It was hypothesized that the intravenous injection of multipotent adult progenitor cells (MAPCs) after traumatic brain injury (TBI) attenuates the inflammatory response and improves performance at motor tasks and spatial learning. MAPCs were administered intravenously to rodents 2 and 24 hours after a cortical contusion injury. It was found that intravenous MAPC treatment offers long-term improvements in cognitive behavior after TBI.
Keywords: Adult stem cells, Neuroimmune, Rat model, Stem/progenitor cell
Abstract
We previously demonstrated that the intravenous delivery of multipotent adult progenitor cells (MAPCs) after traumatic brain injury (TBI) in rodents provides neuroprotection by preserving the blood-brain barrier and systemically attenuating inflammation in the acute time frame following cell treatment; however, the long-term behavioral and anti-inflammatory effects of MAPC administration after TBI have yet to be explored. We hypothesized that the intravenous injection of MAPCs after TBI attenuates the inflammatory response (as measured by microglial morphology) and improves performance at motor tasks and spatial learning (Morris water maze [MWM]). MAPCs were administered intravenously 2 and 24 hours after a cortical contusion injury (CCI). We tested four groups at 120 days after TBI: sham (uninjured), injured but not treated (CCI), and injured and treated with one of two concentrations of MAPCs, either 2 million cells per kilogram (CCI-2) or 10 million cells per kilogram (CCI-10). CCI-10 rats showed significant improvement in left hind limb deficit on the balance beam. On the fifth day of MWM trials, CCI-10 animals showed a significant decrease in both latency to platform and distance traveled compared with CCI. Probe trials revealed a significant decrease in proximity measure in CCI-10 compared with CCI, suggesting improved memory retrieval. Neuroinflammation was quantified by enumerating activated microglia in the ipsilateral hippocampus. We observed a significant decrease in the number of activated microglia in the dentate gyrus in CCI-10 compared with CCI. Our results demonstrate that intravenous MAPC treatment after TBI in a rodent model offers long-term improvements in spatial learning as well as attenuation of neuroinflammation.
Introduction
Traumatic brain injury (TBI) causes a myriad of complex cellular changes that can lead to devastating long-term physical and cognitive deficits and even permanent loss of function. The initial brain injury is followed by secondary changes, including edema and cell death. A variety of effector cells (neutrophils and monocytes) from the peripheral blood accumulate in the injured regions of the brain, where they participate in activation of microglia, the resident macrophages of the brain. Microglia are integral participants in the postinjury inflammatory response [1], and their activation depends upon the biochemical milieu [2]. Resident microglia in the presence of anti-inflammatory cytokines such as interleukin-4 (IL-4) and IL-10 are generally in a resting/ramified state [3]. In an unperturbed environment, microglia have a distinct morphology, consisting of a small, static cell body with dynamic and branched processes. After a central nervous system (CNS) injury, activated microglia become phagocytic [4], retract their processes, and acquire a distinct amoeboid-like morphology. Although a microglial response is necessary to remove necrotic tissue and induce myelin repair, prolonged microglial activation can potentially lead to phagocytosis of healthy neurons and astrocytes and subsequently affect behavior. Recent evidence demonstrates the role of activated microglia in the development of neuropathic pain behavior [5]. Classically activated microglia participate in the early phase of the immune response to TBI and are responsible for continued production of proinflammatory and potentially cytodestructive substances. In addition to activation of resident microglia due to the injury and subsequent breakdown of the blood-brain barrier (BBB), an influx of infiltrating macrophages [6] and release of proinflammatory cytokines such as IL-1α, IL-1β, IL-6, and tumor necrosis factor-α (TNF-α) have been observed during the first 48 hours after injury as measured in the intracerebral fluid [7, 8].
There are few pharmacological treatments available for TBI; long-term treatments include motor, cognitive, and behavioral rehabilitation. Recently, cellular therapy has been used to attenuate the long-term effects of CNS injuries, in both clinical and preclinical models [9–11]. Multiple investigators have noted in stroke models that mesenchymal stromal cell and multipotent adult progenitor cell (MAPC) therapy leads to decreased tissue levels of proinflammatory cytokines IL-1α, IL-1β, TNF-α, and IL-6 and increased levels of anti-inflammatory cytokines such as IL-10 [11, 12].
Previously, we have demonstrated that an MAPC treatment regimen at 2 and 24 hours after injury preserves the BBB and modifies the inflammatory response systemically in rodents. Biodistribution studies demonstrated that a majority of the injected MAPCs are found in the spleen and lungs [13]. MAPC treatment increases the ratio of anti-inflammatory (alternatively activated) microglia/macrophages versus proinflammatory (activated) microglia/macrophages in mice at 72 hours postinjury [14]. MAPC treatment also preserves the BBB and reduces cerebral edema at early time points [15]. Menge et al. recently demonstrated preservation of BBB using mesenchymal stem cells with a dosing regimen identical to ours, at 2 and 24 hours postinjury [16]. Interestingly, a single dose at 72 hours after injury of autologous mononuclear cells also preserves the BBB and attenuates activated microglia via apoptosis [17].
Whereas MAPC treatment, along with other cell types, has demonstrated beneficial effects acutely after treatment in cortical contusion injury (CCI)-injured rats, the long-term effects of MAPC treatment are unknown. Based on our previous MAPC treatment regimen and acute data, we hypothesized that the intravenous administration of MAPCs after TBI would attenuate the inflammatory response as measured by enumeration of activated microglia/macrophages in brain tissue, leading to improved long-term cognitive function due to decreased activation of microglia/macrophages. To test this hypothesis, a series of experiments was performed to investigate the long-term effects of MAPCs on microglia/macrophage phenotype and on the cognitive and motor functions of rodents after TBI.
