The in vitro culture system presented here provides an inexpensive and simple method for investigating cell attachment, growth, and differentiation on an acellular biological active substrate. It offers a significant reduction in feeder cell contamination and provides a substantially “cleaner” platform for further cultivation and downstream analyses of differentiated cells.
Keywords: Pluripotent stem cells, Technology, Scaffold attachment region, Cell culture, Serum-free media, Stem cell, Stem cell culture, Extracellular matrix
Abstract
Pluripotent cells such as human embryonic stem cells and human induced pluripotent stem cells are useful in the field of regenerative medicine because they can proliferate indefinitely and differentiate into all cell types. However, a limiting factor for maintaining and propagating stem cells is the need for inactivated fibroblasts as a growth matrix, since these may potentially cause cross-contamination. In this study, we aimed to maintain stem cells on the extracellular matrix (ECM) of either nonirradiated or γ-irradiated fibroblasts. It has been demonstrated that the ECM contains factors and proteins vital for the adhesion, proliferation, and differentiation of pluripotent cells. In order to preserve the ECM, the cell layers of the fibroblasts were decellularized by treatment with 0.05% sodium dodecyl sulfate (SDS), which resulted in an absence of DNA as compared with conventional feeder culture. However, SDS treatment did not cause a detectable change in the ECM architecture and integrity. Furthermore, immunohistochemistry demonstrated that expressions of major ECM proteins, such as fibronectin, collagen, and laminin, remained unaltered. The human pluripotent cells cultured on this decellularized matrix maintained gene expression of the pluripotency markers NANOG and OCT4 and had the potency to differentiate to three germ layers. The in vitro culture system shown here has an excellent potential since the main allogeneic components (i.e., DNA of the feeder cells) are removed. It is also a technically easy, fast, safe, and cheap method for maintaining a refined feeder-free stem cell culture for further cell differentiation studies.
Introduction
Pluripotent cells such as human embryonic stem cells (hESCs) [1] and human induced pluripotent stem cells (hiPSCs) [2, 3] are useful in the field of regenerative medicine. Specifically, they can be used as tools when elucidating mechanisms behind diseases and to explore methods to control the differentiation of specific progenitor cells with the ultimate goal of repairing and replacing tissues or organs. Pluripotent cells can be (a) clonally expanded in larger quantities; (b) induced to differentiate into an adequate supply of functional cells; and (c) frozen, banked, and retrieved when required during the tissue engineering process. Furthermore, when using pluripotent cells, harvesting and expanding lineage-restricted cells from patients who might have very low amounts of functional cells can be avoided. hESCs and hiPSCs were initially propagated and maintained on mouse embryonic fibroblast feeder cells. The feeder cell layer was shown to provide an important cellular substrate for stem cell maintenance and differentiation, as they served as a support matrix for the pluripotent cells. In addition, they supply important growth factors, such as fibroblast growth factor and leukemia inhibitory factor [4]. Feeder cells are usually prepared by treatment with mitomycin C or γ-irradiated to mitotically inactivate the cells. Over the years, improvements have been made to the derivation process of pluripotent cells. Such improvements include efforts to avoid contaminants from feeder cells, removal of animal byproducts in defined culture media [5–7], and avoidance of animal-derived substrates that possibly contain undefined growth factors. This is important since such factors may confound both the differentiation processes and subsequent downstream analyses.
For transplantation of a tissue-engineered organ, it is important to avoid allo- or xenogeneic factors, as these can potentially trigger an immune response in the recipient. There are several studies proving that the removal of resident cells by decellularizing a tissue or organ could still preserve the architecture and integrity of the extracellular matrix (ECM). These matrices can support cell adhesion, proliferation, and differentiation of seeded cells on a tissue-engineered organ [8–12].
Here, we describe an uncomplicated method to decellularize human fibroblast culture to remove genomic DNA while preserving the fiber architecture and bioactive molecules. The yielded matrix is shown to support the maintenance and differentiation of both hESCs and hiPSCs.
We believe that this is a useful in vitro technique to culture and test the differentiation ability of stem cell lines where the risk of contamination from allogenic components from feeder cells is eliminated. This should be of importance both for basic studies of stem cell biology and in clinical applications directed at stem cell-based therapy.
Materials and Methods
Preparation of Feeder Cells
Human foreskin fibroblasts (HFFs) (catalog no. CRL-2429; American Type Culture Collection, Manassas, VA, http://www.atcc.org) were used as feeder cells from passage 10 to passage 16 for culturing the pluripotent stem cells. HFFs were grown to confluence and were either nonirradiated or γ-irradiated with a total cumulative dose of 30 Gy for 20 minutes to induce mitotic inactivation. Feeder cells were seeded at a cell density of 3 × 104 cells per cm2.
