renal injury. When injected in vivo, renal stem/progenitor cells contributed to injury repair by integrating into the tubular cells and interstitial space, and when transplanted into the metanephric kidney, they integrated into the epithelial components of the nephron [49–54].
Investigating the mechanisms of proximal tubular cell regeneration, Humphreys et al. [55] showed that the repair was mediated by scattered resident injured tubular cells that had survived the injury, suggesting that regeneration should be ascribed to differentiated tubular cells undergoing dedifferentiation and therefore excluding the presence of a cell niche of stem cells with slow cycling characteristics. In contrast, experiments from Langworthy et al. [52] demonstrated a specific resident population of cells identified by the transcription factor Nfatc1. This cell population survived to apoptosis and promoted proliferation and tubule repair in a mercury chloride-induced acute kidney injury model. In particular, 75% of reconstitution of proximal tubular cells derived from Nfatc1+ cells, indicating their role as the predominant source of proliferating cells required for tubular repair [52]. A possible explanation for these apparent discrepancies is the presence of a cell population with progenitor characteristics not in quiescence but rather in proliferation, which is able to survive and repopulate the nephron after injury.
We first identified and characterized a resident population with progenitor characteristics along the human nephron using the CD133 stem cell marker [56]. CD133+ cells were subsequently identified in different segments of nephron, being localized in the Bowman's capsule of the glomeruli, in the proximal tubules as well as in the inner medullary papilla region, including Henle's loop and the S3 limb segment [57, 58]. CD133+ progenitors exhibited the embryonic renal markers Pax-2, Six 1, and Six 2 and several mesenchymal stem cell markers, such as CD29, CD73, and CD90 [56–58].
The exact nature of CD133+ progenitors is uncertain: they could represent a pre-existing population able to survive injury, or rather a dedifferentiated population acquiring progenitor characteristics after damage. Moreover, the different populations of CD133+ that were reported in different nephron segments exhibit different properties and possibly different functions. Cortical CD133+ cells in proximal tubules were reported to proliferate in response to renal damage [59, 60]. In fact, the number of CD133+ cells was increased in the tubules of transplanted patients undergoing delayed graft function as a result of acute renal injury [59], as well as in tubules of patients with proteinuric glomerular diseases [60].
CD133+ cells isolated from the Henle's loop showed higher differentiation ability and stemness markers compared with those in tubules [58]. This may be due to the hypoxic environment of the inner medulla, which has been reported to induce the stem cell transcription factor OCT4A under the control of hypoxia inducible factor-1 [58]. The presence of cells with higher activation of hypoxia inducible and stem cell programs could explain the increased ability of the medulla to resist insults with respect to the cortex. In addition, these medullary CD73+/CD133+ cells were recently shown to synthesize and release erythropoietin under hypoxia [61]. Beside erythropoiesis, CD133+cell-released erythropoietin may play tissue-specific physiologic roles such as modulation of angiogenesis and cell survival.
Experiments on the administration of CD133+ cells in murine models of glycerol-induced acute kidney injury and of adriamycin-induced glomerular damage showed improvement of the renal function, suggesting the feasibility of cell therapy with human isolated CD133+ renal progenitors [56, 57, 61, 62]. However, at present, the possible application of these cells cannot be envisaged, for several reasons such as the scarce availability of the cells, the need for compatibility among patients, and the availability of other sources of effective cells as MSCs. In any case, the possible modulation of renal progenitors in situ using pharmacological approaches, or alternatively the modulation of tubular cells toward the acquisition of progenitor characteristics to repair kidney injury, is of interest. It can also be speculated that mesenchymal-like CD133 progenitors might be directed to divergent fates, including fibrosis formation, depending on a different microenvironment or the duration of the injury [63]. The knowledge of these aspects could lead to new pharmacological approaches targeting renal progenitors. For instance, as the pharmacologic inhibition of prolyl hydroxylase was shown to enhance erythropoietin release by CD73+/CD133+ progenitors [61], a new rationale for the use of prolyl hydroxylase inhibitors in clinical settings of acute or chronic renal injury might be envisaged. In addition, the influence of currently used drugs, such as steroids and calcineurin inhibitors, on adult renal progenitors of glomeruli and tubules is of interest. For instance, angiotensin-converting enzyme inhibitors were reported to limit an excessive proliferation of glomerular progenitors in a rat model of progressive glomerular injury and to favor glomerular repair [64]. Finally, MSC therapy itself may promote progenitor cell survival and proliferation, and dedifferentiation of resident cells. The possible identification of new pharmacological therapies for progenitor cell manipulation is a promising approach for kidney regeneration.