Materials and Methods
Controlled Cortical Injury
A pneumatic controlled cortical impact device (Pittsburgh Precision Instruments, Inc., Pittsburgh, PA, http://pghprecision.tripod.com) was used to administer a unilateral brain injury as described previously [18]. Male rats weighing 250–300 g were anesthetized with 4% isoflurane. The head was mounted in a stereotactic frame and held in a horizontal plane. A midline incision was made for exposure, and a 6–7-mm-diameter craniectomy was performed on the right cranial vault. The center of the craniectomy was placed at the midpoint between bregma and lambda, 3 mm lateral to the midline, overlying the temporoparietal cortex. Animals received a single impact of 2.7 mm depth of deformation with an impact velocity of 5.6 m/second and a dwell time of 150 milliseconds (moderate-severe injury) to the parietal association cortex orthogonal to the surface and at an angle of 10° from the vertical plane. A 6-mm-diameter flat impactor tip was used. Sham injuries were performed by anesthetizing the animals, making the midline incision, and separating the skin, connective tissue, and aponeurosis from the cranium. Incisions in both the sham-injured and TBI groups were closed using skin clips.
MAPC Preparation and Administration
Vials of frozen MAPCs were obtained from Athersys, Inc. (Cleveland, OH, http://www.athersys.com) and stored in liquid nitrogen until shortly before needed. MAPCs were quickly thawed in a water bath set at 37°C when needed. Cells were washed twice in phosphate-buffered saline (PBS), counted on a hemacytometer, checked for viability via Trypan blue exclusion, and finally resuspended in 1 ml of PBS at concentrations corresponding to a dose of either 2 million MAPCs per kilogram (CCI-2 group) or 10 million MAPCs per kilogram (CCI-10 group). Immediately prior to intravenous injection, suspensions were gently triturated 8–10 times to ensure a homogeneous mixture of cells. Each treatment animal received two doses of its assigned MAPC concentration via tail vein injection at 2 hours and 24 hours after CCI injury, whereas rats in the CCI group received vehicle (PBS) injections at the same time points.
Assessment of Motor Function
Rotarod
An accelerating rotarod, a 10-cm rotating cylinder connected to an electronic engine, was used to measure motor balance and coordination. Each rat underwent two trials per day for 5 days starting 48 hours after injury. Animals were placed on the stationary rotarod platform for 10 seconds. For each 10-second interval that the rat remained on the platform, the engine was accelerated three revolutions per minute (RPM) for a total of 120 seconds. If the animal fell off the platform or if the platform completed two full revolutions without the animal correcting itself, the test was stopped. The time and maximum RPM achieved were measured.
Balance Beam
After rotarod testing the animals were allowed to rest for 30–45 minutes before starting balance beam testing. Animals were placed on a 1.5-cm-wide beam for 60 seconds, or until they fell off, for three trials per day. Time on the beam before falling, number of times an animal's limb slipped off the beam while the animal was stationary on the beam or attempting to traverse it, and a novel left hind deficit measure regarding posture on the beam specific to location of the left hind limb were recorded. For the left hind deficit, we observed the animal's left hind limb placement on the balance beam. A score of 1 was given if the left hind limb was on the narrow portion on top the beam, indicating normal posture. If it was not on the narrow top portion of the beam, it was given a score of 0, indicating motor deficit.
Assessment of Cognitive Function
To assess spatial memory and learning, cognitive function was tested with the Morris water maze (MWM) 120 days after injury. Animals were given four trials per day over five consecutive days during each week of testing. Each trial consisted of gently placing the animal in the tank facing the wall in one of four starting locations (south, southeast, southwest, and northwest) chosen at random and allowing it to search for the platform for up to 60 seconds. If the animal failed to find the platform, it was placed upon the platform and allowed to remain for 30 seconds. Animal movement within the maze was monitored by a video camera linked to tracking software (EthoVision 3.1; Noldus Information Technology, Asheville, NC, http://www.noldus.com) to measure latency and distance traveled to platform. To examine memory retrieval, each animal was given a single probe trial 24 hours after completion of the platform testing. Probe trials consisted of removing the platform from the tank, then observing the movements of each rat when placed inside. Calculations were completed to determine the seconds the animal spent in the same quadrant as the platform, its first appearance in an area with a diameter three times the size of the platform (proximity measure), and the number of times the animal crossed over the previous location of the platform. In addition, animal swimming speed was calculated via the tracking software to verify that all animals were swimming at similar speeds.
Tissue Harvest
Following completion of all behavior testing on day 126, the animals were sacrificed. Under isoflurane anesthesia, each animal's chest was opened, and then using a right ventricle puncture technique the animals were simultaneously exsanguinated and perfused with 60 ml of ice-cold PBS followed by 60 ml of ice-cold 4% paraformaldehyde (PFA). After fixation, the brains were removed, postfixed in 4% PFA for 24 hours, and stored at 4°C.
Immunohistochemistry
After being postfixed in 4% PFA for 24 hours, the brains were transferred to a 30% sucrose solution, where they were maintained at 4°C for at least 72 hours and allowed to sink. Brains were then put in a 3% agar mold and sectioned into 50-μm-thick slices using a vibrating-blade microtome (Leica Microsystems, Bannockburn, IL, http://www.leica.com). Sections were stained using a standard free-floating staining protocol. Sections were washed twice in PBS with 0.01% Triton X-100 (PBST; T-8787; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) for 1 minute, incubated for 20–30 minutes in PBS with 0.2% Triton X-100, and blocked for 1 hour at room temperature (RT) in 3% goat serum (no. 005-000-121; Jackson Immunoresearch Laboratories, West Grove, PA, http://www.jacksonimmuno.com) in PBST. A rabbit polyclonal primary antibody was used to identify microglia/macrophages (IBA1 [1:100]; Wako, Richmond, VA, http://www.wakousa.com). The antibody was prepared in PBTB (PBS with 0.01% Triton X-100, 2% bovine serum albumin [A9647; Sigma-Aldrich]) and 1% goat serum, and sections were incubated at 4°C overnight. The next day the sections were rinsed briefly and then washed with PBST, and incubated with a goat anti-rabbit IgG secondary antibody (1:500; red/568:A 11011; Invitrogen, Carlsbad, CA, http://www.invitrogen.com) in PBTB for 2 hours at RT. The sections were again rinsed briefly, mounted on slides, and coverslipped with Fluoromount-G (SouthernBiotech, Birmingham, AL, http://www.southernbiotech.com).