Feeder Cell Culture Medium
Iscove's modified Dulbecco's medium (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) was supplemented with 10% fetal bovine serum (FBS), and 1× penicillin-streptomycin, 1× GlutaMAX, and 1× nonessential amino acid solution (all from Invitrogen).
Extracellular Matrix Obtainment
After culturing nonirradiated or γ-irradiated HFFs for 6 days, the cells were treated with 0.01%, 0.05%, and 0.1% sodium dodecyl sulfate (SDS) (Ambion, Austin, TX, http://www.ambion.com) for 15 minutes at 37°C to remove the cells. The treated cells were washed three times with Dulbecco's phosphate-buffered saline (DPBS) with calcium and magnesium (Invitrogen) and were placed onto a 37°C rocking platform for 25 minutes to thoroughly remove SDS that may still have been retained on the ECM. The ECM was kept in culture media prior to cell seeding. The ECM plates were equilibrated on the day of seeding for 30 minutes with stem cell medium prior to seeding the decellularized matrices with pluripotent stem cells.
Pluripotent Cell Lines
hESC line HS181 was used in this study and was derived at Karolinska Institutet, Karolinska University Hospital (Huddinge, Sweden) [13]. The Ethics Committee of Karolinska Institutet gave approval for establishing and differentiating hESC lines (Dnr 454/02). Integration-free hiPSCs were also used in this study. ShiPS-miFF1 (abbreviated as miFF1) was derived at the University of Sheffield (Sheffield, U.K.). The hiPSCs were mRNA transduced with five factors (OCT4, SOX2, KLF4, cMYC, and LIN28) according to the manufacturer's instructions (Stemgent, Cambridge, MA, https://www.stemgent.com). Briefly, the mRNA of five factors was serially transfected for 18 days into HFFs. Colonies were picked after 22 days and expanded for more than 20 passages, and they underwent standard characterization showing expression of pluripotency markers with a normal karyotype.
Pluripotent Cell Cultures
HS181 and miFF1 were initially cultured on γ-irradiated HFFs, and after 5–6 days of culture, the undifferentiated stem cells were enzymatically passaged with 1 mg/ml collagenase type IV (Invitrogen). The cells were incubated for 5 minutes at 37°C, cell strained with 70 μm (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com), centrifuged at 400g for 10 minutes (Eppendorf, Hamburg, Germany, http://www.eppendorf.com), resuspended in stem cell culture medium, and reseeded on the freshly prepared ECM plates.
Stem Cell Culture Medium
Knockout Dulbecco's modified Eagle's medium was supplemented with 20% Knockout serum replacement, 2 mM GlutaMAX, 0.5% penicillin-streptomycin, 1% nonessential amino acids (all from Invitrogen), 0.5 mM 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), and 8 ng/ml basic fibroblast growth factor (bFGF) (R&D Systems, Minneapolis, MN, http://www.rndsystems.com) at 37°C in 5% CO2.
Differentiation of Pluripotent Cells In Vitro
Pluripotent cells were cultured on decellularized human matrices and were differentiated for 7 days in vitro to the three different germ lineages using growth factors: 100 ng/ml retinoic acid [14] (ectoderm), 100 ng/ml bone morphogenetic protein 4 (BMP4) [15] (endoderm), and 100 ng/ml Activin A [16] (mesoderm) (all from R&D Systems). The stem cell culture medium without bFGF was replaced every second day.
Genomic DNA Purification
Total genomic DNA was purified with the DNeasy tissue kit (Qiagen, Hilden, Germany, http://www.qiagen.com) according to the manufacturer's instructions.
RNA Isolation and cDNA Amplification
The cells were harvested and total RNA was purified with the RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. One hundred nanograms of RNA was reverse-transcribed with Superscript III (Invitrogen) according to the manufacturer's instructions.
Quantitative Reverse Transcription-Polymerase Chain Reaction
The samples were run on a 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com). Reactions were performed in triplicate, using approximately 20 ng/ml cDNA obtained as described above. TaqMan probes for pluripotency markers OCT4 (HS03005111_g1) and NANOG (HS04260366_g1) were used from Applied Biosystems. The housekeeping gene GAPDH (HS02758991_g1) was used as an endogenous control. The expression level for each sample was normalized to GAPDH, relative quantification of expression was estimated using the ΔΔCT method, and results were presented as relative fold change. Water was used as a negative control to ensure that there was no artifactual expression.
