Abstract
Background
In a previous study, we showed that individuals who had participated in oil clean-up tasks after the wreckage of the Prestige presented an increase of structural chromosomal alterations two years after the acute exposure had occurred. Other studies have also reported the presence of DNA damage during acute oil exposure, but little is known about the long term persistence of chromosomal alterations, which can be considered as a marker of cancer risk.
Objectives
We analyzed whether the breakpoints involved in chromosomal damage can help to assess the risk of cancer as well as to investigate their possible association with DNA repair efficiency.
Methods
Cytogenetic analyses were carried out on the same individuals of our previous study and DNA repair errors were assessed in cultures with aphidicolin.
Results
Three chromosomal bands, 2q21, 3q27 and 5q31, were most affected by acute oil exposure. The dysfunction in DNA repair mechanisms, expressed as chromosomal damage, was significantly higher in exposed-oil participants than in those not exposed (p= 0.016).
Conclusion
The present study shows that breaks in 2q21, 3q27 and 5q31 chromosomal bands, which are commonly involved in hematological cancer, could be considered useful genotoxic oil biomarkers. Moreover, breakages in these bands could induce chromosomal instability, which can explain the increased risk of cancer (leukemia and lymphomas) reported in chronically benzene-exposed individuals. In addition, it has been determined that the individuals who participated in clean-up of the oil spill presented an alteration of their DNA repair mechanisms two years after exposure.
Introduction
In 2002, the oil tanker Prestige foundered and spilled more than 67,000 tons of the tanker´s oil, which contaminated more than 1,000 km of the coast of Galicia (North-West Spain). In response more than 300,000 clean-up workers were mobilized. The fact that the oil had a high content of aromatic hydrocarbons (50% by weight), saturated hydrocarbons, heavy metals, resins and asphaltenes, which are classified by the International Agency for Research on Cancer [1] as carcinogens or potential/probable carcinogens, alerted the scientific community to the value of investigating the genotoxic effects on human exposure to the Prestige oil.
Genotoxic studies conducted on populations exposed to the clean-up of oil spills are scarce [2-10]. Two of these studies had been performed before the Prestige accident [2,3], and seven more were performed on clean-up workers of the Prestige oil spill [4-10]. Different types of biomarkers were analyzed to address the potential genotoxic effects of acute oil-exposure research. DNA adducts were analyzed by Cole et al. [3], while sister chromatid exchanges, micronucleus and comet assay tests were used as biomarkers by others [4-9], while only two groups analyzed chromosomal damage [2,10]. Not all of the biomarkers analyzed showed significant differences between exposed and non-exposed individuals, although in the majority of them, increased genomic damage in exposed individuals has been documented. Moreover, most of these studies were carried out during the oil exposure [2-9]. No information is available regarding the reversibility or persistence of the negative oil effects. So far, only our study, reported by Rodríguez-Trigo et al. [10] has revealed an increase of chromosomal alterations (CAs) in circulating lymphocytes in exposed individuals two years after oil exposure. The findings were unexpected, due to the long time-period that had passed following exposure, and relevant due to the fact that a high number of CAs is associated with a higher risk of developing cancer, as described in the literature [11-15]. These observations led us to make a complete cytogenetic study of the same individuals.
The aim of the present study is to identify if there are specific chromosomal regions especially affected by oil exposure in the same chromosomal preparations of individuals in which an increase of chromosomal damage was found [10]. In addition, we also determined the possible existence of errors in DNA repair mechanisms by analyzing the chromosomal damage in cultures with aphidicolin, an inhibitor of DNA polymerase α and other polymerases.
Materials and Methods
Study population
In this study, an accurate selection of individuals highly exposed to the oil was performed [10,16]. Only slightly over 1% (137/10,000) of the individuals, who were non-smokers and had been initially invited, were included in the study. The collection of the samples was performed between 22 to 27 months after the Prestige disaster. The project was approved by the Ethics Committee on Clinical Research of Galicia and all participants provided written informed consent.
Cytogenetic analysis
Peripheral blood (PB) was cultured in supplemented RPMI-1640 medium (GIBCO Invitrogen Cell Culture, Invitrogen; Carlsbad, California) and then harvested according to standard procedures. For the study of chromosomal bands, PB standard culture at 37°C for 72h was used. The cytogenetic banded preparations previously studied in 91 exposed and 46 non-exposed individuals [10] were re-examined for an accurate identification of breakpoints involved in chromosomal damage.