Renal Pericytes/MSCs
Resident MSCs have been described in almost all organs as a multipotent population adjacent to endothelial cells in the microvasculature and characterized by expression of platelet-derived growth factor receptor, CD146, neuron glial antigen-2, and coexpression of other mesenchymal markers [65]. In the kidney, resident MSCs have been first isolated in the murine glomeruli [66], in the light of its vascular nature. MSCs were subsequently confirmed in the human glomeruli as CD133−/CD146+ cells with self-renewal capability, clonogenicity, and multipotency [67]. Recently, murine renal MSCs cells isolated from the adult whole kidney were shown to differentiate into rennin-producing cells [68]. The comparative evaluation of gene expression in MSCs of renal and bone marrow origin identified a selected patterns of genes in renal MSCs possibly related to a memory of tissue origin [69]. This suggests that renal MSCs might display organ-specific regenerative capacities that could overcome those of MSCs from unrelated organs and that could be exploited for therapeutic applications [69].
Organ Generation
Renal Organoids
Although the different cell-based experimental approaches mentioned above may represent an optimistic prospect of renal disease management, the unmet need of whole-organ renal transplants forces us to consider other approaches of tissue engineering and whole organ culture (Fig. 1). Different strategies have been tested for constructing renal organoids from the recombination of cultured renal progenitor cells and/or primordial tissue in vitro on three-dimensional (3D) scaffolds [70–73]. A renal assist device was generated using cloned metanephros cultured on collagen-coated cylindrical polycarbonate membrane and subsequently transplanted in mice [70]. The subcutaneous implants retrieved showed functional properties, with urine-like liquid production six times higher than the control, and generated vascularized structures with tubules and glomeruli [70]. Rosines et al. [71] established in vitro a coculture system of ureteric bud and metanephric mesenchyme cells recapitulating the reciprocal induction of kidney development observed in the embryo. In the presence of growth factors, Wolffian duct was induced to bud, and each ureteric bud was induced to branch and subsequently recombined with metanephric mesenchyme. After a few days of mutual induction, cells developed a branched collecting duct system and formation of nascent nephrons resembling a late-stage embryonic kidney [71]. In a subsequent study, the authors demonstrated the feasibility to recapitulate 3D growth and branching morphogenesis in vitro when kidney rudiments were embedded in a 3D matrix [72]. Similarly, Joraku et al. [73] developed a 3D in vitro culture system of murine primary renal cells to form tubules and glomerulus-like structures using rat tail collagen type 1 as supporting matrix. When in vitro generated kidney rudiments were implanted under the renal capsule of host rat, they vascularized, formed glomeruli [71], and survived for more than 5 weeks [72].
Recently, a new method for the development of functional organoids in the absence of 3D scaffolds was reported using single-cell suspensions of fully dissociated murine embryonic day 11.5 kidneys [74], by modification of a single cell-based dissociation-reassociation method of producing embryonic renal tissues from suspensions of embryonic mouse renal cells [75]. When this 5-day in vitro-cultured structure was injected under the renal capsule of athymic rats, it developed vascularized glomeruli, mature tubular structures, and a functional engineered nephron with the capacity for filtration, reabsorption, and erythropoietin release [74]. Interestingly, human kidney epithelial cells isolated from tissues obtained from nephrectomized patients showed the ability to generate renal organoids in the absence of 3D scaffolds [76]. These cellular aggregates or spheroids could be regenerated even upon enzymatic disaggregation in vitro and expressed nephron progenitor genes such as Pax2 and Sal1. In addition, in comparison with monolayer cells, the human spheroids increased the expression of previously described renal progenitor markers, including the epithelial cell adhesion molecule, CD24 and CD133, and aldehyde dehydrogenase 1 activity [76]. However, the functional properties of these organoids were not tested.