Quantification of Immunohistochemistry
Photomicrographs were taken of the hippocampus at a magnification of ×20 using a Nikon fluorescent microscope (TE2000-U; Nikon, Tokyo, Japan, http://www.nikon.com). A single slice per animal from approximately midinjury was examined; the dentate gyrus, CA3, and CA1 regions were photomicrographed; and microglia were quantified. The data shown in the graphs represent cells in the dentate gyrus only. The entire dentate gyrus was photomicrographed (∼5 photomicrographs per slice). Labeled cells were then classified on the basis of morphology: IBA1+ cells with small, static cell bodies and dynamic and branched processes were classified as inactivated, cells with distinct amoeboid morphology were classified as activated, and cell bodies that were amoeboid with some branched processes were classified as slightly activated.
Statistical Analysis
Unless otherwise indicated, all values are presented as mean ± SEM. Values were compared using analysis of variance with a Fisher's least significance difference test. A p value of ≤.05 was used to denote statistical significance.
Results
MAPC Treatment Reduces Left Hind Limb Deficit on the Balance Beam
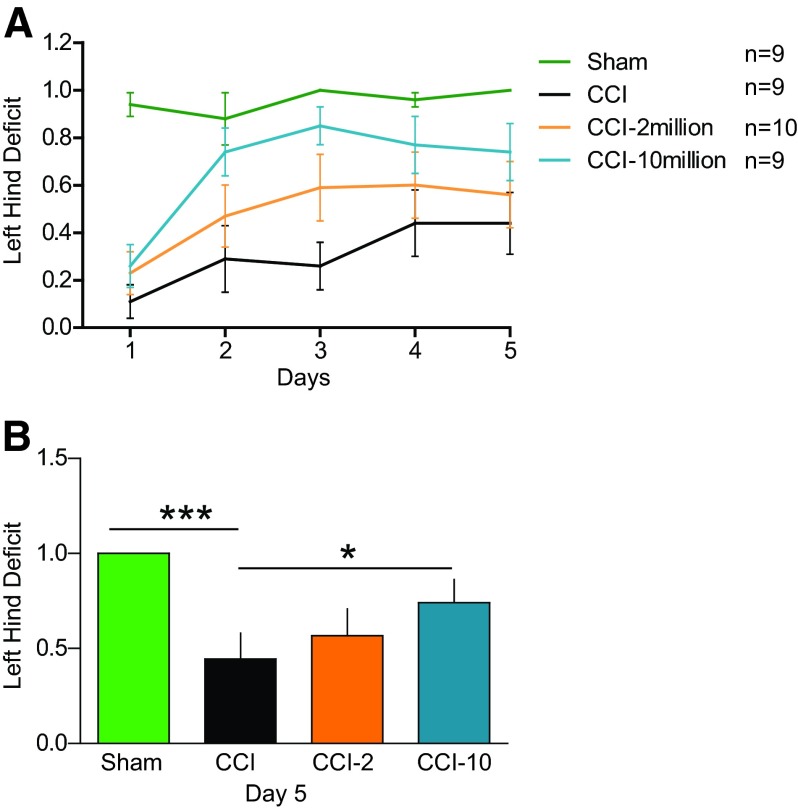
We observed a significant improvement (p < .05) in left hind deficit in the CCI-10 group (0.7 ± 0.1) compared with CCI (0.4 ± 0.1) on the fifth day of balance beam trials (Fig. 1) in addition to the expected significant difference (p < .005) between sham-injured (1.0) and CCI rats, the majority of which could not maintain proper hind limb posture on the beam.
Figure 1.
Intravenous multipotent adult progenitor cell (CCI-10) treatment at 2 and 24 hours after traumatic brain injury improved motor coordination on the balance beam by reducing left hind deficit on the balance beam. (A): Five-day training on the balance beam for left hind deficit. (B): Fifth day data of left hind deficit showing significant differences between CCI-10 versus CCI and sham versus CCI. *, p ≤ .05; ***, p ≤ .005. Abbreviations: CCI, cortical contusion injury (untreated); CCI-2, cortical contusion injury treated with 2 million cells per kilogram; CCI-10, cortical contusion injury treated with 10 million cells per kilogram.
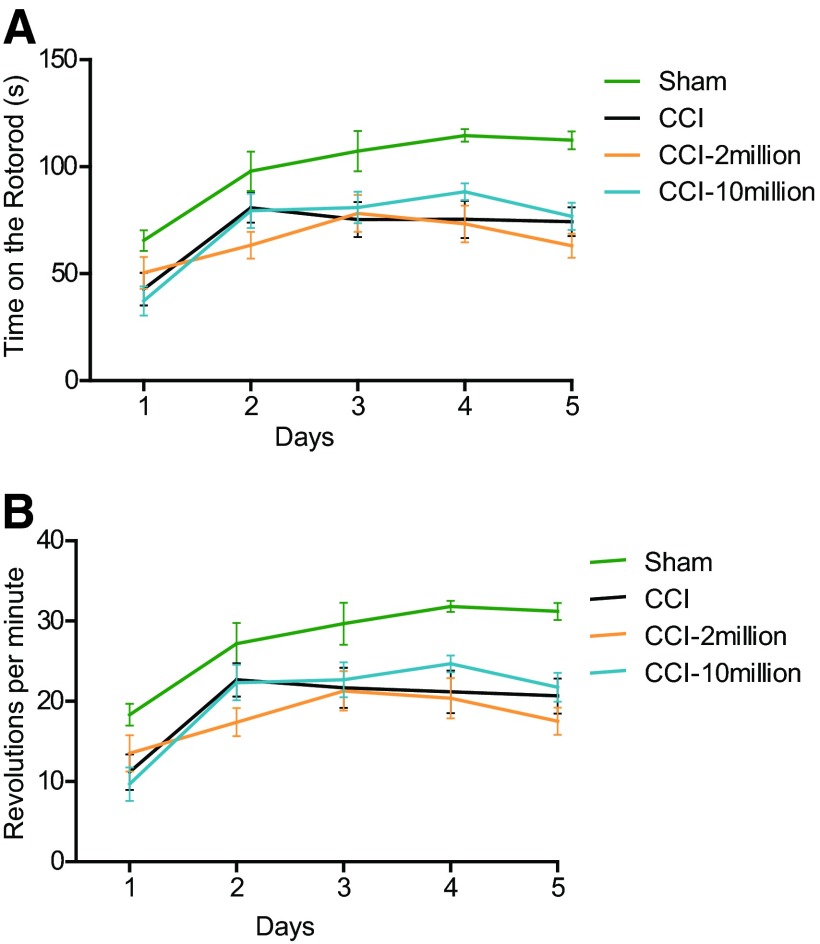
There were no significant differences among the groups for the duration spent on the beam and the number of slips on the fifth day of training (data not shown). In addition, we measured time spent on the rotarod and maximum RPM reached on the fifth day (Fig. 2). There were no significant differences in either measurement between CCI and treatment groups.