For the study of DNA repair efficiency, PB obtained in the same extraction was cultured at 37°C for 96h, and aphidicolin (Sigma Aldrich), an inhibitor of DNA polymerase α and other polymerases, was added to the cultures 24h before harvesting at a final concentration of 0.2µM. The cellular suspension was keep frozen until cytogenetic results without aphidicolin were obtained. Dysfunction in DNA repair mechanisms was studied only in randomly selected female subsamples because women were more prevalent than man is both the samples of exposed and non-exposed individuals [10]. A total of 14 exposed and 14 non-exposed individuals were studied and compared with standard culture without aphidicolin. Chromosomal preparations were uniformly stained with Leishman (1:4 in Leishman buffer) to detect chromosomal damage, expressed mainly as chromosomal lesions (gaps and breaks). Moreover, apparent or large structural CAs (rings, marker chromosomes, dicentric translocations, etc.) were also detected. In these cases, a posterior G banding technique was applied in order to clarify if the apparent CAs were a marker chromosome, a reciprocal translocation, a duplication, etc. A minimum of 100 metaphases were analyzed in each participant according to conventional criteria.
Criteria for cytogenetic evaluations were determined according to the International System for Human Cytogenetic Nomenclature [17].
Statistical analysis
To identify which chromosomal bands were involved in chromosomal damage using standard culture, two statistical methods were used. First, the Fragile Site Multinomial method, FSM version 995, [18-20], specifically used to determine chromosomal regions with a greater propensity to break. Second, a chi-square test was performed to test the null hypothesis of a uniform distribution among the chromosomal bands, where the bands were corrected by their length. The relative length of the affected bands in relation to total genome was estimated using the diagram of the standardized human karyotype [17]. A generalized estimating equation, GEE [20,21], was used for assessing the differences between the exposed and non-exposed groups for the different types of chromosomal damage induced by aphidicolin. The GEE approach is an extension of generalized linear models designed to account for repeated, within-individual measurements. This technique is particularly indicated when the normality assumption is not reasonable, as happens, for instance, with discrete data. The GEE model was used instead of the classic Fisher exact test because the former takes into account the possible within-individual correlation, whereas the latter assumes that all observations are independent. Since several metaphases were analyzed per individual, the GEE model is more appropriate. Statistical significance was set at p< 0.05. Statistical analyses were carried out with SAS/STAT release 9.02 (SAS Institute Inc; Cary, NC). The GEE model was fitted using the REPEATED statement in the GENMOD procedure. The conservative Type 3 statistics score was used for the analysis of the effects in the model.
Results
Chromosomal bands most affected by oil exposure
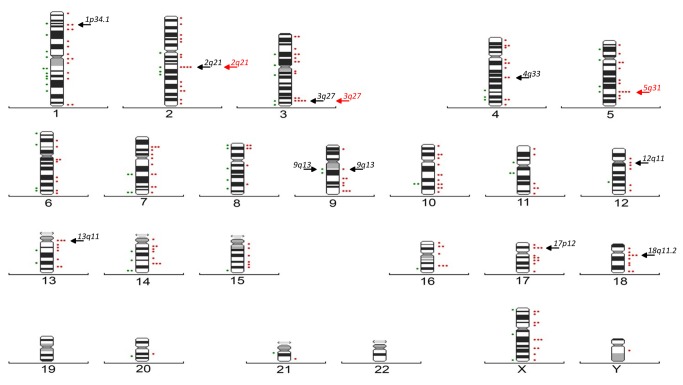
A total of 9,520 and 4,859 metaphases from standard culture were analyzed in 91 exposed and 46 non-exposed individuals, respectively. Table 1 shows the same results described in our previous report [10] using the conventional cytogenetic frequencies in order to compare them with other genotoxic studies. A total of 203 breakpoints in exposed and 61 in non-exposed individuals involved in chromosomal damage (lesions and structural CAs) were detected. The breakpoints distribution in the human ideogram (at the 400-band resolution level) is shown in Figure 1. The breakpoints involved in the chromosomal damage were mainly located on chromosomes 3, 10, 17 and 18 in exposed individuals (vs. 1, 8 and 14 in non-exposed). To identify those chromosomal bands that significantly expressed breakpoints in exposed and non-exposed participants, two statistical methods were used. In the first one, the FSM method, the number of breaks required to consider a band as being non-randomly affected was four or more. The most affected bands in the exposed group were 2q21, 3q27 and 5q31 (vs. none in non-exposed). On the other hand, the second statistical method, using the chi-square test, was applied considering the relative length of chromosomal bands identified as 1p34.1, 2q21, 3q27, 4q33, 9q13, 12q11, 13q11, 17p12 and 18q11.2 bands in exposed vs the 9q13 band in non-exposed individuals. It is interesting to note that only 2q21 and 3q27 were considered to be bands affected by both methods.