Among the different strategies that aim to build efficient kidney tissue, the generation of renal organoids in the absence of 3D scaffolds showed interesting aspects of cell differentiation into a complex nephron-like structure, with functional properties [74, 75]. This might be due to the recapitulation of nephrogenesis mechanisms within the assay. The use of different cell types, such as induced pluripotent stem (iPS) cells, eventually differentiated in the renal lineages, could further improve this technology. However, at the moment, the use of small organoids appears quite far from the generation of the critical mass of nephrons required for renal function. Therefore, the use of decellularized kidneys engineered with different cell types, described below, appears more promising.
Decellularized Kidney
The kidney has a complex anatomical and physiological structure that leads to various difficulties in engineering a whole organ ex vivo. However, advances have been obtained using decellularized tissue matrices that mimic native 3D architecture to generate in vitro renal tissues in rat [77], rhesus monkey [78], and pig [79]. Kidneys were decellularized using washing detergents and seeded with different cell types, including renal fetal kidney explants and embryonic cells [77, 78]. Interestingly, decellularized matrix was reported to contain growth factors, in addition to matrix proteins [78]. More recently, a functional kidney was generated by recellularization of a rat decellularized kidney using both human endothelial cells injected through the renal artery and rat neonatal kidney cells retrogradely injected through the ureter [80]. It appears therefore that rather than stem cells, endothelial and epithelial cells, possibly of neonatal tissue, are sufficient to repopulate a kidney [80]. However, this method of generating whole organ culture is still in its early stage, and extensive research is needed to identify the optimal sample source, age, decellularization protocol, viability, and functional parameters of these cultures.
Toward Programming: Embryonic Stem Cells and Induced Pluripotent Stem Cells
Embryonic stem (ES) cells are pluripotent stem cells able to give rise under appropriate culture conditions to all cell lineages of the body [81]. Although research on human ES cells is limited by ethical concerns, extensive research was conducted on murine ES cells to establish methods to efficiently induce differentiation into renal cells (reviewed in [82]). In vitro, ES cells were able to generate both renal progenitors with markers of intermediate mesoderm or fully differentiated renal-like cells expressing WT-1 and rennin [83, 84]. In addition, mouse ES cells have been successfully integrated into renal structures [85–87], suggesting a possible efficacy for kidney repair. In particular, labeled ES cells microinjected into developing metanephros in organ culture were exclusively located in the cortical nephrogenic zone and differentiated into epithelial cells resembling renal tubules and, occasionally, into glomerular tufts [85–87]. Furthermore, when injected in vivo in mice, ES cells integrated into proximal tubules of newborn mice and were detectable until 7 months without teratoma formation [87].
At present, ES cell application is considered to be impractical for application in clinics because of both ethical and compatibility issues. These problems have become resolvable by using iPS cells, which can be generated from somatic cells of patients and subsequently redifferentiated/dedifferentiated in the different cell types required [88]. Because a persistent genome-wide epigenetic memory of the somatic cell of origin can be retained in iPS cells [89], the possible renal origin of iPS cells could provide some advantages for renal cell therapy or organ reconstruction. This was achieved, for instance, using iPS cells from human mesangial cells that were differentiated in podocyte and, once engrafted in a metanephric kidney, integrated in the developing glomeruli [90, 91]. iPS cells have been also obtained from renal tubular epithelial cells [92] and, interestingly, from human exfoliated renal epithelial cells present in the urine [93, 94]. The function of kidney lineage cells derived from ES or iPS cells remains to be determined [82].
In addition, it appears difficult to replicate in vitro the complex interactions occurring during organogenesis among cells and tissues to generate a whole organ from iPS cells. A novel possible approach for organ generation is the de novo blastocyst complementation, in which cells are microinjected into a blastocyst in which the development of a certain organ is precluded by genetic manipulation [95, 96]. Using this technique, chimeric kidneys were successfully obtained by injecting murine iPS cells into the blastocysts of anephric Sall1−/− mice [97]. In addition, the collecting ducts and the vascular system of the iPS-derived kidneys were of both donor and host recipient origin, suggesting the possibility of integrating these different cell sources [97]. The iPS-derived kidneys appeared to be functional, as shown by urine accumulation in the bladders of neonatal Sall1−/− mice derived from the complemented blastocysts. However, it was not possible to evaluate the proper function of the iPS-derived kidneys because of the lack of progression of the mice to adulthood, and further studies will be essential to dissect this point [97].