Histological Staining
Nonirradiated and γ-irradiated HFFs that were treated with 0.01%, 0.05%, and 0.1% SDS were fixed with Bouin's solution (Histolab, Gothenburg, Sweden, http://www.histolab.se) overnight at room temperature. Masson's trichrome staining (Sigma-Aldrich) procedures were carried out according to the manufacturer's instructions.
Immunocytochemistry Staining
To identify the bioactive proteins within the HFFs and pluripotency and differentiation in stem cells, cells were fixed with 4% formalin (Histolab) at room temperature for 10 minutes. Cells were blocked with 5% FBS in DPBS (Invitrogen) for 1 hour at room temperature on a rocking platform. The cells were stained with the following primary antibodies: decellularized HFFs were stained for rabbit polyclonal to collagen I (1:100) (catalog no. ab34710; Abcam, Cambridge, U.K., http://www.abcam.com), rabbit polyclonal to collagen IV (1:100) (catalog no. ab6586; Abcam), rabbit polyclonal to laminin (1:100) (catalog no. ab11575; Abcam), rabbit polyclonal to elastin (1:50) (catalog no. ab21610; Abcam), and mouse monoclonal to fibronection (1:100) (catalog no. ab6328; Abcam). Stem cells were stained for rabbit monoclonal to OCT4A (1:200) (catalog no. 2840; Cell Signaling Technology, Beverly, MA, http://www.cellsignal.com), and differentiated stem cells were stained for rabbit monoclonal to Desmin (1:100) (catalog no. 5332; Cell Signaling Technology) (mesoderm), rabbit monoclonal to GATA6 (1:1,600) (catalog no. 5851; Cell Signaling Technology) (endoderm), and rabbit polyclonal to Nestin (1:100) (catalog no. AB5922; Millipore) (ectoderm) on a rocking platform at room temperature for 1 hour. The cells were washed with DPBS with 0.1% Tween 20 (Sigma-Aldrich). The corresponding secondary antibodies, Alexa 488 goat anti-rabbit (1:500), Alexa 546 goat anti-mouse (1:500), and Alexa 546 goat anti-rabbit (1:500) (Invitrogen) were incubated on a rocking platform at room temperature for 1 hour. The cells were washed with DPBS with 0.1% Tween 20. The cells were counterstained with nuclear marker 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich) and visualized in an Olympus TE microscope (Olympus, Tokyo, Japan, http://www.olympus-global.com). Negative controls for each secondary antibody were performed without the addition of primary antibody, and no nonspecific binding was detected.
Statistical Analysis
The results were presented as mean ± standard error of the mean. Statistical analyses were performed using analysis of variance with GraphPad Prism, version 5.0 (GraphPad Software, Inc., San Diego, CA, http://www.graphpad.com). Statistical significance was defined as p < .05.
Results
Extracellular Matrix
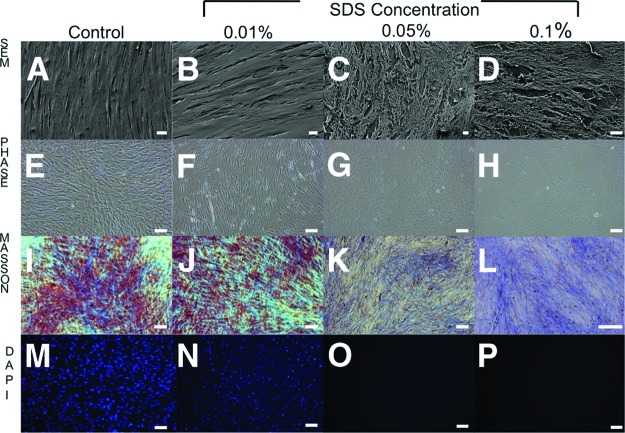
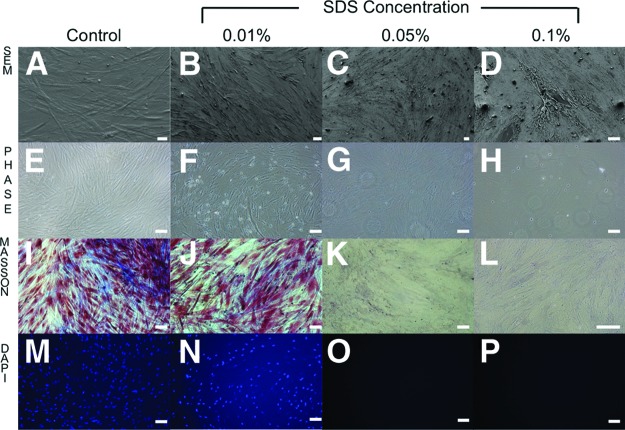
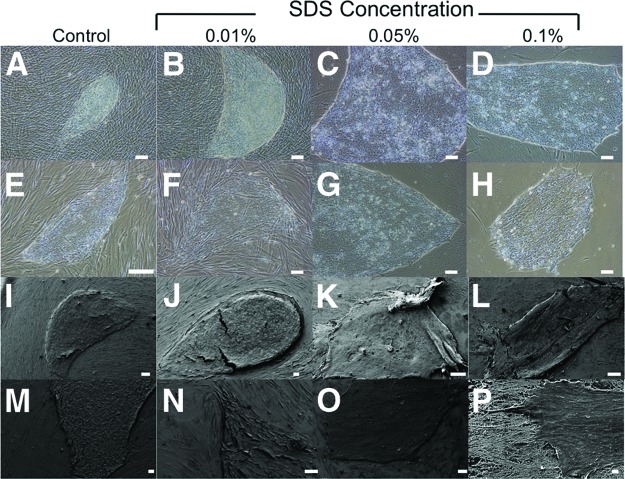
