Abstract
The liver plays a central role in cholesterol homeostasis. It exclusively receives and metabolizes oxysterols, which are important metabolites of cholesterol and are more cytotoxic than free cholesterol, from all extrahepatic tissues. Hepatocellular carcinomas (HCCs) impair certain liver functions and cause pathological alterations in many processes including cholesterol metabolism. However, the link between an altered cholesterol metabolism and HCC development is unclear. Human ACAT2 is abundantly expressed in intestine and fetal liver. Our previous studies have shown that ACAT2 is induced in certain HCC tissues. Here, by investigating tissue samples from HCC patients and HCC cell lines, we report that a specific cholesterol metabolic pathway, involving induction of ACAT2 and esterification of excess oxysterols for secretion to avoid cytotoxicity, is established in a subset of HCCs for tumor growth. Inhibiting ACAT2 leads to the intracellular accumulation of unesterified oxysterols and suppresses the growth of both HCC cell lines and their xenograft tumors. Further mechanistic studies reveal that HCC-linked promoter hypomethylation is essential for the induction of ACAT2 gene expression. We postulate that specifically blocking this HCC-established cholesterol metabolic pathway may have potential therapeutic applications for HCC patients.
Keywords: ACAT2, CpG methylation, oxysterol secretion, inhibition of tumor growth, HCC
Introduction
The human liver is the central organ for cholesterol homeostasis (Dietschy et al., 1993). In extrahepatic tissues, oxysterols are derived from cholesterol through either enzymatic oxidation or non-enzymic oxidation (Brown and Jessup, 2009), among which 27-hydroxycholesterol (27OH, from peripheral macrophages) and 24S-hydroxycholesterol (24sOH, from brain tissues) are the most dominant ones (Schroepfer, 2000). Oxysterols exhibit important biological activities in the induction of cell apoptosis, inhibition of cell growth, and regulation of cholesterol metabolism (Panini and Sinensky, 2001; Gill et al., 2008). However, excess oxysterols are very toxic to cells (Bjorkhem et al., 2002). Therefore, under physiological conditions, excess oxysterols in all extrahepatic tissues are transported to the liver and further metabolized by catabolism into bile acids, esterification to esterified oxysterols, sulfation for excretion, and direct efflux from liver cells (Bjorkhem et al., 1999; Russell, 2003; Brown and Jessup, 2009).
Hepatocellular carcinoma (HCC) is the sixth most prevalent cancer and the third leading cause of cancer-related deaths in the world (Parkin et al., 2005). The causative factors for HCC include chronic hepatitis B virus, hepatitis C virus, alcohol, and aflatoxin (El-Serag, 2012). However, detailed molecular pathways through which HCC develops and progresses remain elusive. Until recently, main curative therapies include surgical resection, liver transplantation, and local ablation. Unfortunately, these curative therapies are only effective to one third of HCC patients. For other patients with intermediate- and advanced-stage carcinomas, few effective treatments are available (de Lope et al., 2012; Forner et al., 2012). Although sorafenib, a multikinase inhibitor with antiangiogenic and antiproliferative effects, has been found to improve the survival of patients with advanced HCC (Llovet et al., 2008), more effective therapies are urgently needed for HCC patients at intermediate and advanced stages. As many other tumors, HCCs are known to undergo metabolic alterations to sustain faster proliferation (DeBerardinis et al., 2008; Tennant et al., 2009). Thus, treatments targeting these metabolic alterations may be a new therapeutic strategy (Tennant et al., 2010). HCCs impair certain cellular functions of the liver and cause pathological alterations in many processes including cholesterol metabolism (Monte et al., 2005; Wu et al., 2010). However, the pathological role of altered cholesterol metabolism in HCC development is still unclear.
Acyl-coenzyme A:cholesterol acyltransferase (ACAT) is the exclusive intracellular enzyme that catalyzes the formation of cholesteryl esters from cholesterol and long-chain fatty acyl-CoA (Chang et al., 2009). The ACAT family includes two members, ACAT1 and ACAT2. ACAT1 produces steryl esters that are incorporated into cellular lipid droplets and is expressed ubiquitously in almost all human tissues, while ACAT2 gene expression is tissue-specific (Chang et al., 2009). In humans, ACAT2 is abundantly expressed in the intestine and fetal liver, but not in the adult liver (Chang et al., 2000, 2009). However, it is highly induced in pathological liver tissues from certain HCC patients (Song et al., 2006). This is controversial with other reports that human ACAT2 can be detected in normal adult livers at very low levels but in liver biopsy samples from patients afflicted with gallstone disease at higher levels (Parini et al., 2004; Smith et al., 2004). ACAT2 is responsible for the synthesis of cholesteryl esters followed by their incorporation into chylomicrons and very low-density lipoproteins (VLDLs) in the intestine and liver, respectively (Buhman et al., 2000; Repa et al., 2004; Lee et al., 2005). Under in vitro assay conditions, both ACATs utilize certain oxysterols as substrates more efficiently than cholesterol itself, while ACAT2 but not ACAT1 is involved in oxysterol secretion (Cases et al., 1998; Liu et al., 2005; Chang et al., 2009). So far, two isotype-specific ACAT inhibitors, K-604 (Ikenoya et al., 2007) and pyripyropene A (Ohshiro et al., 2007), have been characterized for ACAT1 and ACAT2, respectively.
In this study, we investigated the pathological role of induced ACAT2 in the altered cholesterol metabolism for HCC development and revealed possible mechanisms underlying HCC-linked ACAT2 induction.
Results
ACAT2 induction with oxysterol accumulation in HCC
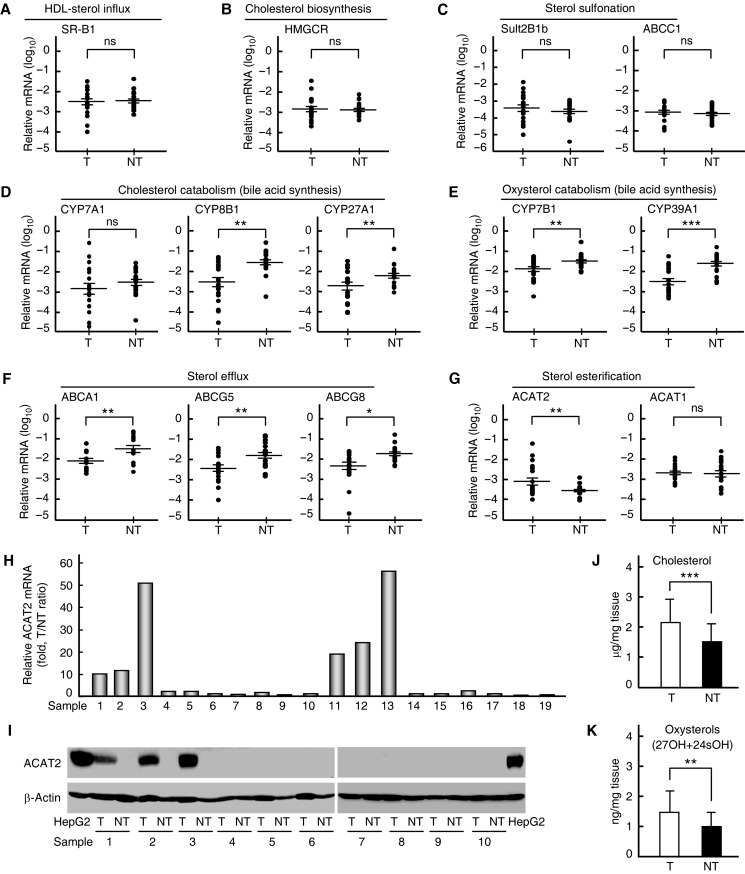
To monitor cholesterol metabolism in HCC, we first analyzed relevant gene expressions and sterol amounts in 19 paired samples of HCC and adjacent non-tumorous tissues from HCC patients. RT–PCR results showed that the expression of genes responsible for HDL-sterol influx (SR-B1), cholesterol biosynthesis (HMGCR), and sterol sulfonation (SULT2B1b and ABCC1) was not affected (Figure 1A–C), while the expression of those involved in sterol catabolism (CYP8B1, CYP27A1, CYP7B1, and CYP39A1) and sterol efflux (ABCA1, ABCG5, and ABCG8) was all significantly reduced in HCC tissues (Figure 1D–F). These data indicate that the sterol influx by SR-B1 from the reverse cholesterol transport (RCT) pathway is functioning normally, while the sterol catabolism (bile acid synthesis) and sterol efflux pathways are impaired, implying that sterols may accumulate in HCC tissues.