Conclusion
In conclusion, stem/progenitor cells offer an intriguing opportunity to provide regeneration and repair of the renal tissue (Table 1). However, the high complexity of this organ and the absence of a population with the classic stem cell capability of organ homeostasis and nephron regeneration render this approach still distant. The most convincing data at the moment are the use of stem cells to enhance the intrinsic reparative capabilities of the kidney (Fig. 1). MSCs represent a promising cell resource for this approach, and the different characteristics of MSCs from different sources could be useful in defining different applications. MSCs from fetal tissues deserve a great interest for their enhanced potency, whereas those from adipose tissue present advantages of accessibility and could be ideal for autotransplants. Indeed, information from clinical trials is available only for BM-MSCs. Renal progenitors, on the other hand, do not seem to represent a potential cell source for cell therapy. Understanding the molecular mechanisms involved in their proliferation and differentiation could lead to the generation of specific pharmacological approaches to enhance renal repair. It is possible, however, that renal progenitors, as well as embryonic renal cells or induced renal progenitors, could be of interest in the generation of bioengineered kidneys. Bioproducts from stem/progenitor cells, such as microvesicles and exosomes, are likely a new promising approach of reprogramming resident cells. Several issues need to be solved before a possible use of EVs can be envisaged, including the elucidation of their mechanisms of action, their biodistribution, and, in particular, their dosage. Finally, the possibility of generating renal organoids or bioengineered decellularized kidneys could represent the cure for conditions of chronic renal damage, where the endogenous mechanisms of repair cannot be stimulated for loss of renal tissue, fibrosis, and progenitor cell exhaustion. Although several cell sources are candidates for the bioengineering studies, recent studies suggest that renal differentiated cells could be sufficient to repopulate a kidney, limiting the need and related problems of stem cells.
Acknowledgments
This work was supported by the European Union through the Research Training Network (RTN) Project Nephrotools. S.A. is a Marie Curie Fellow within the Nephrotools Project.
Author Contributions
S.A. and A.M.: provision of study material, manuscript writing; B.B.: conception and design, financial support, manuscript writing.
Disclosure of Potential Conflicts of Interest
S.A. has compensated employment and research funding.
References
- 1.Hass R, Kasper C, Böhm S, et al. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chamberlain G, Fox J, Ashton B, et al. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 3.Herrera MB, Bussolati B, Bruno S, et al. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14:1035–1041. [PubMed] [Google Scholar]
- 4.Morigi M, Imberti B, Zoja C, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–1804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 5.Morigi M, Introna M, Imberti B, et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075–2082. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- 6.Lange C, Tögel F, Ittrich H, et al. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68:1613–1617. doi: 10.1111/j.1523-1755.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 7.Morigi M, Rota C, Montemurro T, et al. Life-sparing effect of human cord blood-mesenchymal stem cells in experimental acute kidney injury. Stem Cells. 2010;28:513–522. doi: 10.1002/stem.293. [DOI] [PubMed] [Google Scholar]
- 8.Panepucci RA, Siufi JL, Silva WA, Jr., et al. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells. 2004;22:1263–1278. doi: 10.1634/stemcells.2004-0024. [DOI] [PubMed] [Google Scholar]
- 9.Hauser PV, De Fazio R, Bruno S, et al. Stem cells derived from human amniotic fluid contribute to acute kidney injury recovery. Am J Pathol. 2010;177:2011–2021. doi: 10.2353/ajpath.2010.091245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunswig-Spickenheier B, Boche J, Westenfelder C, et al. Limited immune-modulating activity of porcine mesenchymal stromal cells abolishes their protective efficacy in acute kidney injury. Stem Cells Dev. 2010;19:719–729. doi: 10.1089/scd.2009.0494. [DOI] [PubMed] [Google Scholar]