Standard propagation and culturing conditions of feeder-dependent pluripotent stem cells would require either expensive extracellular matrices or mitotically inactive fibroblasts (feeder cells) that are mitomycin C-treated or γ-irradiated. However, such feeder cells are problematic because they contaminate stem cell differentiation and downstream analyses. Thus, through decellularization, we have provided a new method that removes the cellular components of feeder cells but leaves behind an intact ECM. After 15 minutes of exposure to different concentrations of SDS (0.01%, 0.05%, and 0.1%) on nonirradiated (Fig. 1) and γ-irradiated (Fig. 2) HFFs, it was shown by scanning electron microscopy (Figs. 1A–1D, 2A–2D) and phase contrast microscopy (Figs. 1E–1H, 2E–2H) that the matrix morphology and architecture were increasingly disrupted with higher concentration of SDS. Nonirradiated HFFs better preserved ECM architecture and integrity compared with γ-irradiated HFFs (Figs. 1I–1J, 2I–2J). These findings were confirmed by Masson's trichrome staining: Biebrich scarlet-acid fuchsin (which stains epithelium and muscle cells red) and aniline blue (which stains connective tissue blue). In the case of nonirradiated HFFs, no cells were observed and there was no DAPI nuclei staining in the 0.05% and 0.1% SDS treatment groups (Figs. 1M–1P, 2M–2P).
Figure 1.
Morphological and histological images of human foreskin fibroblasts treated with 0% (control), 0.01%, 0.05%, and 0.1% concentrations of SDS. (A–D): Morphological images by scanning electron microscopy. Magnifications: ×200 (A), ×1,600 (B), ×900 (C), ×1,700 (D). (E–H): Phase contrast microscopy. Magnification, ×20. (I–P): Decellularization of feeder extracellular matrix was assessed histologically by Masson's trichrome staining (I–L) and 4′,6-diamidino-2-phenylindole (DAPI) staining (M–P). (I, M): Control feeder cells were prepared with the Dulbecco's phosphate-buffered saline (DPBS) protocol; the DPBS contained intact nuclei visible by Masson's trichrome and DAPI staining. Magnifications: ×20 (I–K, M–P), ×40 (L). Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; SDS, sodium dodecyl sulfate; SEM, scanning electron microscopy.
Figure 2.
Morphological and histological images of γ-irradiated human foreskin fibroblasts treated with 0% (control), 0.01%, 0.05%, and 0.1% concentrations of SDS. (A–D): Morphological images by scanning electron microscopy. Magnifications: ×400 (A), ×250 (B), ×250 (C), ×600 (D). (E–H): Phase contrast microscopy. Magnification, ×20. (I–P): Decellularization of feeder extracellular matrix was assessed histologically by Masson's trichrome staining (I–L) and 4′,6-diamidino-2-phenylindole (DAPI) staining (M–P). (I, M): Control feeder cells were prepared with the Dulbecco's phosphate-buffered saline (DPBS) protocol; the DPBS contained intact nuclei visible by Masson's trichrome and DAPI staining. Magnification, ×20 (I–P). Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; SDS, sodium dodecyl sulfate; SEM, scanning electron microscopy.