Figure 1.
Determination of cholesterol metabolism-related gene expressions and sterols in human tissues. (A–G) Quantitative RT–PCR analysis of mRNA levels for cholesterol metabolism-related genes in 19 paired samples of human HCC tissues (T) and adjacent non-tumorous tissues (NT). Statistical analysis was performed with a two-tailed ratio t-test; data are shown as mean ± SEM after log transformation (n = 19). Each dot represents the mean of relative mRNA levels (log10) in triplicates for the indicated gene in each tissue sample. *P < 0.05, **P < 0.01, ***P < 0.001, ns, not significant. (H) Quantitative RT–PCR analysis of ACAT2 mRNA levels in 19 paired samples (1–19) of human T and NT. T/NT ratio of each pair of samples indicates relative ACAT2 mRNA level, expressed as fold changes, in each T sample. (I) Immunoblots of ACAT2 protein in 10 paired samples (1–10) of human T and NT. Data are representative of two independent experiments. (J and K) Cholesterol (J) and oxysterol (K) contents in 10 paired samples (1–10) of human T and NT. Statistical analysis was performed with a two-tailed paired t-test, and data are shown as mean ± SD (n = 10). **P < 0.01, ***P < 0.001.
Interestingly, among all the genes examined, ACAT2 that directly controls the synthesis of cholesteryl esters followed by their incorporation into VLDL (Buhman et al., 2000; Repa et al., 2004; Lee et al., 2005) was significantly upregulated in HCC tissues compared with adjacent non-tumorous tissues (Figure 1G). The induction of ACAT2 in HCC tissues (6 of 19 samples, Figure 1H) is in a similar rate to that observed previously (5 of 14 samples, Song et al., 2006). The clinicopathological feature analysis also indicated the highest incidence of ACAT2 elevation in the advanced HCCs (50% in Edmonson stage III, Supplementary Figure S1), implying that ACAT2 induction might be correlated with the pathological stage of HCC patients. In addition, ACAT2 gene expression in non-HCC tumor tissues and cell lines was not detectable (Supplementary Figure S2). These results suggest that elevated ACAT2 expression in a specific subset of HCCs might play an important role in HCC sterol metabolism.
Next, western blotting analysis of 10 paired samples with adequate tissue amounts confirmed that human ACAT2 protein was induced in 3 HCC tissues (Figure 1I, samples 1–3) that showed high ACAT2 mRNA levels (T/NT ratio >10, Figure 1H, samples 1–3). We further determined cholesterol and oxysterol contents in these 10 paired samples. Both cholesterol (Figure 1J) and oxysterols (27OH + 24sOH, Figure 1K) were significantly increased in HCC tissues than in adjacent non-tumorous tissues, which were correlated with expression changes of cholesterol metabolism-related genes (Figure 1A–F). Most likely, an increase in cholesterol is required for the faster proliferation of HCC cells. However, accumulated oxysterols are toxic to cells and may affect HCC growth. Interestingly, of the eight HCC tissues with higher levels of oxysterols (Supplementary Figure S3), three exhibited elevated human ACAT2 (Figure 1H and I, samples 1–3). These results further suggest that the induced ACAT2 in certain HCCs might play a role in oxysterol esterification for secretion.
ACAT2-mediated oxysterol secretion in HCC cell lines
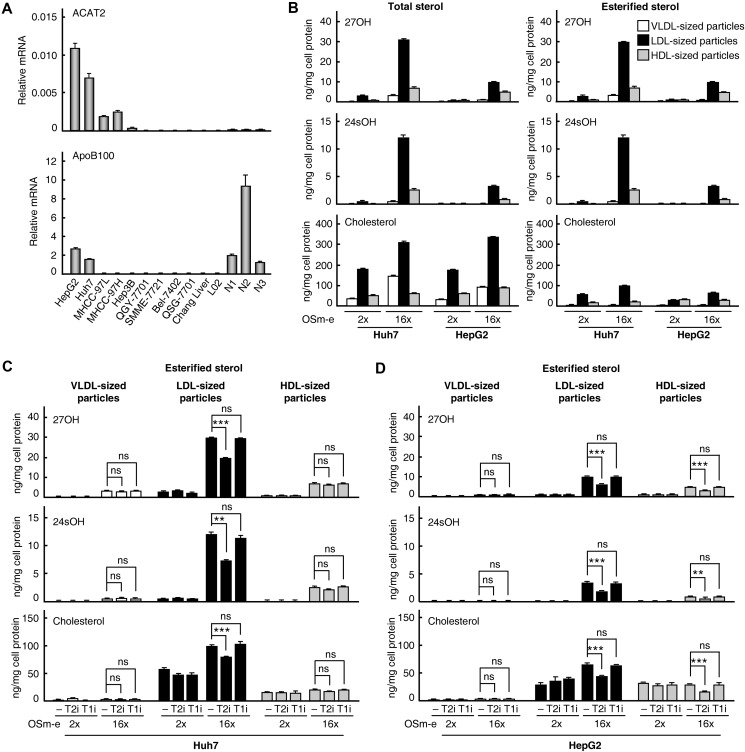
As shown in Figure 2A, only Huh7 and HepG2 cells highly express both ACAT2 and APOLIPOPROTEIN-B100 (APOB100). The protein product of latter gene is essential for the assembly and secretion of VLDL from the liver (Sundaram and Yao, 2010). Thus, these two HCC cell lines were used to study the role of ACAT2 in oxysterol metabolism in HCC cells. Most of the genes involved in sterol catabolism (bile acid synthesis) and sterol efflux were downregulated (except for CYP39A1 in Huh7 cells and CYP27A1 in HepG2 cells, Supplementary Figure S4), indicating that these two pathways are impaired in Huh7 and HepG2 cells as in HCC tissues. Furthermore, they are cell models for studying liver VLDL secretion (Meex et al., 2011), and they secreted VLDL-, LDL-, and HDL-sized particles that could be separated by FPLC under our experiment condition (Supplementary Figure S5). According to previous reports that VLDL particles secreted by Huh7 and HepG2 cells were predominant in the LDL range (Higashi et al., 2002; Meex et al., 2011), LDL-sized particles separated in our study were similarly considered to be VLDL and were used for sterol assays by GC–MS.
Figure 2.
Determination of oxysterols secreted from HCC cell lines. (A) Quantitative RT–PCR analysis of ACAT2 and APOB100 mRNA levels in different liver cell lines and three normal liver tissues (N1-N3). Data are shown as mean ± SD of triplicates. (B–D) VLDL-, LDL-, and HDL-sized particles in serum-free media culturing Huh7 (B and C) or HepG2 (B and D) cells with indicated treatments were separated by FPLC. The contents of total and unesterified sterols were determined by GC–MS and used to calculate the content of esterified sterols. Data are shown as mean ± SD of three independent experiments with statistical analysis by two-way ANOVA (Bonferroni post hoc test). **P < 0.01, ***P < 0.001, ns, not significant. OSm-e, oxysterol mixture of 27OH and 24sOH in ethanol; T2i, ACAT2-specific inhibitor; T1i, ACAT1-specific inhibitor; 2×, 0.2 μg/ml 27OH and 0.1 μg/ml 24sOH; 16×, 1.6 μg/ml 27OH and 0.8 μg/ml 24sOH.