Figure 2.
Multipotent adult progenitor cell treatment did not affect endurance, balance, or coordination after traumatic brain injury as measured by the rotarod. (A): There were no significant differences in the time spent on the rotarod among the groups. (B): There were no significant differences in the revolutions per minute on the rotarod among the groups. Abbreviations: CCI, cortical contusion injury (untreated); CCI-2, cortical contusion injury treated with 2 million cells per kilogram; CCI-10, cortical contusion injury treated with 10 million cells per kilogram.
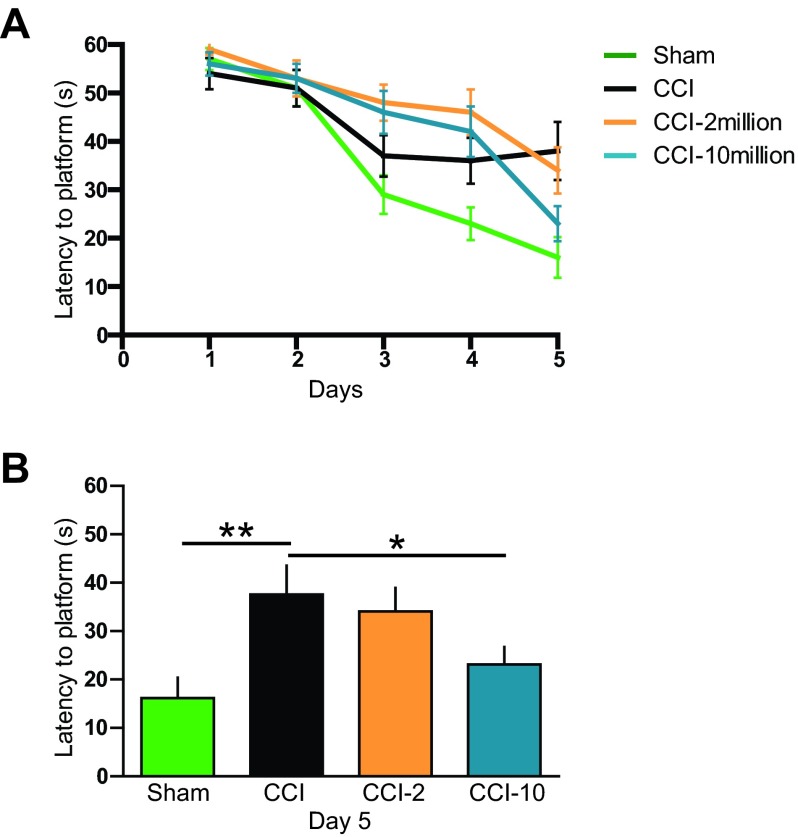
MAPC Treatment Improves Latency to Hidden Platform
Animals treated with the higher dose (CCI-10) showed significant improvement in the latency (23 ± 4 seconds; p < .05) to find the platform compared with CCI (38 ± 5.9 seconds) on the fifth day of training (Fig. 3B), approaching the performance of sham-injured animals (16 ± 4 seconds; p > .05), which was also significantly different from that of the CCI group (p < .01). There was no difference between the lower dose (CCI-2) and untreated CCI rats in latency to platform.
Figure 3.
Intravenous multipotent adult progenitor cell (CCI-10) treatment at 2 and 24 hours after traumatic brain injury improved spatial learning and memory as measured by the attenuation in latency to find the hidden platform. (A): Five-day spatial training in the Morris water maze. (B): Fifth-day comparison of latency among groups indicating a significant decrease in latency in CCI-10 in comparison with CCI. *, p ≤ .05; **, p ≤ .01. Abbreviations: CCI, cortical contusion injury (untreated); CCI-2, cortical contusion injury treated with 2 million cells per kilogram; CCI-10, cortical contusion injury treated with 10 million cells per kilogram.
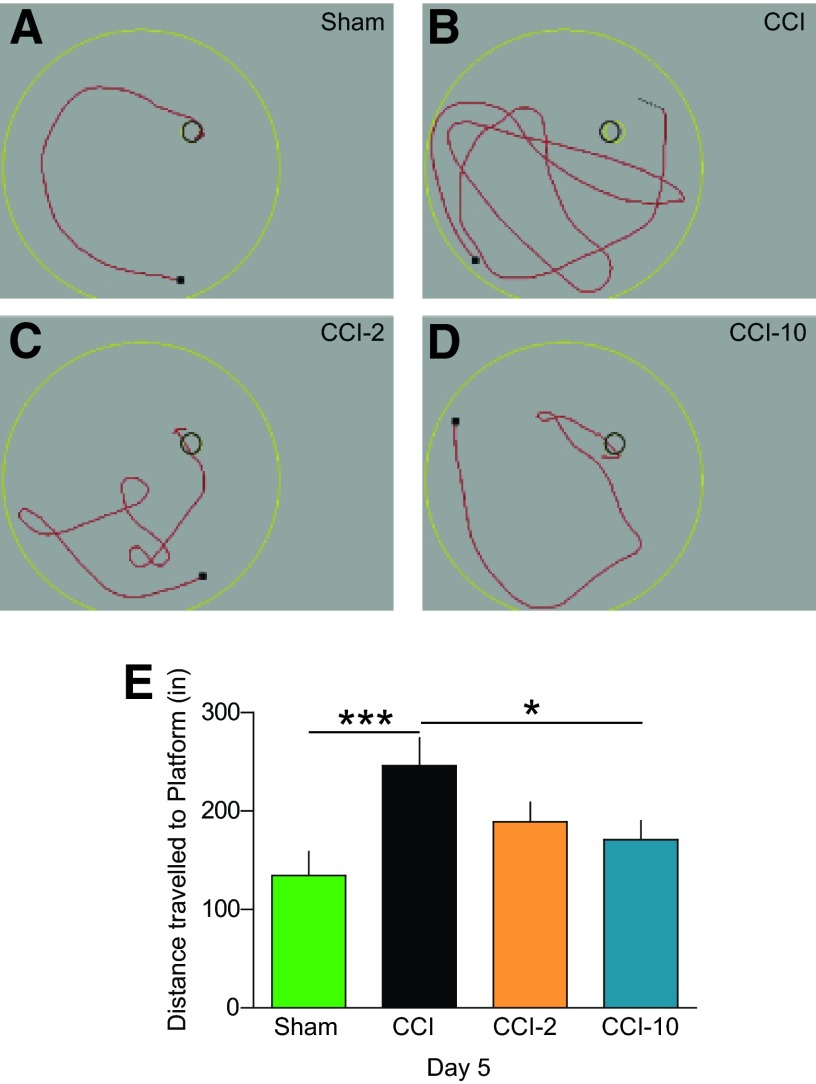
MAPC Treatment Reduces Distance Traveled to Hidden Platform
In addition to improving latency to hidden platform on the fifth day of training, MAPC-treated (CCI-10) rats traveled significantly less distance (171 ± 19 inches; p < .05) than CCI animals (246 ± 28 inches) to find the hidden platform (Fig. 4). Again, we observed the expected significant difference between sham-injured (135 ± 24 inches; p < .005) and CCI animals, but no significant differences between CCI and CCI-2 in distance traveled.