Genomic DNA Quantification
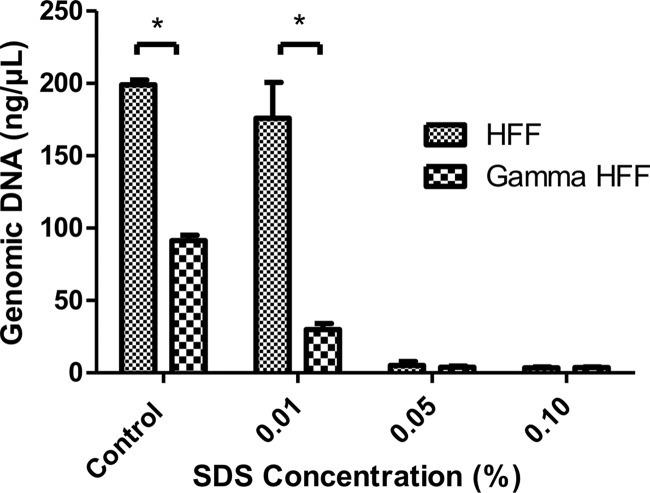
Next, we assessed the genomic DNA to evaluate the efficiency of the decellularization protocol. The amount of genomic DNA that was extracted from decellularized HFFs correlated well with the remaining cellular material that was present in the ECM. (Fig. 3). Both 0.05% and 0.1% SDS treatment groups for nonirradiated and γ-irradiated HFFs yielded an extracellular matrix that consisted of no residual DNA compared with conventional feeder culture (p < .05).
Figure 3.
Concentration of remnant amounts of genomic DNA in the extracellular matrix scaffolds of HFFs (i.e., nonirradiated or γ-irradiated). The difference in the amount of genomic DNA for the control group is due to the difference in cell numbers prior to the decellularization process. *, p < .05. Abbreviations: HFF, human foreskin fibroblast; SDS, sodium dodecyl sulfate.
Immunohistochemistry of Extracellular Matrices
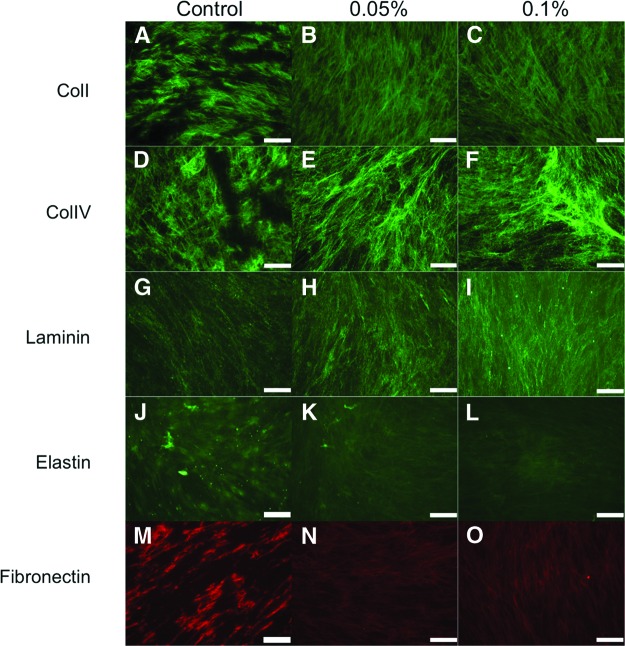
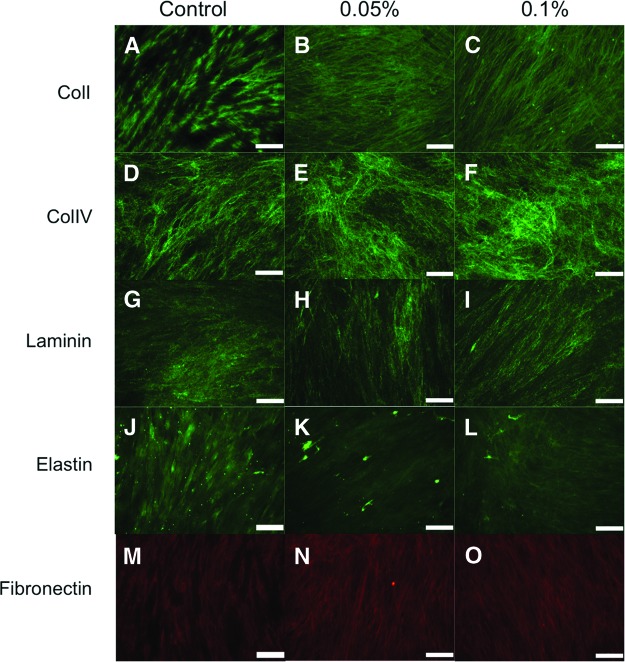
Another important issue to address after the decellularization process with SDS is whether the feeder cell matrices can preserve bioactive proteins in the matrix, since these are essential for culturing pluripotent stem cells (i.e., hESCs and hiPSCs). The 0.05% and 0.1% SDS treatment groups on nonirradiated (Fig. 4) and γ-irradiated (Fig. 5) HFFs were characterized by immunohistochemistry to examine the composition and structure of the matrix. The assessment of ECM content demonstrated that the configurations of collagen I (Figs. 4A–4C, 5A–5C), collagen IV (Figs. 4D–4F, 5D–5F), laminin (Figs. 4G–4I, 5G–5I), and fibronectin (Figs. 4M–4O, 5M–5O) were unaffected by the decellularization process. Although still present to a moderate degree, the level of elastin expression (Figs. 4J–4L, 5J–5L) was decreased compared with the control group. The decrease seen was due to the decellularization process for both nonirradiated and γ-irradiated HFF conditions.