- 11.Choi S, Park M, Kim J, et al. The role of mesenchymal stem cells in the functional improvement of chronic renal failure. Stem Cells Dev. 2009;18:521–529. doi: 10.1089/scd.2008.0097. [DOI] [PubMed] [Google Scholar]
- 12.Asanuma H, Vanderbrink BA, Campbell MT, et al. Arterially delivered mesenchymal stem cells prevent obstruction-induced renal fibrosis. J Surg Res. 2011;168:e51–e59. doi: 10.1016/j.jss.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eirin A, Zhu XY, Krier JD, et al. Adipose tissue-derived mesenchymal stem cells improve evascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells. 2012;30:1030–1041. doi: 10.1002/stem.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franquesa M, Herrero E, Torras J, et al. Mesenchymal stem cell therapy prevents interstitial fibrosis and tubular atrophy in a rat kidney allograft model. Stem Cells Dev. 2012;21:3125–3135. doi: 10.1089/scd.2012.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quimby JM, Webb TL, Habenicht LM, et al. Safety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: Results of three sequential pilot studies. Stem Cell Res Ther. 2013;4:48. doi: 10.1186/scrt198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramann R, Couson SK, Neuss S, et al. Exposure to uremic serum induces a procalcific phenotype in human mesenchymal stem cells. Arterioscler Thromb Vasc Biol. 2011;31:e45–e54. doi: 10.1161/ATVBAHA.111.228601. [DOI] [PubMed] [Google Scholar]
- 17.Kramann R, Couson SK, Neuss S, et al. Uraemia disrupts the vascular niche in a 3D co-culture system of human mesenchymal stem cells and endothelial cells. Nephrol Dial Transplant. 2012;27:2693–2702. doi: 10.1093/ndt/gfr656. [DOI] [PubMed] [Google Scholar]
- 18.Reinders ME, Roemeling-van Rhijn M, Khairoun M, et al. Bone marrow-derived mesenchymal stromal cells from patients with end-stage renal disease are suitable for autologous therapy. Cytotherapy. 2013;15:663–672. doi: 10.1016/j.jcyt.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Roemeling-van Rhijn M, Reinders ME, de Klein A, et al. Mesenchymal stem cells derived from adipose tissue are not affected by renal disease. Kidney Int. 2012;82:748–758. doi: 10.1038/ki.2012.187. [DOI] [PubMed] [Google Scholar]
- 20.Villanueva S, Carreño JE, Salazar L, et al. Human mesenchymal stem cells derived from adipose tissue reduce functional and tissue damage in a rat model of chronic renal failure. Clin Sci. 2013;125:199–210. doi: 10.1042/CS20120644. [DOI] [PubMed] [Google Scholar]
- 21.Gao J, Dennis JE, Muzic RF, et al. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 22.Furlani D, Ugurlucan M, Ong L, et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res. 2009;77:370–376. doi: 10.1016/j.mvr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Caldas HC, Fernandes IM, Gerbi F, et al. Effect of whole bone marrow cell infusion in the progression of experimental chronic renal failure. Transplant Proc. 2008;40:853–855. doi: 10.1016/j.transproceed.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Cavaglieri RC, Martini D, Sogayar MC, et al. Mesenchymal stem cells delivered at the subcapsule of the kidney ameliorate renal disease in the rat remnant kidney model. Transplant Proc. 2009;41:947–951. doi: 10.1016/j.transproceed.2009.01.072. [DOI] [PubMed] [Google Scholar]
- 25.Zhuo W, Liao L, Fu Y, et al. Efficiency of endovenous versus arterial administration of mesenchymal stem cells for ischemia-reperfusion-induced renal dysfunction in rats. Transplant Proc. 2013;45:503–510. doi: 10.1016/j.transproceed.2012.07.162. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, He J, Pei X, et al. Systematic review and meta-analysis of mesenchymal stem/stromal cells therapy for impaired renal function in small animal models. Nephrology. 2013;18:201–208. doi: 10.1111/nep.12018. [DOI] [PubMed] [Google Scholar]
- 27.Gnecchi M, Zhang Z, Ni A, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 29.Tögel FE, Westenfelder C. Mesenchymal stem cells: A new therapeutic tool for AKI. Nat Rev Nephrol. 2010;6:179–183. doi: 10.1038/nrneph.2009.229. [DOI] [PubMed] [Google Scholar]
- 30.Perico N, Casiraghi F, Introna M, et al. Autologous mesenchymal stromal cells and kidney transplantation: A pilot study of safety and clinical feasibility. Clin J Am Soc Nephrol. 2011;6:412–422. doi: 10.2215/CJN.04950610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan J, Wu W, Xu X, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: A randomized controlled trial. JAMA. 2012;307:1169–1177. doi: 10.1001/jama.2012.316. [DOI] [PubMed] [Google Scholar]