Table 1. Frequency and types of chromosomal damage observed in standard culture.
| Exposed | Non-Exposed | |
|---|---|---|
| Total individuals, No. | 91 | 46 |
| Total metaphases analyzed (uniform stain), No. | 9520 | 4859 |
| Total metaphases karyotyped (G-banded), No. | 2448 | 1285 |
| Chromosomallesion (uniform stain), No. (%) | 100/9520 (1.05) | 35/4859 (0.72) |
| Gaps | 48 (0.5) | 19 (0.39) |
| Breaks | 52 (0.55) | 16 (0.33) |
| Structuralchromosomalalterations (G-banded), No. (%) | 196/2448 (8) | 33/1285 (2.56) |
| Balanced | 12/196 (6.1) | 7/33 (21.2) |
| Reciprocal translocations | 10 | 7 |
| Robertsonian translocations | 2 | 0 |
| Imbalanced | 184/196 (93.9) | 26/33(78.8) |
| Deletions | 23 | 6 |
| Deletions + acentric fragments | 9 | 3 |
| Acentric fragments | 42 | 0 |
| Imbalanced translocations | 23 | 3 |
| Dicentric translocations | 3 | 0 |
| Dicentric translocations+acentric fragment | 4 | 1 |
| Rings | 9 | 0 |
| Markers | 68 | 13 |
| Additional material of unknown origin | 2 | 0 |
| Isochromosomes | 1 | 0 |
| Totalbreakpointsidentified | 203 | 61 |
| Chromosomal lesion (after G-banded) | 98 | 34 |
| Structural chromosomal alterations (G-banded) | 109 | 27 |
Figure 1. Distribution of chromosome breakpoints observed in exposed (right) and non-exposed individuals (left) in the human ideogram (400-band resolution).
The most affected bands using the FSM statistical method are indicated by red arrows, and when using another statistical method that takes into account the relative length of chromosomal bands, by black arrows.
DNA repair efficiency
Table 2 shows the chromosomal damage detected using uniform staining in cultures with aphidicolin and standard culture from the same exposed and non-exposed individuals. A total of 1,441 and 1,410 metaphases from cultures with aphidicolin were analyzed in 14 exposed and 14 non-exposed participants, respectively. The chromosomal damage induced by aphidicolin is usually expressed by chromosomal lesions. The number of chromosomal lesions was higher and statistically significant in exposed vs. non-exposed individuals (p= 0.023), and almost the same findings were observed in relation to apparently structural CAs (p= 0.024). Chromosomal damage (lesions and structural CAs) was also statistically higher in exposed than in non-exposed individuals (p= 0.016). Due to the large number of chromosomal lesions induced by aphidicolin, their average number is shown per 100 metaphases in Table 2.
Table 2. Chromosomal damage observed in E and NE individuals using uniform staining in cultures with aphidicolin vs standard culture.
| Culture with aphidicolin |
|
Standard culture |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposed |
Non-Exposed |
p-value |
|
Exposed | Non-Exposed |
p-value | |||||||
| Total individuals | 14 | 14 | 14 | 14 | |||||||||
| Total metaphases analyzed | 1441 | 1410 | 1500 | 1463 | |||||||||
| Total metaphases with lesions (%) | 947/1441 (65.7) | 699/1410 (49.6) | 0.0141 | 12/1500 (0.8) | 8/1463 (0.5) | 0.4775 | |||||||
| Total metaphase with structural alterations No./total (%) | 46/1441 (3.2) | 16/1410 (1.1) | 0.0376 | 15/1500 (0.01) | 1/1463 (0.07) | 0.0594 | |||||||
| Chromosomallesions (% in 100 cells) | 1864/1441 (129.3) | 1216/1410 (86.2) | 0.0231 | 13/1500 (0.9) | 10/1463 (0.7) | 0.6455 | |||||||
| Gaps | 1007 | 623 | 6 | 6 | |||||||||
| Chromatid gap | 496 | 346 | 5 | 6 | |||||||||
| Chromosome gap | 511 | 277 | 1 | 0 | |||||||||
| Breaks | 857 | 593 | 7 | 4 | |||||||||
| Chromatid break | 239 | 107 | 5 | 2 | |||||||||
| Chromosome break | 618 | 487 | 2 | 2 | |||||||||
| Apparent structural chromosomal alterations (%) | 63/1441 (4.4) | 19/1410 (1.4) | 0.0239 | 24/1500 (1.6) | 1/1463 (0.07) | 0.0791 | |||||||
| Balanced | |||||||||||||
| Translocations | 7 | 2 | 0 | 0 | |||||||||
| Imbalanced | |||||||||||||
| Deletions | 12 | 0 | 0 | 0 | |||||||||
| Acentric fragments | 11 | 3 | 16 | 1 | |||||||||
| Imbalanced translocations | 9 | 0 | 0 | 0 | |||||||||
| Dicentric translocations | 15 | 12 | 0 | 0 | |||||||||
| Rings | 7 | 1 | 2 | 0 | |||||||||
| Markers | 1 | 1 | 6 | 0 | |||||||||
| Duplications | 1 | 0 | 0 | 0 | |||||||||
| Total chromosomal damage (%) | 1927/1441 (1.33) | 1235/1410 (0.87) | 0.0161 | 37/1500 (0.025) | 11/1500 (0.07) | 0.1080 | |||||||
Discussion
A previous study reported by our group [10] has revealed an increase of structural CAs in circulating lymphocytes in exposed individuals two years after the Prestige oil exposure. The present study, carried out on the same individuals, shows that a few chromosomal bands exist in the human genome which are particularly sensitive to breakage in acute oil exposure. Furthermore, we also found that the increase in chromosomal damage is due to the existence of a statistically significant reduction of DNA repair efficiency in exposed as compared to non-exposed individuals.