Figure 4.
Characterization of nonirradiated human foreskin fibroblasts treated with 0% (control), 0.05%, and 0.1% sodium dodecyl sulfate. Representative pictures of the decellularized feeder matrices are shown: collagen I (A–C), collagen IV (D–F), laminin (G–I), elastin (J–L), and fibronectin (M–O). Magnification, ×20. Abbreviations: ColI, collagen I; ColIV, collagen IV.
Figure 5.
Characterization of γ-irradiated human foreskin fibroblasts treated with 0% (control), 0.05%, and 0.1% sodium dodecyl sulfate. Representative pictures of the decellularized feeder matrices are shown: collagen I (A–C), collagen IV (D–F), laminin (G–I), elastin (J–L), and fibronectin (M–O). Magnification, ×20. Abbreviations: ColI, collagen I; ColIV, collagen IV.
Morphology of Pluripotent Cells Is Maintained After Seeding on Decellularized Matrix
It was also important to address whether the matrices supported attachment and proliferation of pluripotent stem cells, including hESCs (Fig. 6B–6D, 6F–6H) and hiPSCs (Fig. 6J–6L, 6N–6P). Pluripotent cells were grown on the matrices, and they showed unaltered growth rates and retained similar morphological characteristics compared with cells grown by a conventional culture system (Fig. 6E, 6M). After 5–6 days in culture, the stem cell colonies were still compacted with a high cytoplasmic to nuclei ratio in all SDS treatment groups (i.e., 0.01%, 0.05%, and 0.1%) for both nonirradiated fibroblasts (number of colonies: 12 ± 6, 10 ± 3, and 3 ± 3, respectively) and γ-irradiated fibroblasts (number of colonies: 7 ± 2, 7 ± 3, and 3 ± 2, respectively). The fewest colonies were formed and the most were differentiating in the 0.1% SDS-treated group (Fig. 6D, 6H, 6L, 6P).
Figure 6.
Seeding pluripotent cells on nonirradiated (A–D, I–L) and γ-irradiated (E–H, M–P) human foreskin fibroblasts that were treated with 0% (control), 0.01%, 0.05%, and 0.1% SDS. Magnifications: ×20, (A–D, E–H), ×200 (I, O), ×300 (K, L, N, P), ×350 (J, M). Shown are morphological images of seeded human embryonic stem cells by phase contrast microscopy (A–H) and morphological images of seeded induced pluripotent stem cells by scanning electron microscopy (I–P). Abbreviation: SDS, sodium dodecyl sulfate.