- 32.Casiraghi F, Perico N, Remuzzi G. Mesenchymal stromal cells to promote solid organ transplantation tolerance. Curr Opin Organ Transplant. 2013;18:51–58. doi: 10.1097/MOT.0b013e32835c5016. [DOI] [PubMed] [Google Scholar]
- 33.Reinders ME, de Fijter JW, Roelofs H, et al. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: Results of a phase I study. Stem Cells Translational Medicine. 2013;2:107–111. doi: 10.5966/sctm.2012-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imberti B, Morigi M, Tomasoni S, et al. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007;18:2921–2928. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- 35.Bi B, Schmitt R, Israilova M, et al. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18:2486–2496. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- 36.Eliopoulos N, Zhao J, Bouchentouf M, et al. Human marrow-derived mesenchymal stromal cells decrease cisplatin renotoxicity in vitro and in vivo and enhance survival of mice post-intraperitoneal injection. Am J Physiol Renal Physiol. 2010;299:F1288–F1298. doi: 10.1152/ajprenal.00671.2009. [DOI] [PubMed] [Google Scholar]
- 37.Tögel F, Zhang P, Hu Z, et al. VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J Cell Mol Med. 2009;13:2109–2114. doi: 10.1111/j.1582-4934.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.György B, Szabó TG, Pásztói M, et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruno S, Grange C, Collino F, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reis LA, Borges FT, Simões MJ, et al. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One. 2012;7:e44092. doi: 10.1371/journal.pone.0044092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomasoni S, Longaretti L, Rota C, et al. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013;22:772–780. doi: 10.1089/scd.2012.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gatti S, Bruno S, Deregibus MC, et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26:1474–1483. doi: 10.1093/ndt/gfr015. [DOI] [PubMed] [Google Scholar]
- 44.Del Tatto M, Ng T, Aliotta JM, et al. Marrow cell genetic phenotype change induced by human lung cancer cells. Exp Hematol. 2011;39:1072–1080. doi: 10.1016/j.exphem.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang JW, Tsai HL, Chen CW, et al. Conditioned mesenchymal stem cells attenuate progression of chronic kidney disease through inhibition of epithelial-to-mesenchymal transition and immune modulation. J Cell Mol Med. 2012;16:2935–2949. doi: 10.1111/j.1582-4934.2012.01610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costantini F, Kopan R. Patterning a complex organ: Branching morphogenesis and nephron segmentation in kidney development. Dev Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kobayashi A, Valerius MT, Mugford JW, et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartman HA, Lai HL, Patterson Cessation of renal morphogenesis in mice. Dev Biol. 2007;310:379–387. doi: 10.1016/j.ydbio.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maeshima A, Yamashita S, Nojima Y. Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol. 2003;14:3138–3146. doi: 10.1097/01.asn.0000098685.43700.28. [DOI] [PubMed] [Google Scholar]
- 50.Dekel B, Zangi L, Shezen E, et al. Isolation and characterization of nontubular sca-1+lin-multipotent stem/ progenitor cells from adult mouse kidney. J Am Soc Nephrol. 2006;17:3300–3314. doi: 10.1681/ASN.2005020195. [DOI] [PubMed] [Google Scholar]
- 51.Kinomura M, Kitamura S, Tanabe K, et al. Amelioration of cisplatin-induced acute renal injury by renal progenitor-like cells derived from the adult rat kidney. Cell Transplant. 2008;17:143–158. doi: 10.3727/000000008783907008. [DOI] [PubMed] [Google Scholar]
- 52.Langworthy M, Zhou B, de Caestecker M, et al. NFATc1 identifies a population of proximal tubule cell progenitors. J Am Soc Nephrol. 2009;20:311–321. doi: 10.1681/ASN.2008010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oliver JA, Maarouf O, Cheema FH, et al. The renal papilla is a niche for adult kidney stem cells. J Clin Invest. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maeshima A, Sakurai H, Nigam SK. Adult kidney tubular cell population showing phenotypic plasticity, tubulogenic capacity and integration capability into developing kidney. J Am Soc Nephrol. 2006;17:188–198. doi: 10.1681/ASN.2005040370. [DOI] [PubMed] [Google Scholar]