To analyze oxysterol secretion, ethanol-dissolved oxysterols 27OH and 24sOH were delivered into Huh7 or HepG2 cells at a high concentration (16× OSm-e, approximately eight times of the concentration in human plasma); then the serum-free media culturing cells for a short time (3 h) were collected to determine the sterol amounts in the secreted lipoproteins with minimized transfer of steryl esters among lipoproteins. The results showed that total oxysterols (27OH and 24sOH) and cholesterol in secreted lipoproteins were significantly increased after the delivery of high-level oxysterols (16× OSm-e) and were distributed mainly in LDL-sized particles and, to a less extent, in VLDL- or HDL-sized particles (Figure 2B, left panels). Notably, in LDL-sized particles of secreted lipoproteins, both 27OH and 24sOH were mostly in esterified form (∼100%, Figure 2B, top and middle of right panels), while cholesterol was only partially in its esterified form (<35%, Figure 2B, bottom of right panels). In addition, both free and esterified cholesterols were increased after the delivery of high-level oxysterols (Figure 2B, bottom).
Next, when we treated cells with ACAT1-specific inhibitor K-604 or ACAT2-specific inhibitor pyripyropene A individually after the delivery of oxysterols, the ACAT2-specific inhibitor, but not the ACAT1-specific inhibitor, led to a significant reduction of esterified sterols (27OH, 24sOH, and cholesterol) in LDL-sized particles from both Huh7 (Figure 2C) and HepG2 (Figure 2D) cells. Additionally, a reduction of esterified sterols was also observed in HDL-sized particles from HepG2 cells treated with ACAT2-specific inhibitor (Figure 2D), which was possibly caused by transferring steryl esters among lipoproteins in the light of the Lecithin:cholesterol acyltransferase and cholesteryl ester transfer protein expression in HepG2 cells (Supplementary Figure S6).
These data demonstrate that human ACAT2 expressed in HCC cell lines can mediate oxysterol secretion through the synthesis of oxysteryl esters followed by their incorporation into VLDL. Therefore, we hypothesize that ACAT2-mediated oxysterol secretion might protect HCC cells from the cytotoxicity of excess oxysterols; thus, inhibiting ACAT2 would suppress the growth of HCC cell lines.
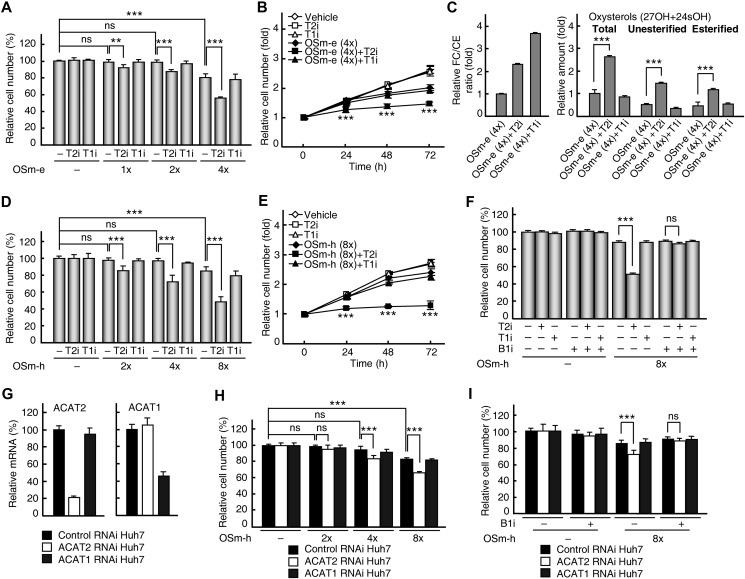
Growth suppression in HCC cell lines by blocking ACAT2-mediated oxysterol secretion
To understand the role of ACAT2-mediated oxysterol secretion in HCC cell growth, ethanol-dissolved oxysterols 27OH and 24sOH were delivered into HCC cell lines at low levels (1×, 2×, and 4× OSm-e) to mimic the oxysterol influx through the RCT pathway in vivo; then the inhibitor pyripyropene A or K-604 was individually added and the growth of HCC cell lines was examined. With exogenous oxysterols at relative lower levels (1× and 2× OSm-e, less than or equal to the concentration in human plasma), the growth of control cell lines QGY-7701 and SMME-7721 that do not express ACAT2 was suppressed (Figure 2A, top panel and Supplementary Figure S7), while the growth of Huh7 cells that express ACAT2 was not suppressed (Figure 2A, top panel and Figure 3A); this indicates that ACAT2 may protect HCC cells from growth suppression caused by excess oxysterols. As expected, the ACAT2-specific inhibition by pyripyropene A, but not the ACAT1-specific inhibition by K-604, significantly suppressed the growth of Huh7 cells with exogenous oxysterols at proximate plasma concentrations (1×, 2×, and 4× OSm-e, Figure 3A and B). However, this was not observed in QGY-7701 or SMME-7721 cells (Supplementary Figure S7). When intracellular sterol contents in Huh7 cells were examined by GC–MS, the enzymatic effects of ACAT2 or ACAT1 inhibition were first confirmed by the increased ratio of free cholesterol/esterified cholesterol with the treatment of ACAT2- or ACAT1-specific inhibitor (Figure 3C, left panel). Further results showed that the ACAT2-specific inhibitor, but not the ACAT1-specific inhibitor, led to the intracellular accumulation of unesterified 27OH and 24sOH (Figure 3C, middle of right panel), indicating that ACAT2-mediated oxysterol secretion is blocked by the ACAT2-specific inhibitor. Similar results were obtained by using HepG2 cells (Supplementary Figure S8A–C), confirming that ACAT2 leads to the accumulation of unesterified oxysterols and specifically suppresses the growth of ACAT2-expressing HCC cell lines.
Figure 3.
Growth suppression by ACAT2 inhibition in Huh7 cells. (A–E) Effects of the ACAT2-specific inhibitor on Huh7 cell growth and intracellular sterols. Huh7 cells were incubated with OSm-e (A and B) or OSm-h (D and E) for 24 h followed by treatment with specific inhibitors (T2i or T1i) for another 48 h (A and D) or for the indicated times (B and E). The relative cell number was expressed as the percentage to controls without any treatment (A and D) or fold change to 0 h of inhibitor treatment (B and E). The free cholesterol/esterified cholesterol ratio (FC/CE; C, left) and the oxysterol contents (C, right) were expressed as fold changes to the group treated with OSm-e (4×) alone. (G) Quantitative RT–PCR analysis confirmed knockdown of ACAT2 and ACAT1 mRNA levels in different RNAi Huh7 stable cell lines. (H) Effects of ACAT2 knockdown on Huh7 cell growth. Different RNAi Huh7 stable cells were incubated with OSm-h for 72 h and the relative cell number was determined as percentage to control RNAi Huh7 cells without any treatment. (F and I) ACAT2 inhibition-induced growth suppression was eliminated by an SR-B1-specific inhibitor. Huh7 cells treated with OSm-h for 24 h followed by T2i or T1i for another 48 h (F) or different RNAi Huh7 cells treated with OSm-h for 72 h (I) were incubated with additional SR-B1-specific inhibitors (B1i). The relative cell number was expressed as the percentage to the control (F) or control RNAi Huh7 (I) without any treatment. Data are shown as mean ± SD of three independent experiments with statistical analysis by two-way ANOVA (Bonferroni post hoc test). **P < 0.01, ***P < 0.001, ns, not significant. B1i, SR-B1-specific inhibitor; OSm-h, oxysterol mixture of 27OH and 24sOH in HDL; control RNAi Huh7, ACAT2 RNAi Huh7, and ACAT1 RNAi Huh7 are Huh7 stable cell lines knockdown for control, ACAT2 and ACAT1.