Figure 4.
Animals treated with multipotent adult progenitor cells (MAPCs) (CCI-10) traveled less distance to find a hidden platform. (A–D): Examples of distance traveled to find the hidden platform on the fifth day of training in the various groups. (E): Measurement of distance traveled. The treated animals traveled significantly less distance to the platform when comparing CCI-10 versus CCI. *, p ≤ .05; ***, p ≤ .005. Abbreviations: CCI, cortical contusion injury (untreated); CCI-2, cortical contusion injury treated with 2 million cells per kilogram; CCI-10, cortical contusion injury treated with 10 million cells per kilogram.
MAPC Treatment Improves Proximity Measure
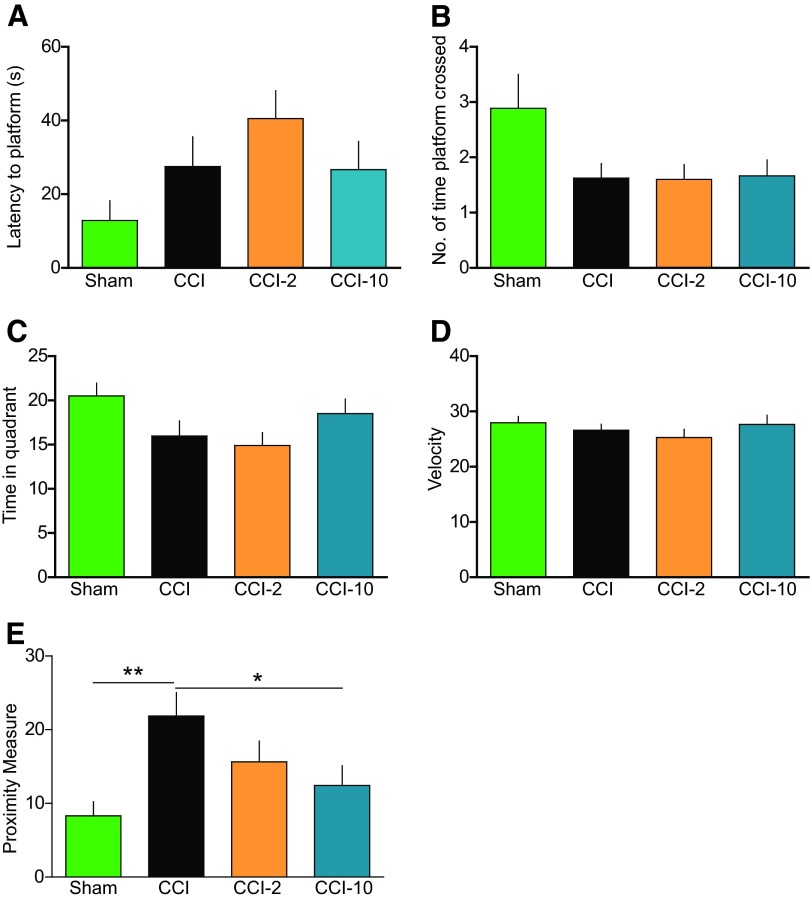
In probe trials with the platform removed after 5 days of learning, initial entrance of rats in the CCI group (22 ± 3 seconds) to the area 3 times the platform diameter (Fig. 5E) occurred significantly later than sham-injured animals (8 ± 2 seconds; p < .01), but animals treated with the higher-dose MAPCs (CCI-10) showed significant improvement (12 ± 3 seconds; p < .05) compared with injured untreated. We observed no significant differences in latency to platform (Fig. 5A), number of times the platform was crossed (Fig. 5B), time spent in quadrant (Fig. 5C), or swim speeds (velocity) (Fig. 5D) among the groups.
Figure 5.
Multipotent adult progenitor cell (MAPC) treatment improved proximity measure during probe trial. (A): Latency to the location of the previously hidden platform. (B): Number of times the platform was crossed. (C): Time spent in the quadrant where the hidden platform was previously located. (D): Velocity of animals. (E): Proximity measure indicating significant differences between CCI-10 versus CCI and sham versus CCI. MAPC-treated (CCI-10) animals found the area of the previously hidden platform in a significantly shorter time period (seconds) than animals without treatment (CCI) as measured by the proximity measure test. MAPC treatment (CCI-10) improved retrieval of memory of the hidden platform. *, p ≤ .05; **, p ≤ .01. Abbreviations: CCI, cortical contusion injury (untreated); CCI-2, cortical contusion injury treated with 2 million cells per kilogram; CCI-10, cortical contusion injury treated with 10 million cells per kilogram.