- 55.Humphreys BD, Czerniak S, DiRocco DP, et al. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci USA. 2011;108:9226–9231. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bussolati B, Bruno S, Grange C, et al. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166:545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sagrinati C, Netti GS, Mazzinghi B, et al. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 58.Bussolati B, Moggio A, Collino F, et al. Hypoxia modulates the undifferentiated phenotype of human renal inner medullary CD133+ progenitors through Oct4/miR-145 balance. Am J Physiol Renal Physiol. 2012;302:F116–F128. doi: 10.1152/ajprenal.00184.2011. [DOI] [PubMed] [Google Scholar]
- 59.Loverre A, Capobianco C, Ditonno P, et al. Increase of proliferating renal progenitor cells in acute tubular necrosis underlying delayed graft function. Transplantation. 2008;85:1112–1119. doi: 10.1097/TP.0b013e31816a8891. [DOI] [PubMed] [Google Scholar]
- 60.Smeets B, Boor P, Dijkman H, et al. Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J Pathol. 2013;229:645–659. doi: 10.1002/path.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bussolati B, Lauritano C, Moggio A, et al. Renal CD133+/CD73+ progenitors produce erythropoietin under hypoxia and prolyl hydroxylase inhibition. J Am Soc Nephrol. 2013;24:1234–1241. doi: 10.1681/ASN.2012080772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ronconi E, Sagrinati C, Angelotti ML, et al. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20:322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simone S, Cosola C, Loverre A, et al. BMP-2 induces a profibrotic phenotype in adult renal progenitor cells through Nox4 activation. Am J Physiol Renal Physiol. 2012;303:F23–F34. doi: 10.1152/ajprenal.00328.2011. [DOI] [PubMed] [Google Scholar]
- 64.Benigni A, Morigi M, Rizzo P, et al. G inhibiting angiotensin-converting enzyme promotes renal repair by limiting progenitor cell proliferation and restoring the glomerular architecture. Am J Pathol. 2011;179:628–638. doi: 10.1016/j.ajpath.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 66.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 67.Bruno S, Bussolati B, Grange C, et al. Isolation and characterization of resident mesenchymal stem cells in human glomeruli. Stem Cells Dev. 2009;18:867–880. doi: 10.1089/scd.2008.0320. [DOI] [PubMed] [Google Scholar]
- 68.Wang H, Gomez JA, Klein S, et al. Mesenchymal stem cell-like cells contribute to juxtaglomerular cell recruitment. J Am Soc Nephrol. 2013;24:1263–1273. doi: 10.1681/ASN.2012060596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pelekanos RA, Li J, Gongora M, et al. Comprehensive transcriptome and immunophenotype analysis of renal and cardiac MSC-like populations supports strong congruence with bone marrow MSC despite maintenance of distinct identities. Stem Cell Res. 2012;8:58–73. doi: 10.1016/j.scr.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 70.Lanza RP, Chung HY, Yoo JJ, et al. Generation of histocompatible tissues using nuclear transplantation. Nat Biotechnol. 2002;20:689–696. doi: 10.1038/nbt703. [DOI] [PubMed] [Google Scholar]
- 71.Rosines E, Sampogna RV, Johkura K, et al. Staged in vitro reconstitution and implantation of engineered rat kidney tissue. Proc Natl Acad Sci USA. 2007;104:20938–20943. doi: 10.1073/pnas.0710428105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosines E, Johkura K, Zhang X, et al. Constructing kidney-like tissues from cells based on programs for organ development: Toward a method of in vitro tissue engineering of the kidney. Tissue Eng Part A. 2010;16:2441–2455. doi: 10.1089/ten.tea.2009.0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Joraku A, Stern KA, Atala A, et al. In vitro generation of three-dimensional renal structures. Methods. 2009;47:129–133. doi: 10.1016/j.ymeth.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 74.Xinaris C, Benedetti V, Rizzo P, et al. In vivo maturation of functional renal organoids formed from embryonic cell suspensions. J Am Soc Nephrol. 2012;23:1857–1868. doi: 10.1681/ASN.2012050505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Unbekandt M, Davies JA. Dissociation of embryonic kidneys followed by reaggregation allows the formation of renal tissues. Kidney Int. 2010;77:407–416. doi: 10.1038/ki.2009.482. [DOI] [PubMed] [Google Scholar]