HDL is the physiological carrier of oxysterols and the RCT pathway is the most important way to transfer sterols from HDL into liver cells. The liver cell receptor SR-B1 is indispensable in the RCT pathway. However, its expression was not affected in HCC tissues or cell lines compared with normal liver tissues (Figure 1A and Supplementary Figure S4A). Therefore, we used HDL to deliver oxysterols (2×, 4×, and 8× OSm-h) in the following experiments to verify the role of ACAT2 in HCC cell growth. The growth of Huh7 cells was not suppressed with HDL-carried oxysterols at relative lower levels (2× and 4× OSm-h) but was slightly suppressed with high level (8× OSm-h) of HDL-carried oxysterols (Figure 3D). The ACAT2-specific inhibitor, but not the ACAT1-specific inhibitor, significantly suppressed the growth of Huh7 cells with HDL-carried oxysterols at all concentrations (Figure 3D and E). However, when the SR-B1-specific inhibitor was applied to disrupt the transfer of steryl esters from HDL into Huh7 cells (Rothblat and Phillips, 2010), the growth suppression in Huh7 cells by the ACAT2-specific inhibitor disappeared (Figure 3F). Similar results were obtained from HepG2 cells except for more cytotoxicity of the SR-B1-specific inhibitor to HepG2 cell line (Supplementary Figure S8D–F). These data confirm the suppressive effect of inhibiting ACAT2 on the growth of HCC cell lines under physiological oxysterol delivery conditions.
Unexpectedly, there was also an increase in esterified oxysterols with the treatment of the ACAT2-specific inhibitor (Figure 3C, right of right panel and Supplementary Figure S8C, right of right panel). This was possibly caused by an enhanced ACAT1-mediated esterification of cytotoxic intracellular oxysterols when ACAT2-mediated oxysterol secretion was blocked. This speculation could be supported by the observation that a combination of both ACAT2-specific and ACAT1-specific inhibitors eliminated the increase in esterified oxysterols (Supplementary Figure S9).
Further experiments using the RNAi approach in Huh7 cells showed that when ACAT2 mRNA level was reduced to 20% (Figure 3G), the growth of ACAT2 RNAi Huh7 stable cells was significantly suppressed with oxysterols at higher levels (4× and 8× OSm-h) compared with that of control RNAi Huh7 stable cells (Figure 3H). Additionally, the SR-B1-specific inhibitor was able to rescue the growth suppression in ACAT2 RNAi Huh7 stable cells (Figure 3I).
The cytotoxicity by oxysterols is mostly attributed to their ability to induce cell apoptosis (Panini and Sinensky, 2001; Rusinol et al., 2004; Gill et al., 2008), so we next examined the effect of inhibiting ACAT2 on cell apoptosis in Huh7 cells. The results from both flow cytometric analysis and DAPI staining assay showed that the inhibition of ACAT2 led to a significant increase in cell apoptosis with delivery of either ethanol-dissolved or HDL-carried oxysterols (Supplementary Figure S10), indicating that the expressed ACAT2 can protect HCC cell lines from the cytotoxicity of excess oxysterols.
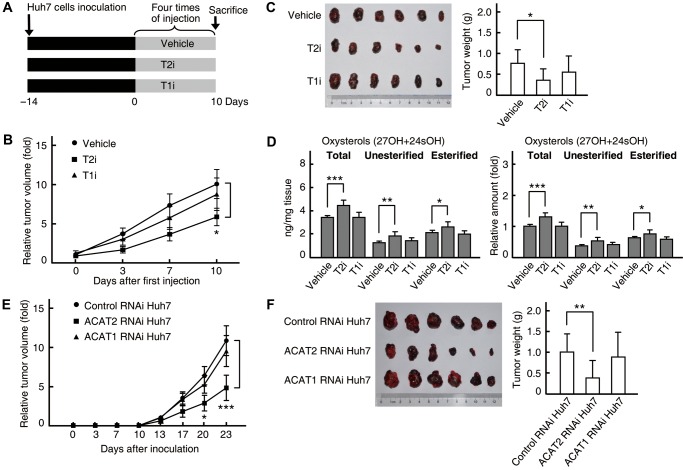
Growth suppression in HCC xenograft tumors by blocking ACAT2-mediated oxysterol secretion
In mice, ACAT2 is highly expressed in normal adult livers for steryl ester secretion in VLDL (Chang et al., 2009), indicating that mouse hepatocarcinogenesis models are not suitable for studying the induced expression of ACAT2 in HCC. Therefore, we used xenograft tumor models to further verify the in vivo role of ACAT2-mediated oxysterol secretion in HCC. Huh7 cells were transplanted into nude mice to form xenograft tumors, and the tumor growth was measured in nude mice with different treatments (Figure 4A). The growth of Huh7 xenograft tumors was significantly suppressed by the ACAT2-specific inhibitor in the absence of exogenous oxysterols (Figure 4B and C), implying that Huh7 xenograft tumors might absorb oxysterols from the blood circulation of nude mice. We then determined oxysterol contents in Huh7 xenograft tumors and found that the treatment with the ACAT2-specific inhibitor, but not the ACAT1-specific inhibitor, led to the accumulation of unesterified oxysterols (Figure 4D), indicating that ACAT2-mediated oxysterol secretion is blocked by ACAT2-specific inhibition in Huh7 xenograft tumors. Similar results were obtained by using HepG2 cells (Supplementary Figure S11). Additionally, an increase in esterified oxysterols was observed in xenograft tumors treated with the ACAT2-specific inhibitor (Figure 4D and Supplementary Figure S11D). This is similar to that occurred in HCC cell lines (Figure 3C and Supplementary Figure S8C) and may be related to the ACAT1-mediated esterification of cytotoxic intracellular oxysterols. Interestingly, the oxysterol contents in Huh7 and HepG2 xenograft tumors from nude mice (Figure 4D and Supplementary Figure S11D, left panels) are comparable to those in human HCC tissues (Figure 1K).
Figure 4.
Growth suppression by ACAT2 inhibition in Huh7 xenograft tumors. (A–D) Effects of the ACAT2-specific inhibitor on Huh7 xenograft tumor growth and intracellular sterols. Huh7 xenograft tumors were established in nude mice and treatments with specific inhibitors (T2i or T1i) were performed as in A. Tumor volumes were assessed and calculated on the indicated days (B) and, at the end of the experiment, tumors were dissected, weighed (C) and determined for oxysterol contents, expressed as fold changes to the total oxysterol content from tumors in control mice receiving vehicle (D). (E and F) Effects of ACAT2 knockdown on Huh7 xenograft tumor growth. Different RNAi Huh7 stable cell lines were used to establish xenograft tumors in nude mice. Tumor volumes were assessed on the indicated days (E) and tumors were dissected for size and weight (F). The average tumor volume was expressed as fold change to that on Day 0 (B) or that of control RNAi Huh7 group on Day 13 (E). Statistical analysis was performed with repeated-measures two-way ANOVA (Bonferroni post hoc test; B and E, mean ± SEM, n = 11) and one-way ANOVA (Bonferroni post hoc test; C and F, mean ± SD, n = 11; D, mean ± SD, n = 8). *P < 0.05, **P < 0.01, ***P < 0.001.
Next, we examined the effect of ACAT2 RNAi on Huh7 xenograft tumor growth. Notably, the growth of xenograft tumors from the ACAT2 RNAi Huh7 stable cell line was significantly suppressed compared with that from the control RNAi Huh7 stable cell line (Figure 4E and F). Importantly, the animals remained healthy without obvious weight loss upon treatments throughout all experiments (data not shown).
Collectively, these findings support the idea that a specific cholesterol metabolic pathway, involving ACAT2 induction and oxysterol secretion to avoid cytotoxicity, is established in certain HCCs for tumor growth, while blocking this pathway by the ACAT2-specific inhibition leads to the accumulation of cytotoxic oxysterols and the suppression of HCC xenograft tumor growth in vivo.