Expression of Pluripotency Markers and Differentiation Capacities of Pluripotent Cells on Decellularized Human Matrix
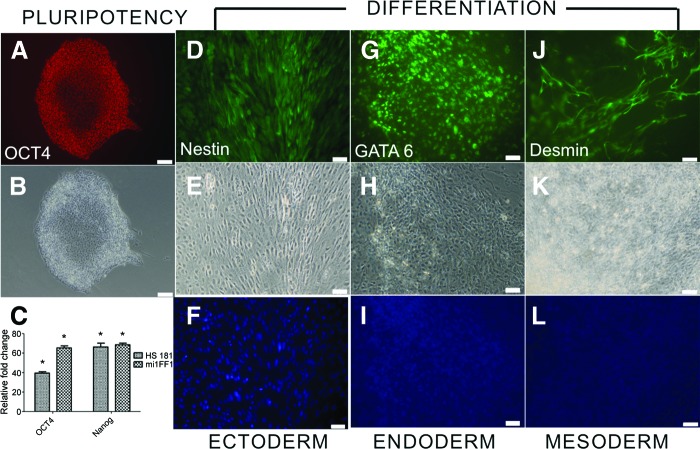
Since we found that SDS treatment at 0.05% of nonirradiated HFFs was most optimal for the in vitro cell seeding, we further characterized the pluripotent cells for expression of undifferentiated stem cell markers by immunocytochemistry (Fig. 7A, 7B) and quantitative reverse transcription-polymerase chain reaction (Fig. 7C) after one passage after seeding from feeders. Pluripotent cells showed no significant differences in expression for OCT4 and NANOG between nonirradiated HFFs as compared with conventional culture plates. Nonirradiated and γ-irradiated HFFs were used as negative controls, and there was no expression of the selected genes. The pluripotent cells were further confirmed by immunocytochemistry staining, and the cells were able to differentiate toward three different germ layers using the following growth factors: retinoic acid (ectoderm) (Fig. 7D–7F), BMP4 (endoderm) (Fig. 7G–7I), and Activin A (mesoderm) (Fig. 7J–7L). There was also no interference from the matrices.
Figure 7.
Evaluation of human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) on nonirradiated human foreskin fibroblasts. (A, B): A representative image of immunocytochemistry staining for a pluripotency marker OCT4 (A) and phase (B). Magnification, ×20. (C): Gene expression of OCT4 and NANOG in hESCs and hiPSCs, normalized to conventional culture plates. *, p < .05. (D–L): In vitro differentiation of the pluripotent cells toward three different germ layers that stained positive for ectoderm (nestin [D], phase [E], and 4′,6-diamidino-2-phenylindole [DAPI] [F]), endoderm (GATA6 [G], phase [H], and DAPI [I]), and mesoderm (desmin [J], phase [K], and DAPI [L]). Magnification, ×20.
Discussion
The present study was designed to develop a platform for stem cell culture by seeding directly on the ECM of a decellularized feeder layer. This short-term differentiation initiation system that is free from feeder cell contamination could offer the possibility of better understanding cell-to-matrix interactions. Here we have shown the functional support of the matrix for pluripotent stem cells, but it might also be applicable for other cell types that require collagen, fibronectin, or laminin matrices.
The detergent SDS was demonstrated in this study to be capable of removing allogeneic components from both nonirradiated and γ-irradiated HFFs, by the successful removal of genomic DNA. This suggests that an unfavorable immunogenic response is unlikely to occur when the cultured cells are transplanted [17, 18]. In this system, no tedious washing protocol is involved, and there is no use of mitogenic or cytotoxic substances such as mitomycin C or need for radiation exposure. It is not always necessary to use expensive defined ECM, such as laminin or variable-matrix Matrigel (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com), which may have batch-to-batch variations that could affect experimentation. Although our feeder cell matrix is also not a completely defined system because of possible differences between feeder cell batches, it is still easy and cheap to produce.
Our present system is fast, safe, cheap, and reproducible for seeding pluripotent cells on xeno-free and feeder-free culture systems, but there are still some issues to consider. For example, it is important to note that with increasing concentrations of SDS, there was a reduction in the number of colonies as compared with conventional feeder cultures. This may be due to the decreased expression of elastin in SDS treatment groups of both nonirradiated and γ-irradiated HFFs. Thus, elastin could be one of the key proteins that is responsible for the colony formation of pluripotent cells on the ECM and should then preferably be maintained [19]. This could be done by using various methodologies other than SDS for feeder cell layer decellularization, either chemical processes (such as enzymatic, ionic, nonionic, alkaline, acidic, zwitterionic, or chelation) or physical processes (such as agitation), which might improve and refine the system. The modification of the decellularization protocol might also be important for other applications to different cell types.
Conclusion
The culture system presented here provides an inexpensive and simple method for investigating cell attachment, growth, and differentiation on an acellular biological active substrate. Its major advantage over other feeder cell systems is that it offers a significant reduction in feeder cell contamination. This provides a substantially “cleaner” platform for further cultivation and downstream analyses of differentiated cells (e.g., microarray and/or gene expression studies). The decellularized feeders could also be prepared in advance and stored, making it an off-the-shelf alternative to conventional feeder layers or more expensive matrices.