MAPC Treatment Reduces the Number of Activated Microglia in the Dentate Gyrus
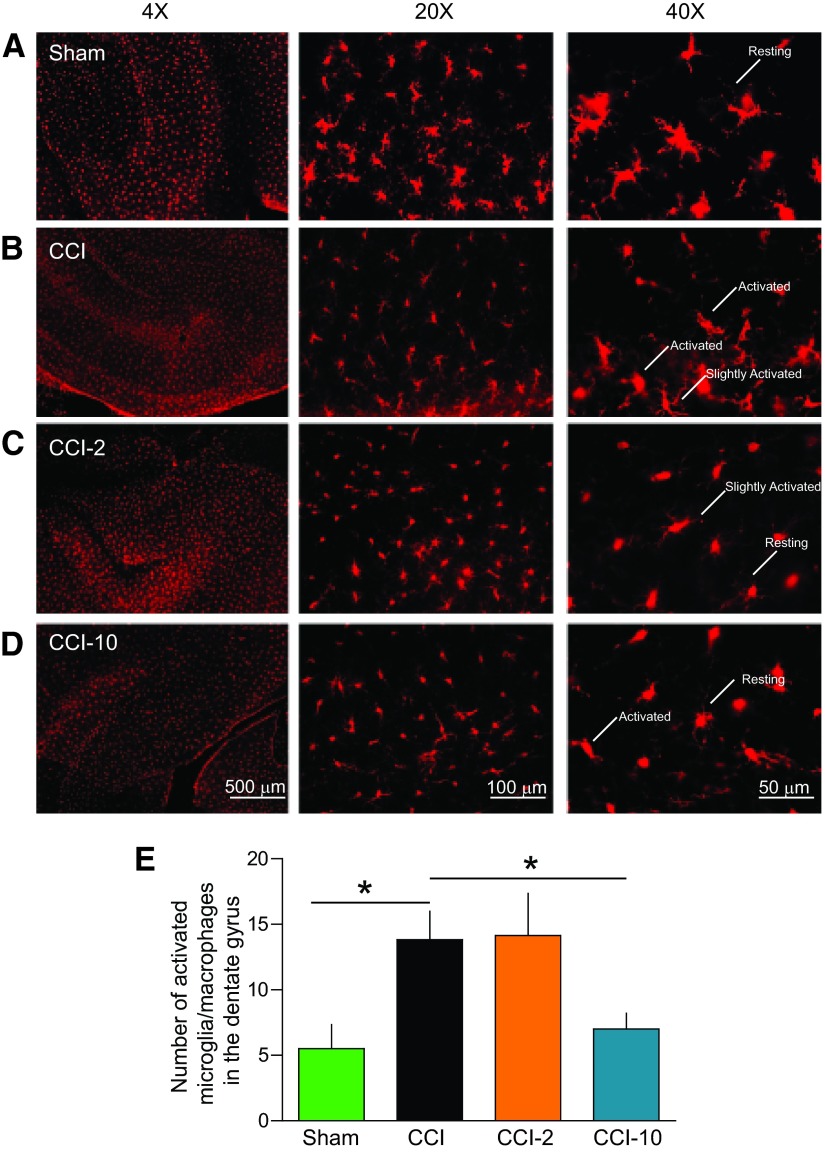
We found a substantial increase in the total number of activated microglia (Fig. 6) in the dentate gyrus of the ipsilateral hippocampus in untreated injured brains (n = 6, 14 ± 2) compared with sham-injured brains (n = 4, 6 ± 2, p < .05), but MAPC therapy significantly reduced this total in the CCI-10 group (n = 9, 7 ± 1, p < .05). The total number of microglia in the right hippocampus did not significantly differ among the groups (sham, 169 ± 9; CCI, 184 ± 13; CCI-2, 203 ± 20; CCI-10, 162 ± 26; p > .05). We also measured the number of activated microglia in the CA1 and CA3 regions, and there were no differences among the groups. In addition, there were no significant differences in the number of slightly activated or resting microglia among all groups (data not shown).
Figure 6.
Multipotent adult progenitor cell (MAPC) treatment reduced the number of activated microglia/macrophages in the dentate gyrus. Traumatic brain injury resulted in chronic activation of microglia/macrophages that was attenuated with MAPC treatment (CCI-10) in the dentate gyrus. The attenuation of activated microglia due to treatment potentially contributed to the improvement in cognitive behavior (Figs. 3–5). (A–D): Photomicrographs of microglia/macropahges from sham (A), CCI (B), CCI-2 (C), and CCI-10 (D) animals using IBA1 antibody (red). Scale bars = 500 μm (×4), 100 μm (×20), and 50 μm (×40). (E): Number of activated microglia/macropahges in the dentate gyrus of the ipsilateral hippocampus. *, p ≤ .05. Abbreviations: CCI, cortical contusion injury (untreated); CCI-2, cortical contusion injury treated with 2 million cells per kilogram; CCI-10, cortical contusion injury treated with 10 million cells per kilogram.
Discussion
Our data demonstrate that intravenous MAPC (CCI-10) treatment at 2 and 24 hours after TBI improved cognitive function as measured by MWM performance and motor function revealed by posture on the balance beam. Improvements in cognitive and motor function were accompanied by localized reduction in numbers of activated microglia 120 days after injury. Cellular therapy also improved spatial learning, information retention, and memory retrieval of the former location of the hidden platform (Figs. 3, 5). Left hind deficit, a measure of motor function, improved significantly after MAPC treatment (Fig. 1). The number of activated microglia in the dentate gyrus of the hippocampus, ipsilateral to the injury, decreased after MAPC treatment (Fig. 6). These improvements constitute a long-term benefit of MAPC therapy.
TBI generally results in gross vestibular motor dysfunction that can be measured by balance beam and rotarod. Injured and sham-injured animals do not differ significantly 4 days postinjury in terms of latency [19], but others have observed significant differences in latency 14 days after injury [20]. The CCI in our experiments overlay the temporoparietal cortex near the motor cortex. Although we did not observe any effects of injury or treatment on latency (data not shown), balance beam trials revealed a novel deficit in which most of the injured rats were not able to maintain the proper placement of left hind limb observed in sham-injured animals. Differences were maintained over the testing period between the injured and sham-injured animals. However, animals in the MAPC treatment (CCI-10) group showed a significant improvement in this motor function deficit (Fig. 1).