Epigenetic mechanism of the induced ACAT2 gene expression in HCC
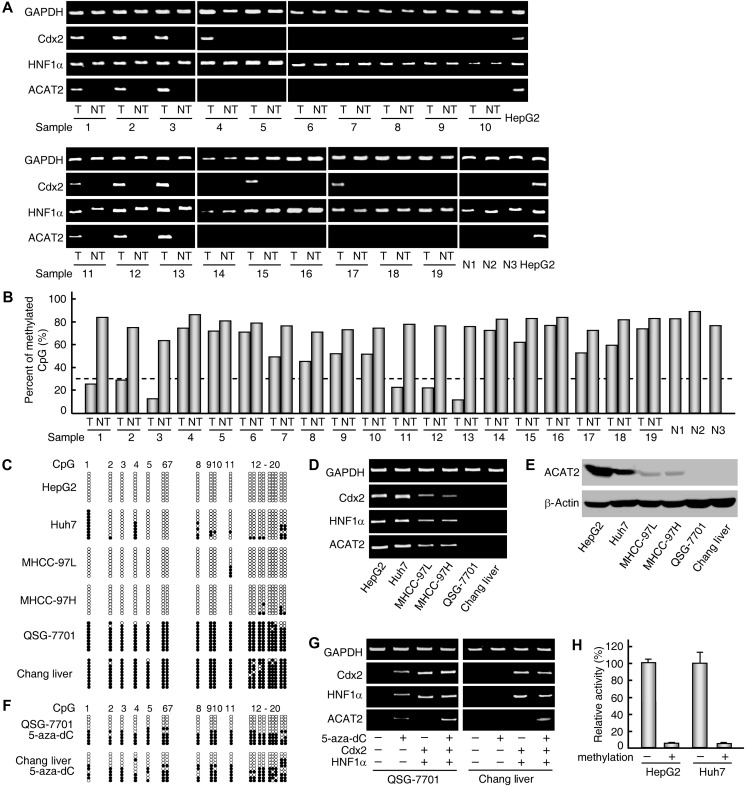
Our previous study revealed that two transcription factors, Cdx2 and HNF1α, synergistically regulate ACAT2 gene expression (Song et al., 2006). We first examined the expression of ACAT2, Cdx2, and HNF1α in 19 paired samples of human HCC and adjacent non-tumorous tissues and 3 normal liver tissues by RT–PCR analysis. Significant CDX2 and HNF1α mRNA signals were detected not only in six HCC tissues with the induced ACAT2 expression (Figures 1H and 5A, samples 1–3 and 11–13), but also in three HCC tissues without ACAT2 expression (Figures 5A and 1H, samples 4, 15, and 17), suggesting that an epigenetic mechanism might be involved in the elevation of human ACAT2 gene expression in HCC. We then examined the CpG methylation status of the ACAT2 gene promoter region in 19 paired samples using bisulfite genomic sequencing. The ACAT2 gene promoter region was hypomethylated (<30%) in 6 HCC tissues (Figure 5B, samples 1–3 and 11–13 and Supplementary Table S1) with induced ACAT2 expression (Figures 1H, I and 5A, samples 1–3 and 11–13) but was hypermethylated in the other 13 HCC tissues, all 19 adjacent non-tumorous tissues, and 3 normal liver tissues (Figure 5B) lacking detectable ACAT2 expression (Figures 1H and 5A), indicating that the induced expression of ACAT2 gene is correlated with promoter hypomethylation in certain HCC tissues.
Figure 5.
HCC-linked promoter hypomethylation of human ACAT2 gene. (A and B) Analyses in 19 paired samples of human T and NT and 3 normal liver tissues (N1-N3). ACAT2, CDX2, and HNF1α mRNA levels were analyzed by RT–PCR and representative results from at least three experiments are shown (A). The methylation status of the ACAT2 gene promoter region was determined by bisulfite genomic sequencing analysis and indicated by the percentage of methylated CpGs to total CpGs (B). (C–E) Analyses in six different liver cell lines. ACAT2, CDX2, and HNF1α were analyzed by RT–PCR (D) and western blotting (E), and methylation status of the ACAT2 gene promoter region was examined by bisulfite genomic sequencing analysis (C). Each row of circles represents a single cloned allele, and each circle indicates a single CpG site. The filled and open circles represent methylated and unmethylated CpG sites, respectively. (F and G) ACAT2 gene expression was induced in two liver cell lines by 5-aza-dC treatment and two exogenous transcriptional factors. ACAT2, CDX2, and HNF1α expressions were analyzed by RT–PCR (G) and methylation status of the ACAT2 gene promoter region was examined by bisulfite genomic sequencing analysis (F). Representative results from at least three experiments are shown. (H) Effects of CpG methylation on human ACAT2 gene promoter activity. The relative activity was expressed as the percentage to that of the unmethylated ACAT2 gene promoter-luciferase reporter construct. Data are shown as mean ± SD of triplicates.
Additionally, the hypomethylation or hypermethylation of the ACAT2 gene promoter region in four HCC cell lines (Huh7, HepG2, MHCC-97L, and MHCC-97H) and two non-HCC liver cell lines (QSG-7701 and Chang Liver) was clearly correlated with ACAT2 expression in these cell lines (Figure 5C–E). Furthermore, this phenomenon was validated by the fact that in these two non-HCC liver cell lines, the treatment of DNA methyltransferase inhibitor 5-aza-deoxycytidine (5-aza-dC) led to promoter hypomethylation (Figure 5F), and the treatment of 5-aza-dC combined with exogenous CDX2 and HNF1α expression induced ACAT2 gene expression (Figure 5G). On the other hand, in vitro CpG methylation of the ACAT2 gene promoter led to an inhibition of the promoter activity in both Huh7 and HepG2 cells (Figure 5H) that abundantly expressed CDX2 and HNF1α (Figure 5D). These data demonstrate that promoter hypomethylation is required for the elevated expression of human ACAT2 gene in a subset of HCCs.
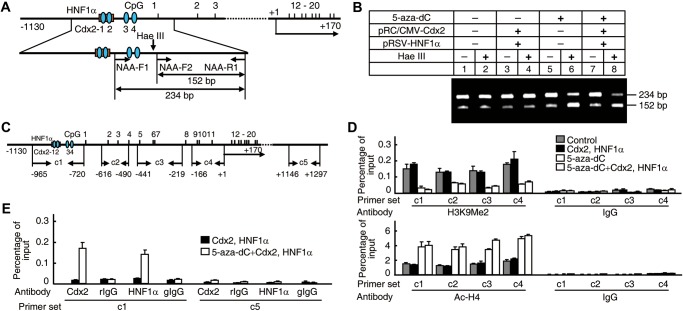
Next, we further investigated how promoter hypomethylation induced ACAT2 gene expression. One reported mechanism of CpG methylation-induced transcriptional repression was that methyl-CpG-binding proteins bind to methylated CpG sites and form complexes with histone deacetylases, which in turn results in a closed chromatin conformation (Jones and Takai, 2001). Therefore, we examined the chromatin conformation and histone modification status of the ACAT2 gene promoter by nuclease accessibility assay and ChIP assay with different primer sets (Figure 6A and C). The treatment of 5-aza-dC on Chang Liver cells that exhibit an absence of ACAT2 gene expression and promoter hypermethylation showed an enhanced nuclease accessibility (Figure 6B, lanes 6 and 8), a reduced H3 dimethyl K9 level (Lachner et al., 2003) and an increased acetylated H4 level (Struhl, 1998) at the ACAT2 gene promoter region (Figure 6D), demonstrating that promoter hypomethylation leads to an open chromatin conformation and contributes to the induction of human ACAT2 gene expression. Meanwhile, transcriptional factors Cdx2 and HNF1α were also bound to the ACAT2 gene promoter region (Figure 6E). In light of all mechanistic data described above, we conclude that HCC-linked promoter hypomethylation is essential for the induced human ACAT2 gene expression.
Figure 6.
Binding of transcriptional factors Cdx2 and HNF1α to the hypomethylated ACAT2 gene promoter. (A and B) Examination of the chromatin conformation of the ACAT2 gene promoter region by 5-aza-dC treatment. Primers indicated by arrows in the schematic representation of the human ACAT2 gene promoter region (A) were used in PCR for the nuclease accessibility assay (B). (C–E) Examination of histone modification status and binding of Cdx2 and HNF1α to the ACAT2 gene promoter region by 5-aza-dC treatment. Primer sets c1 to c5 indicated by arrows in the schematic representation of the human ACAT2 gene promoter region (C) were used individually in real-time PCR with input and ChIP DNAs as templates. ChIP assay using IgG as control was performed with antibodies against H3K9me2 (D, top), Ac-H4 (D, bottom), Cdx2, or HNF1α (E). Representative results from at least three experiments are shown.