- 76.Buzhor E, Harari-Steinberg O, Omer D, et al. Kidney spheroids recapitulate tubular organoids leading to enhanced tubulogenic potency of human kidney-derived cells. Tissue Eng Part A. 2011;17:2305–2319. doi: 10.1089/ten.TEA.2010.0595. [DOI] [PubMed] [Google Scholar]
- 77.Ross EA, Williams MJ, Hamazaki T, et al. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol. 2009;20:2338–2347. doi: 10.1681/ASN.2008111196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakayama KH, Batchelder CA, Lee CI, et al. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng Part A. 2010;16:2207–2216. doi: 10.1089/ten.tea.2009.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sullivan DC, Mirmalek-Sani SH, Deegan DB, et al. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials. 2012;33:7756–7764. doi: 10.1016/j.biomaterials.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 80.Song JJ, Guyette JP, Gilpin SE, et al. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19:646–651. doi: 10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 82.Osafune K. In vitro regeneration of kidney from pluripotent stem cells. Exp Cell Res. 2010;316:2571–2577. doi: 10.1016/j.yexcr.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 83.Bruce SJ, Rea RW, Steptoe AL, et al. In vitro differentiation of murine embryonic stem cells toward a renal lineage. Differentiation. 2007;75:337–334. doi: 10.1111/j.1432-0436.2006.00149.x. [DOI] [PubMed] [Google Scholar]
- 84.Schuldiner M, Yanuka O, Itskovitz-Eldor J. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2000;97:11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Steenhard BM, Isom KS, Cazcarro P, et al. Integration of embryonic stem cells in metanephric kidney organ culture. J Am Soc Nephrol. 2005;16:1623–1631. doi: 10.1681/ASN.2004070584. [DOI] [PubMed] [Google Scholar]
- 86.Vigneau C, Polgar K, Striker G, et al. Mouse embryonic stem cell-derived embryoid bodies generate progenitors that integrate long term into renal proximal tubules in vivo. J Am Soc Nephrol. 2007;18:1709–1720. doi: 10.1681/ASN.2006101078. [DOI] [PubMed] [Google Scholar]
- 87.Kim D, Dressler GR. Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Nephrol. 2005;16:3527–3534. doi: 10.1681/ASN.2005050544. [DOI] [PubMed] [Google Scholar]
- 88.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 89.Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Song B, Niclis JC, Alikhan MA, et al. Generation of induced pluripotent stem cells from human kidney mesangial cells. J Am Soc Nephrol. 2011;22:1213–1220. doi: 10.1681/ASN.2010101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Song B, Smink AM, Jones CV, et al. The directed differentiation of human iPS cells into kidney podocytes. PLoS One. 2012;7:e46453. doi: 10.1371/journal.pone.0046453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Montserrat N, Ramírez-Bajo MJ, Xia Y, et al. Generation of induced pluripotent stem cells from human renal proximal tubular cells with only two transcription factors, OCT4 and SOX2. J Biol Chem. 2012;287:24131–24138. doi: 10.1074/jbc.M112.350413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang WW, Wang W, Jiang Y, et al. Reprogramming of mouse renal tubular epithelial cells to induced pluripotent stem cells. Cytotherapy. 2013;15:578–585. doi: 10.1016/j.jcyt.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 94.Zhou T, Benda C, Dunzinger S. Generation of human induced pluripotent stem cells from urine samples. Nat Protoc. 2012;7:2080–2089. doi: 10.1038/nprot.2012.115. [DOI] [PubMed] [Google Scholar]
- 95.Kobayashi T, Yamaguchi T, Hamanaka S, et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142:787–799. doi: 10.1016/j.cell.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 96.Matsunari H, Nagashima H, Watanabe M, et al. Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc Natl Acad Sci USA. 2013;110:4557–4562. doi: 10.1073/pnas.1222902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Usui J, Kobayashi T, Yamaguchi T, et al. Generation of kidney from pluripotent stem cells via blastocyst complementation. Am J Pathol. 2012;180:2417–2426. doi: 10.1016/j.ajpath.2012.03.007. [DOI] [PubMed] [Google Scholar]