Spatial learning, as measured by the Morris water maze, is critically dependent upon the hippocampus [21]. Spatial learning encompasses both acquisition and spatial localization of relevant visual cues that are consolidated, retained, and retrieved over the training and probe trials [22]. The dentate gyrus and N-methyl-d-aspartate receptor (NMDA-R) are key components of the hippocampus that are deeply involved in the acquisition of spatial memory [23, 24]. As a result, rats with lesions of the hippocampus, dentate gyrus, and subiculum generally do poorly in post-training probe trials [24]. Our proximity measure results confirm these observations and provide evidence for the long-term efficacy of MAPC therapy for spatial learning; CCI animals took much longer than sham-injured animals to enter to the area 3 times the platform diameter, but CCI-10 rats were able to arrive at the area significantly more quickly (Fig. 5E), suggesting successful retrieval of memories of the former location of the missing platform. Additionally, on the fifth and final day of the training period 120 days after CCI, MAPC treatment (CCI-10) significantly decreased both distance traveled and latency to find the hidden platform (Fig. 3). Swim speeds were similar across all experimental groups (Fig. 5D), so these results suggest that the animals that received cell therapy learned to find the shortest distance to the platform.
MAPC Therapy Has Both Short-Term and Long-Term Effects on Microglia After TBI
Activation states of microglia can be categorized by morphological features and/or expression of surface or intracellular markers, as previously described [2, 4, 25]. Twenty-four to 120 hours after TBI, MAPC treatment alters the ratio of proinflammatory (CD86+ “M1”) microglia/macrophages to anti-inflammatory (CD206+ “M2”) microglia/macrophages in mice [14]. One hundred twenty days after injury, we observed a similar attenuation of the inflammatory response in rat microglia/macrophages based on morphology; since rat-specific monoclonal antibodies for M1/M2 markers are not yet available, we used morphological features to characterize cells for the present work. MAPC treatment (CCI-10) results in a significant decrease in the number of activated (amoeboid-like) microglia in the ipsilateral dentate gyrus (Fig. 6E) compared with CCI. Previous in vitro analyses have also provided evidence that MAPC treatment can induce microglial apoptosis [14]. We are currently developing immunomagnetic enrichment and flow cytometry protocols to obtain, classify, and characterize infiltrating versus resident microglial/macrophage populations after injury in mice [26]. In addition, potential future experiments include delineating the relative contributions of peripheral versus resident microglia/macrophages. Central depletion studies will include circulating macrophage depletion using dichloromethylene diphosphonate-containing liposomes [27] and examining the morphological and functional outcomes after TBI and MAPC treatment. Another potential method for delineating the contributions of peripheral versus resident microglia/macrophages is by using the CD11-HSTVK knockout mouse, which can undergo transient microglial paralysis [28].
A critical component of MAPC therapy might be the timing of the MAPC injections. We had previously observed improvements in BBB and modifications of the microglial response 72 hours after injury with injections 2 and 24 hours after injury [14]. We observed comparable effects on microglia 120 days after injury with identical MAPC dosing intervals (Fig. 6). The blood-brain barrier was also preserved with mesenchymal stem cells with identical dosing intervals (2 and 24 hours) [16]. Interestingly, a single dose of autologous mononuclear cells 72 hours after injury also preserves the BBB and causes microglial apoptosis [17]. It is indeed a possibility that a single dose of MAPCs could attenuate the inflammatory effects and improve cognitive and motor functions. Evidence from Harting et al. demonstrated significant increases in proinflammatory cytokines 6 hours after injury in the penumbra [8]; therefore, the 2-hour dose may not be critical to attenuate the inflammatory effects of microglia/macrophages. One interesting possibility is that in order for an MAPC therapeutic response, initially an inflammatory signal (not yet precisely defined) is required to prompt the systemic anti-inflammatory effects of MAPC therapy. Future experiments will test the importance of single and multiple doses at varying time points and their effects on cognitive behavior and the microglia/macrophage inflammatory response.
Chronic neuroinflammation negatively affects neuronal function and behavior [29, 30]. Activated microglia, a part of the neuroinflammatory response, are known to affect neurons and subsequently behavior [5]. Long-term persistent activation can cause further neurodegeneration and contribute to cognitive decline [31]. Case analyses of TBI patients have shown the presence of activated microglia with white matter degeneration ranging from months up to 47 years. Specifically, there is a reduction in corpus callosum thickness surrounded by activated microglia [32, 33]. A potential mechanism for the efficacy following MAPC treatment in TBI is attenuation of the prolonged neuroinflammatory response via increased apoptosis of activated microglia/macrophages, leading to a preservation of normal neuronal and astrocytic functions. We have recently demonstrated a similar mechanism using autologous bone marrow-derived mononuclear cell treatment after TBI [17]. It is important to clear necrotic tissue [34], but sustained proinflammatory immune activation can be detrimental to healthy tissue. In other neurodegenerative diseases such as Alzheimer's disease, chronic neuroinflammation plays a vital role in contributing to the decay of healthy cells [35]. Inhibition of proinflammatory cytokines such as TNF-α can restore neuronal function and reverse cognitive deficits [30]. It is possible that the decrease in the number of activated microglia seen in our experiments (Fig. 6) contributes to the improvement in latency of treated animals to find the hidden platform. Similar decreases in activated microglia/macrophages [36] in spinal cord injury were recently observed after treatment with neural precursor cells.
Conclusion
Our data provide evidence of improved spatial learning, improved motor deficits, and reduction in activated microglia/macrophages in the dentate gyrus of the hippocampus after cell therapy, demonstrating the utility of intravenous administration of MAPCs to improve long-term cognitive behavior after TBI.
Acknowledgments
We thank Dr. Pramod Dash for advice on the spatial learning experiments. This project was funded by a Small Business Innovation Research (SBIR) agreement with Athersys (NIH SBIR 1U44-NS077511-01) and by grants from the Brown Foundation, Inc., and the Children's Memorial Hermann Hospital Foundation.