Discussion
The current work shows that cholesterol and oxysterols are accumulated in HCC tissues due to impaired sterol catabolism (bile acid synthesis) and sterol efflux pathways. While the accumulation of cholesterol may be required for a faster HCC cell proliferation, a specific cholesterol metabolic pathway is established in a subset of HCCs for tumor growth by protecting HCC cells from excess oxysterol-induced cytotoxicity. Thus, cholesterol metabolic alterations involving induction of ACAT2 and esterification of excess oxysterols for secretion are linked to HCC development.
In this study, we found that human ACAT2 was induced in a subset of HCCs. Fifty percent of HCC samples (two of four samples) at advanced stages (Edmondson stages III) exhibit high levels (T/NT ratio >10) of ACAT2 mRNA (Supplementary Figure S1 and Table S1), suggesting a correlation between ACAT2 induction and HCC progression involving more serious alternations in cholesterol metabolism and continuous accumulation of cytotoxic oxysterols from extrahepatic tissues. Among eight HCC tissues with accumulation of oxysterols (Supplementary Figure S3), three exhibited induced ACAT2 at both protein and mRNA levels (Figure 1H and I, samples 1–3). The correlation between oxysterol accumulation and ACAT2 induction in HCCs is to be investigated in a large-scale survey in the future. Although further studies are needed to reveal whether ACAT2 is directly induced by the accumulation of cytotoxic oxysterols in HCC cells, we postulate that specifically blocking this HCC-established cholesterol metabolic pathway could be used as a novel therapeutic strategy for the treatment of certain HCCs.
In this study, we also observed many HCC tissues without detectable ACAT2 expression (Figures 1H, I and 5A, samples 4–10 and 14–19). This raises several possibilities. First, we showed that promoter hypomethylation (methylated CpGs <30%) is required for the induced expression of ACAT2 gene in HCC. Of 19 HCC tissues examined, 13 HCC tissues without detectable ACAT2 expression also exhibited different extents of CpG demethylation (methylated CpGs >30%) in the ACAT2 gene promoter region compared with matched adjacent non-tumorous tissues and normal liver tissues (Figure 5B, samples 4–10 and 14–19), suggesting that the induction of promoter hypomethylation and subsequent human ACAT2 gene expression might be a long period-consuming process during the development of HCC. Second, oxysterols can be metabolized through other pathways, such as catabolism into bile acids, HDL-mediated efflux and sulfonation by SULT2B1b (Brown and Jessup, 2009). Certain HCC tissues may utilize these pathways instead of ACAT2-mediated oxysterol secretion to address excess oxysterols. Third, two HCC tissues exhibited neither accumulated oxysterols (Supplementary Figure S3, samples 6 and 7) nor ACAT2 expression (Figure 1H and I, samples 6 and 7), suggesting a less advanced stage for these HCC tissues.
ACAT inhibitors have been used in animal models for treating coronary atherosclerosis (Bocan et al., 2000; Kusunoki et al., 2001) and Alzheimer's disease (Hutter-Paier et al., 2004), in clinical trials for atherosclerosis treatment (Tardif et al., 2004), and in numerous cell models for anticancer activities (Bemlih et al., 2010); however, most inhibitors target ACAT1 but not ACAT2 in these cases. To our knowledge, an ACAT2-specific inhibitor was only used in an atherogenic mouse model (Ohshiro et al., 2011) and has not been used for HCC treatment.
Oxysterols are natural ligands for LXR (Bjorkhem et al., 2002). Many genes involved in cholesterol efflux and catabolism (such as ABCA1 and CYP7A1) are targets of LXR (Bjorkhem et al., 2002; Gill et al., 2008). However, our finding revealed an uncoupling between the accumulation of oxysterols and the decrease of LXR target gene expression in 19 HCC tissues (Figure 1). Further analysis of the expression of LXRα and LXRβ, two isoforms of LXR, in HCC and adjacent non-tumorous tissues showed that the mRNA level of LXRα was significantly reduced in HCC tissues (Supplementary Figure S12), suggesting that the decrease of LXR target gene expression might be attributed to the reduction of LXRα expression in HCC. Previous studies have revealed that ACAT2 is responsible for the synthesis of cholesteryl esters, followed by their incorporation into VLDL in the liver (Buhman et al., 2000; Repa et al., 2004; Lee et al., 2005). In our study, although the two HCC cell lines Huh7 and HepG2 are poor secretors of VLDL compared with liver tissues in vivo (Meex et al., 2011), we still observed significant effects of the ACAT2-specific inhibitor on oxysterol secretion in these cell lines (Figures 2–4). Thus, the ACAT2-specific inhibitor might affect in vivo oxysterol secretion more effectively. Interestingly, as shown in Figure 2B–D, the delivery of oxysterols at high levels increased both free and esterified cholesterols in LDL-sized VLDL particles, indicating that oxysterols also promote VLDL secretion, which is consistent with previous reports (Dashti, 1992; Grefhorst et al., 2002).
Gene expression data have indicated an impaired sterol catabolism (bile acid synthesis) pathway in HCC tissues (Figure 1). A further analysis in five paired samples with adequate tissues showed that total bile acid contents were significantly reduced in HCC tissues compared with those in adjacent non-tumorous tissues (data not shown), confirming that the sterol catabolism pathway is impaired in HCC tissues. Interestingly, the induced ACAT2 expression may be associated with abnormal bile acid metabolism in livers of certain patients with HCC or gallstone disease. The pathological mechanism and role of induced ACAT2 in liver tissues of patients with gallstone disease need further studies.
We have previously reported the genomic organization of the human ACAT2 gene and that Cdx2 and HNF1α are responsible for intestine-specific expression and HCC-related induction of ACAT2 (Song et al., 2001, 2006). It is also reported that ACAT2 gene expression is controlled by HNF1α and HNF1β in the liver (Pramfalk et al., 2005). Here, we demonstrate that HCC-linked promoter hypomethylation plays a conclusive role in the induction of human ACAT2 gene expression. In normal liver tissues and adjacent non-tumorous tissues, the hypermethylation of the ACAT2 gene promoter region was clearly correlated with the undetectable ACAT2 gene expression (Figure 5A and B). Notably, some HCC tissues with abundant expression of both Cdx2 and HNF1α (Figure 5A, samples 4, 15, and 17) also exhibit undetectable ACAT2 gene expression due to the hypermethylation of the ACAT2 gene promoter region in these HCC tissues (Figure 5B, samples 4, 15, and 17). This also confirms that the ACAT2 gene is not expressed in adult human liver. Future studies would focus on the mechanism underlying CpG demethylation of the ACAT2 gene promoter region and whether it directly results from the accumulation of oxysterols in HCC.
Materials and methods
Materials and plasmids
Lipoprotein-deficient serum (LPDS, density >1.215 g/ml) was prepared from fetal bovine serum (FBS) by ultracentrifugation (Goldstein et al., 1983). Oxysterols 27OH (Medical Isotope) and 24sOH (Enzo Life Science) and cholesterol (Sigma) were used. OSm-e was oxysterol mixture of 27OH and 24sOH in ethanol; OSm-h was oxysterol mixture in HDL, which was prepared by incubating FBS with oxysterols 27OH and 24sOH at 37°C for 24 h followed with isolation of HDL by sequential ultracentrifugation (Lin and Morel, 1996) and determination of 27OH and 24sOH contents by GC–MS. Concentrations 0.2 μg/ml (27OH) and 0.1 μg/ml (24sOH) were defined as 2× concentrations, which are approximate to the physiological concentrations in human plasma (Schroepfer, 2000; Burkard et al., 2007). ACAT2-specific inhibitor pyripyropene A (ALEXIS Biochemicals), SR-B1-specific inhibitor BLT-1 (Chembridge Corporation), Deuterated internal standards 27OH-D5, 24sOH-D7, and Cholesterol-D6 (Medical Isotope), and N,O-Bis(trimethylsilyl)-trifluoroacetamide (BSTFA; Sigma) were all commercially available. ACAT1-specific inhibitor K-604 was chemically synthesized in Fa-Jun Nan's laboratory (Shanghai Institute of Materia Medica, Shanghai, China). Antibodies against H3K9me2, Ac-H4 (Upstate Biotechnology), Cdx2 (Bethyl Laboratories), and HNF1α (Santa Cruz Biotechnology) were used. The expression plasmids for human HNF1α and Cdx2 were described previously (Song et al., 2006).