Acknowledgments
We gratefully acknowledge the technical assistance that was provided by Marie-Louise Olsson at the Department of Dental Medicine, Center for Oral Biology, Karolinska Institutet (Huddinge, Sweden). We also acknowledge Kjell Hultenby at the Institute for Laboratory Medicine, Karolinska Institutet (Huddinge, Sweden), for processing the samples for scanning electron microscope imaging. The creation and characterization of the hiPSC line, miFF1, was funded by the U.K. Medical Research Council (Peter Andrews, Centre for Stem Cell Biology, University of Sheffield, Sheffield, U.K.). This study was supported by European Project FP7-HEALTH-2012-INNOVATION-2: Ambulung, Ambulatory Bio-Artificial Lung (Grant Agreement No. 304932) and ALF Medicine (Stockholm County Council).
Author Contributions
M.L.L.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; P.J.: conception and design, data analysis and interpretation, review of manuscript; S.S., H.N., K.R.K., I.V.: collection and assembly of data, data analysis, review of manuscript; C.U.: provision of study material, review of manuscript; P.M.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript, financial support.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, Vodyanik Ma, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 4.Soncin FL, Mohamet L, Eckardt D, et al. Abrogation of E-cadherin-mediated cell-cell contact in mouse embryonic stem cells results in reversible LIF-independent self-renewal. Stem Cells. 2009;27:2069–2080. doi: 10.1002/stem.134. [DOI] [PubMed] [Google Scholar]
- 5.Rajala K, Lindroos B, Hussein SM, et al. A defined and xeno-free culture method enabling the establishment of clinical-grade human embryonic, induced pluripotent and adipose stem cells. PLoS One. 2010;5:e10246. doi: 10.1371/journal.pone.0010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ström S, Holm F, Bergström R, et al. Derivation of 30 human embryonic stem cell lines-improving the quality. In Vitro Cell Dev Biol Anim. 2010;46:337–344. doi: 10.1007/s11626-010-9308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergström R, Strom S, Holm F, et al. Xeno-free culture of human pluripotent stem cells. Methods Mol Biol. 2011;767:125–136. doi: 10.1007/978-1-61779-201-4_9. [DOI] [PubMed] [Google Scholar]
- 8.Hakala H, Rajala K, Ojala M, et al. Comparison of biomaterials and extracellular matrices as a culture platform for multiple, independently derived human embryonic stem cell lines. Tissue Eng Part A. 2009;15:1775–1785. doi: 10.1089/ten.tea.2008.0316. [DOI] [PubMed] [Google Scholar]
- 9.Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 10.Badylak SF, Weiss DJ, Caplan A, et al. Engineered whole organs and complex tissues. Lancet. 2012;379:943–952. doi: 10.1016/S0140-6736(12)60073-7. [DOI] [PubMed] [Google Scholar]
- 11.Kim BS, Choi JS, Kim JD, et al. Recellularization of decellularized human adipose-tissue-derived extracellular matrix sheets with other human cell types. Cell Tissue Res. 2012;348:559–567. doi: 10.1007/s00441-012-1391-y. [DOI] [PubMed] [Google Scholar]
- 12.Lin H, Yang G, Tan J, et al. Influence of decellularized matrix derived from human mesenchymal stem cells on their proliferation, migration and multi-lineage differentiation potential. Biomaterials. 2012;33:4480–4489. doi: 10.1016/j.biomaterials.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Hovatta O, Mikkola M, Gertow K, et al. A culture system using human foreskin fibroblasts as feeder cells allows production of hu-man embryonic stem cells. Hum Reprod. 2003;18:1404–1409. doi: 10.1093/humrep/deg290. [DOI] [PubMed] [Google Scholar]
- 14.Guan K, Chang H, Rolletsckek A, et al. Embryonic stem cell-derived neurogenesis. Retinoic acid induction and lineage selection of neuronal cells. Cell Tissue Res. 2001;305:171–176. doi: 10.1007/s004410100416. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi M, Takada T, Takahashi K, et al. BMP4 induces primitive endoderm but not trophectoderm in monkey embryonic stem cells. Cloning Stem Cells. 2008;10:495–502. doi: 10.1089/clo.2008.0030. [DOI] [PubMed] [Google Scholar]
- 16.Abe T, Furue M, Myoishi Y, et al. Activin-like signaling activates Notch signaling during mesodermal induction. Int J Dev Biol. 2004;48:327–332. doi: 10.1387/ijdb.041838ta. [DOI] [PubMed] [Google Scholar]
- 17.Keane TJ, Londono R, Turner NJ, et al. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2012;33:1771–1781. doi: 10.1016/j.biomaterials.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 18.Zheng MH, Chen J, Kirilak Y, et al. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: Possible implications in human implantation. J Biomed Mater Res B Appl Biomater. 2005;73:61–67. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]
- 19.Rodin S, Domogatskaya A, Ström S, et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol. 2010;28:611–615. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]