Author Contributions
S.S.B. and R.H.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; C.T., A.B.O., S.W., H.X., K.A., and K.U.: collection and assembly of data; P.S.: collection and assembly of data, data interpretation, manuscript writing; J.H.: conception and design; R.W.M.: conception and design, financial support, provision of study material, final approval of manuscript; C.S.C.: conception and design, data interpretation, manuscript writing, financial support, provision of study material, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
J.H. has compensated employment and stock options with Athersys, Inc. R.W.M. has compensated employment, intellectual property rights, and stock options with Athersys, Inc. C.S.C. is an uncompensated patent holder and has compensated research funding.
References
- 1.Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7:366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 3.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 4.Smith HS. Activated microglia in nociception. Pain Physician. 2010;13:295–304. [PubMed] [Google Scholar]
- 5.Tsuda M, Shigemoto-Mogami Y, Koizumi S, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 6.Pineau I, Sun L, Bastien D, et al. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL-1 receptor/MyD88-dependent fashion. Brain Behav Immun. 2010;24:540–553. doi: 10.1016/j.bbi.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- 8.Harting MT, Jimenez F, Adams SD, et al. Acute, regional inflammatory response after traumatic brain injury: Implications for cellular therapy. Surgery. 2008;144:803–813. doi: 10.1016/j.surg.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savitz SI, Misra V, Kasam M, et al. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70:59–69. doi: 10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- 10.Cox CS, Jr., Baumgartner JE, Harting MT, et al. Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery. 2011;68:588–600. doi: 10.1227/NEU.0b013e318207734c. [DOI] [PubMed] [Google Scholar]
- 11.Brenneman M, Sharma S, Harting M, et al. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010;30:140–149. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu N, Chen R, Du H, et al. Expression of IL-10 and TNF-alpha in rats with cerebral infarction after transplantation with mesenchymal stem cells. Cell Mol Immunol. 2009;6:207–213. doi: 10.1038/cmi.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker PA, Bedi SS, Shah SK, et al. Intravenous multipotent adult progenitor cell therapy after traumatic brain injury: Modulation of the resident microglia population. J Neuroinflammation. 2012;9:228. doi: 10.1186/1742-2094-9-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker PA, Shah SK, Jimenez F, et al. Intravenous multipotent adult progenitor cell therapy for traumatic brain injury: Preserving the blood brain barrier via an interaction with splenocytes. Exp Neurol. 2010;225:341–352. doi: 10.1016/j.expneurol.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menge T, Zhao Y, Zhao J, et al. Mesenchymal stem cells regulate blood-brain barrier integrity through TIMP3 release after traumatic brain injury. Sci Transl Med. 2012;4:161ra150. doi: 10.1126/scitranslmed.3004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedi SS, Walker PA, Shah SK, et al. Autologous bone marrow mononuclear cells therapy attenuates activated microglial/macrophage response and improves spatial learning after traumatic brain injury. J Trauma Acute Care Surg. 2013;75:410–416. doi: 10.1097/TA.0b013e31829617c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lighthall JW. Controlled cortical impact: A new experimental brain injury model. J Neurotrauma. 1988;5:1–15. doi: 10.1089/neu.1988.5.1. [DOI] [PubMed] [Google Scholar]
- 19.Dixon CE, Bao J, Long DA, et al. Reduced evoked release of acetylcholine in the rodent hippocampus following traumatic brain injury. Pharmacol Biochem Behav. 1996;53:679–686. doi: 10.1016/0091-3057(95)02069-1. [DOI] [PubMed] [Google Scholar]
- 20.Budinich CS, Tucker LB, Lowe D, et al. Short and long-term motor and behavioral effects of diazoxide and dimethyl sulfoxide administration in the mouse after traumatic brain injury. Pharmacol Biochem Behav. 2013;108:66–73. doi: 10.1016/j.pbb.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Morris RG, Garrud P, Rawlins JN, et al. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 22.Terry AV., Jr . Spatial navigation (water maze) tasks. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd ed. Boca Raton, FL: CRC Press; 2009. [PubMed] [Google Scholar]
- 23.Bannerman DM, Bus T, Taylor A, et al. Dissecting spatial knowledge from spatial choice by hippocampal NMDA receptor deletion. Nat Neurosci. 2012;15:1153–1159. doi: 10.1038/nn.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutherland RJ, Whishaw IQ, Kolb B. A behavioural analysis of spatial localization following electrolytic, kainate- or colchicine-induced damage to the hippocampal formation in the rat. Behav Brain Res. 1983;7:133–153. doi: 10.1016/0166-4328(83)90188-2. [DOI] [PubMed] [Google Scholar]
- 25.Graeber MB. Changing face of microglia. Science. 2010;330:783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- 26.Bedi SS, Smith P, Hetz RA, et al. Immunomagnetic enrichment and flow cytometric characterization of mouse microglia. J Neurosci Methods. 2013;219:176–182. doi: 10.1016/j.jneumeth.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaytan F, Bellido C, Morales C, et al. Effects of macrophage depletion at different times after treatment with ethylene dimethane sulfonate (EDS) on the regeneration of Leydig cells in the adult rat. J Androl. 1994;15:558–564. [PubMed] [Google Scholar]
- 28.Heppner FL, Greter M, Marino D, et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 29.Belarbi K, Arellano C, Ferguson R, et al. Chronic neuroinflammation impacts the recruitment of adult-born neurons into behaviorally relevant hippocampal networks. Brain Behav Immun. 2012;26:18–23. doi: 10.1016/j.bbi.2011.07.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belarbi K, Jopson T, Tweedie D, et al. TNF-alpha protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J Neuroinflammation. 2012;9:23. doi: 10.1186/1742-2094-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gentleman SM, Leclercq PD, Moyes L, et al. Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Sci Int. 2004;146:97–104. doi: 10.1016/j.forsciint.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 32.Johnson VE, Stewart JE, Begbie FD, et al. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, et al. Inflammation after trauma: Microglial activation and traumatic brain injury. Ann Neurol. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- 34.Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- 35.Xuan A, Long D, Li J, et al. Hydrogen sulfide attenuates spatial memory impairment and hippocampal neuroinflammation in beta-amyloid rat model of Alzheimer's disease. J Neuroinflammation. 2012;9:202. doi: 10.1186/1742-2094-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cusimano M, Biziato D, Brambilla E, et al. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain. 2012;135:447–460. doi: 10.1093/brain/awr339. [DOI] [PMC free article] [PubMed] [Google Scholar]