Human tissue samples and cell culture
Nineteen paired human HCC and adjacent non-tumorous tissues and three normal liver tissues were obtained with informed consents from patients who underwent curative resection and three healthy individual donors, respectively, at the Liver Cancer Institute, Zhongshan Hospital (Fudan University, Shanghai, China). Primary HCC and normal liver tissues were identified by histological analysis. The study protocols were approved by the Biomedical Research Ethics Committee of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
Human cell lines were obtained from American Type Culture Collection (HepG2, Hep3B, and Chang Liver), Shanghai Cell Bank of Chinese Academy of Sciences (Huh7, QGY-7701, SMME-7721, Bel-7402, QSG-7701, and L02), and Liver Cancer Institute of Fudan University in Shanghai (MHCC-97L and MHCC-97H). Cells were maintained in RPMI 1640 medium (QSG-7701 and Chang Liver) or DMEM (all other cell lines) supplemented with 10% (v/v) FBS at 37°C in 5% CO2. Medium A (DMEM supplemented with 5% LPDS) and B (serum-free DMEM) were also used. All media contain 100 units/ml penicillin and 100 μg/ml streptomycin sulfate.
Cell growth
Huh7 or HepG2 cells were first incubated in FBS-medium overnight for cell adherence, and then in medium A containing OSm-e or OSm-h for 24 h followed with treatment of inhibitors specifically for ACAT2 (5 μM pyripyropene A), ACAT1 (3 μM K-604), and/or SR-B1 (1 μM BLT-1) for another 48 h or indicated times. Different Huh7 stable cell lines were first incubated in FBS-medium overnight for cell adherence, and then in medium A containing OSm-h with or without SR-B1-specific inhibitor (1 μM BLT-1) for 72 h. The viable cell number was measured by Cell Counting Kit CCK8 (Dojin Laboratories) according to the manufacturer's instructions.
Huh7 xenograft tumor model
Six-week-old male athymic nude mice (BALB/c, SIPPR-BK Experimental Animal Co., Shanghai, China) were used for establishing Huh7 xenograft tumors. For procedure 1, Huh7 cells (4 × 106) were injected subcutaneously into flank sides of nude mice to produce xenograft tumors. When palpable tumors were established (about 100 mm3), mice (seven per group) were treated by intratumoral injection with vehicle control, ACAT2-specific inhibitor (60 μg/cm3 tumor), or ACAT1-specific inhibitor (30 μg/cm3 tumor) for four times within 10 days. For procedure 2, control RNAi, ACAT2 RNAi, or ACAT1 RNAi Huh7 stable cells (4 × 106) were injected subcutaneously into flank sides of nude mice to produce xenograft tumors. Tumor volumes were assessed on indicated days by a caliper and calculated using the formula: volume = (Length × Width2)/2. At the end of each procedure, mice were sacrificed and tumors were dissected, weighed, taken photos, and used for analysis of oxysterols. All nude mice were treated in strict accordance with animal care procedures and methods approved by the Institutional Animal Care and Use Committee of the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences.
Statistical analysis
Experimental differences were analyzed as indicated in figure legends. Statistical significance was evaluated with GraphPad Prism 5.01 (GraphPad Software). P-values of <0.05 were considered statistically significant.
Details for RT–PCR analysis, western blotting analysis, establishment of stable knockdown cell lines, determination of oxysterols, cholesterol, and sterols, bisulfite genomic DNA sequencing, luciferase activity assay, demethylation treatment and chromatin status assay are described in Supplementary material.
Supplementary material
Supplementary material is available at Journal of Molecular Cell Biology online.
Funding
This work was supported by grants from the Ministry of Science and Technology of China (2011CB910900 to B.-L.L. and 2009CB919000 to B.-L.S.), National Institutes of Health, USA (HL36709 to T.-Y.C.), and National Natural Science Foundation of China (30771233 to Y.X.).
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
We thank Xin-Ying Yang and Lei Qian for technical assistance, Dr Hong-Bin Ji (Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China) for providing lung cancer tissue samples, and Mr Xiao-Ming Yi (Jin-Ling Hospital, Nanjing, China) for providing kidney cancer tissue samples.
References
- Bemlih S., Poirier M.D., El Andaloussi A. Acyl-coenzyme A: cholesterol acyltransferase inhibitor Avasimibe affect survival and proliferation of glioma tumor cell lines. Cancer Biol. Ther. 2010;9:1025–1032. doi: 10.4161/cbt.9.12.11875. [DOI] [PubMed] [Google Scholar]
- Bjorkhem I., Diczfalusy U., Lutjohann D. Removal of cholesterol from extrahepatic sources by oxidative mechanisms. Curr. Opin. Lipidol. 1999;10:161–165. doi: 10.1097/00041433-199904000-00010. [DOI] [PubMed] [Google Scholar]
- Bjorkhem I., Meaney S., Diczfalusy U. Oxysterols in human circulation: which role do they have? Curr. Opin. Lipidol. 2002;13:247–253. doi: 10.1097/00041433-200206000-00003. [DOI] [PubMed] [Google Scholar]
- Bocan T.M., Krause B.R., Rosebury W.S., et al. The ACAT inhibitor avasimibe reduces macrophages and matrix metalloproteinase expression in atherosclerotic lesions of hypercholesterolemic rabbits. Arterioscler. Thromb. Vasc. Biol. 2000;20:70–79. doi: 10.1161/01.atv.20.1.70. [DOI] [PubMed] [Google Scholar]
- Brown A.J., Jessup W. Oxysterols: sources, cellular storage and metabolism, and new insights into their roles in cholesterol homeostasis. Mol. Aspects Med. 2009;30:111–122. doi: 10.1016/j.mam.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Buhman K.K., Accad M., Novak S., et al. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat. Med. 2000;6:1341–1347. doi: 10.1038/82153. [DOI] [PubMed] [Google Scholar]
- Burkard I., von Eckardstein A., Waeber G., et al. Lipoprotein distribution and biological variation of 24S- and 27-hydroxycholesterol in healthy volunteers. Atherosclerosis. 2007;194:71–78. doi: 10.1016/j.atherosclerosis.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Cases S., Novak S., Zheng Y.W., et al. ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J. Biol. Chem. 1998;273:26755–26764. doi: 10.1074/jbc.273.41.26755. [DOI] [PubMed] [Google Scholar]
- Chang C.C., Sakashita N., Ornvold K., et al. Immunological quantitation and localization of ACAT-1 and ACAT-2 in human liver and small intestine. J. Biol. Chem. 2000;275:28083–28092. doi: 10.1074/jbc.M003927200. [DOI] [PubMed] [Google Scholar]
- Chang T.Y., Li B.L., Chang C.C., et al. Acyl-coenzyme A:cholesterol acyltransferases. Am. J. Physiol. Endocrinol. Metab. 2009;297:E1–E9. doi: 10.1152/ajpendo.90926.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti N. The effect of low density lipoproteins, cholesterol, and 25-hydroxycholesterol on apolipoprotein B gene expression in HepG2 cells. J. Biol. Chem. 1992;267:7160–7169. [PubMed] [Google Scholar]
- DeBerardinis R.J., Lum J.J., Hatzivassiliou G., et al. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- de Lope C.R., Tremosini S., Forner A., et al. Management of HCC. J. Hepatol. 2012;56(Suppl 1):S75–S87. doi: 10.1016/S0168-8278(12)60009-9. [DOI] [PubMed] [Google Scholar]
- Dietschy J.M., Turley S.D., Spady D.K. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J. Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- El-Serag H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forner A., Llovet J.M., Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- Gill S., Chow R., Brown A.J. Sterol regulators of cholesterol homeostasis and beyond: the oxysterol hypothesis revisited and revised. Prog. Lipid Res. 2008;47:391–404. doi: 10.1016/j.plipres.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Goldstein J.L., Basu S.K., Brown M.S. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- Grefhorst A., Elzinga B.M., Voshol P.J., et al. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J. Biol. Chem. 2002;277:34182–34190. doi: 10.1074/jbc.M204887200. [DOI] [PubMed] [Google Scholar]
- Higashi Y., Itabe H., Fukase H., et al. Distribution of microsomal triglyceride transfer protein within sub-endoplasmic reticulum regions in human hepatoma cells. Biochim. Biophys. Acta. 2002;1581:127–136. doi: 10.1016/s1388-1981(02)00157-9. [DOI] [PubMed] [Google Scholar]
- Hutter-Paier B., Huttunen H.J., Puglielli L., et al. The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer's disease. Neuron. 2004;44:227–238. doi: 10.1016/j.neuron.2004.08.043. [DOI] [PubMed] [Google Scholar]
- Ikenoya M., Yoshinaka Y., Kobayashi H., et al. A selective ACAT-1 inhibitor, K-604, suppresses fatty streak lesions in fat-fed hamsters without affecting plasma cholesterol levels. Atherosclerosis. 2007;191:290–297. doi: 10.1016/j.atherosclerosis.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Jones P.A., Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- Kusunoki J., Hansoty D.K., Aragane K., et al. Acyl-CoA:cholesterol acyltransferase inhibition reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2001;103:2604–2609. doi: 10.1161/01.cir.103.21.2604. [DOI] [PubMed] [Google Scholar]
- Lachner M., O'Sullivan R.J., Jenuwein T. An epigenetic road map for histone lysine methylation. J. Cell Sci. 2003;116:2117–2124. doi: 10.1242/jcs.00493. [DOI] [PubMed] [Google Scholar]
- Lee R.G., Shah R., Sawyer J.K., et al. ACAT2 contributes cholesteryl esters to newly secreted VLDL, whereas LCAT adds cholesteryl ester to LDL in mice. J. Lipid Res. 2005;46:1205–1212. doi: 10.1194/jlr.M500018-JLR200. [DOI] [PubMed] [Google Scholar]
- Lin C.Y., Morel D.W. Esterification of oxysterols in human serum: effects on distribution and cellular uptake. J. Lipid Res. 1996;37:168–178. [PubMed] [Google Scholar]
- Liu J., Chang C.C., Westover E.J., et al. Investigating the allosterism of acyl-CoA:cholesterol acyltransferase (ACAT) by using various sterols: in vitro and intact cell studies. Biochem. J. 2005;391:389–397. doi: 10.1042/BJ20050428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet J.M., Ricci S., Mazzaferro V., et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- Meex S.J., Andreo U., Sparks J.D. Huh-7 or HepG2 cells: which is the better model for studying human apolipoprotein-B100 assembly and secretion? J. Lipid Res. 2011;52:152–158. doi: 10.1194/jlr.D008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte M.J., Fernandez-Tagarro M., Macias R.I., et al. Changes in the expression of genes related to bile acid synthesis and transport by the rat liver during hepatocarcinogenesis. Clin. Sci. (Lond) 2005;109:199–207. doi: 10.1042/CS20050035. [DOI] [PubMed] [Google Scholar]
- Ohshiro T., Rudel L.L., Omura S., et al. Selectivity of microbial acyl-CoA: cholesterol acyltransferase inhibitors toward isozymes. J. Antibiot. (Tokyo) 2007;60:43–51. doi: 10.1038/ja.2007.6. [DOI] [PubMed] [Google Scholar]
- Ohshiro T., Matsuda D., Sakai K., et al. Pyripyropene A, an acyl-coenzyme A:cholesterol acyltransferase 2-selective inhibitor, attenuates hypercholesterolemia and atherosclerosis in murine models of hyperlipidemia. Arterioscler. Thromb. Vasc. Biol. 2011;31:1108–1115. doi: 10.1161/ATVBAHA.111.223552. [DOI] [PubMed] [Google Scholar]
- Panini S.R., Sinensky M.S. Mechanisms of oxysterol-induced apoptosis. Curr. Opin. Lipidol. 2001;12:529–533. doi: 10.1097/00041433-200110000-00008. [DOI] [PubMed] [Google Scholar]
- Parini P., Davis M., Lada A.T., et al. ACAT2 is localized to hepatocytes and is the major cholesterol-esterifying enzyme in human liver. Circulation. 2004;110:2017–2023. doi: 10.1161/01.CIR.0000143163.76212.0B. [DOI] [PubMed] [Google Scholar]
- Parkin D.M., Bray F., Ferlay J., et al. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Pramfalk C., Davis M.A., Eriksson M., et al. Control of ACAT2 liver expression by HNF1. J. Lipid Res. 2005;46:1868–1876. doi: 10.1194/jlr.M400450-JLR200. [DOI] [PubMed] [Google Scholar]
- Repa J.J., Buhman K.K., Farese R.V., et al. ACAT2 deficiency limits cholesterol absorption in the cholesterol-fed mouse: impact on hepatic cholesterol homeostasis. Hepatology. 2004;40:1088–1097. doi: 10.1002/hep.20439. [DOI] [PubMed] [Google Scholar]
- Rothblat G.H., Phillips M.C. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr. Opin. Lipidol. 2010;21:229–238. doi: 10.1097/mol.0b013e328338472d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinol A.E., Thewke D., Liu J., et al. AKT/protein kinase B regulation of BCL family members during oxysterol-induced apoptosis. J. Biol. Chem. 2004;279:1392–1399. doi: 10.1074/jbc.M308619200. [DOI] [PubMed] [Google Scholar]
- Russell D.W. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- Schroepfer G.J., Jr Oxysterols: modulators of cholesterol metabolism and other processes. Physiol. Rev. 2000;80:361–554. doi: 10.1152/physrev.2000.80.1.361. [DOI] [PubMed] [Google Scholar]
- Smith J.L., Rangaraj K., Simpson R., et al. Quantitative analysis of the expression of ACAT genes in human tissues by real-time PCR. J. Lipid Res. 2004;45:686–696. doi: 10.1194/jlr.M300365-JLR200. [DOI] [PubMed] [Google Scholar]
- Song B.L., Qi W., Yang X.Y., et al. Organization of human ACAT-2 gene and its cell-type-specific promoter activity. Biochem. Biophys. Res. Commun. 2001;282:580–588. doi: 10.1006/bbrc.2001.4612. [DOI] [PubMed] [Google Scholar]
- Song B.L., Wang C.H., Yao X.M., et al. Human acyl-CoA:cholesterol acyltransferase 2 gene expression in intestinal Caco-2 cells and in hepatocellular carcinoma. Biochem. J. 2006;394:617–626. doi: 10.1042/BJ20051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- Sundaram M., Yao Z. Recent progress in understanding protein and lipid factors affecting hepatic VLDL assembly and secretion. Nutr. Metab. (Lond) 2010;7:35. doi: 10.1186/1743-7075-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif J.C., Gregoire J., L'Allier P.L., et al. Effects of the acyl coenzyme A:cholesterol acyltransferase inhibitor avasimibe on human atherosclerotic lesions. Circulation. 2004;110:3372–3377. doi: 10.1161/01.CIR.0000147777.12010.EF. [DOI] [PubMed] [Google Scholar]
- Tennant D.A., Duran R.V., Boulahbel H., et al. Metabolic transformation in cancer. Carcinogenesis. 2009;30:1269–1280. doi: 10.1093/carcin/bgp070. [DOI] [PubMed] [Google Scholar]
- Tennant D.A., Duran R.V., Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat. Rev. Cancer. 2010;10:267–277. doi: 10.1038/nrc2817. [DOI] [PubMed] [Google Scholar]
- Wu J.M., Skill N.J., Maluccio M.A. Evidence of aberrant lipid metabolism in hepatitis C and hepatocellular carcinoma. HPB (Oxford) 2010;12:625–636. doi: 10.1111/j.1477-2574.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.