Structural CAs are considered to be a highly informative biomarker for detecting adverse health effects, such as the risk of developing cancer [11,13,14]. Individuals chronically exposed to benzene have a 20-fold increased risk of developing cancer compared with the general population, particularly hematologic cancer [22-24]. Additionally, an increase in chromosomal damage, especially imbalanced structural CAs, is crucial in the development of cancer [23]. Several authors have suggested that CAs could be used as a biomarker in cancer initiating events [11,12,14]. For this reason, we proposed to determine if some chromosome bands were especially affected by oil exposure and if the effects upon the genes located in these genomic regions, could explain the cellular disorders involved in cancer. To our knowledge, this is the first study concerning chromosomal breakpoints distribution on the human ideogram in relation to acute oil exposure. This distribution was not uniform, with the 2q, 3p, 5q, 10q, 16p, 17p, 18q chromosomal arms being the most affected in exposed individuals. In two previous works only chromosomes involved in breakages were studied revealing that chromosomes 2, 4 and 7 [25] and chromosomes 5 and 7 [26] were the most frequently affected in chronically benzene-exposed individuals. These findings support the hypothesis that the 2q and 5q regions are targets in both acute and chronic exposure.
Our results show that the 2q21 and 3q27 bands are especially affected in exposed individuals when both the FSM method and an additional method that takes into account the relative bands’ lengths were used, while 5q31 was considered affected only when the FSM method was employed. These bands correspond to regions where fragile sites (FRA2F, FRA3C and FRA5C) are located according to the human genome browsers like NCBI (http://www.ncbi.nlm.nih.gov/). Although not all fragile sites may be equally involved in cancer development, it is known that they are vulnerable targets for various oncogenic agents, and their damage may potentially produce consequences for genomic integrity [27]. There is evidence that these fragile sites are involved in vivo in CAs related to tumor development [28]. According to information available from human genome browsers like NCBI (http://www.ncbi.nlm.nih.gov/), in these bands we can find genes involved in different cellular processes (Table 3) such as cellular cycle control (CCNT2 at 2q21; CDC25C, CDC23 and CDKL3 at 5q31), DNA repair (ERCC3 at 2q21), proto-oncogenes (CH-Ras at 2q21; TGFBI at 5q31), tumor suppressor genes (CXCR4, NMTC1 and TP21 at 2q21; BCL6 at 3q27; IRF1and EGR1 at 5q31), and one gene is involved in the apoptotic process (DND1 at 5q31). Results of the present study support the hypothesis that the oil components, some of them considered to be carcinogenic, may induce mutations, mainly due to breakages in specific chromosome regions (2q21, 3q27, 5q31). The 11q23 band, while especially sensitive to benzene and commonly involved in hematopoietic malignance [22,29,30], was not considered statistically significant in our study, as it was observed only in two CAs in exposed individuals. To some extent such changes, when accumulated, may cause deletions or disruptions of functional genes, thus increasing the risk of cancer, cannot be deduced from the present study. It is of interest to note that much of chromosomal reorganization in hematopoietic pathologies is associated with the same chromosome bands most affected by exposure to oil (Table 3). For example, patients with T-Cell lymphoma present CAs involving 2q21 and 3q27, acute lymphoblastic leukemia presents 5q31 as a specific chromosome region and acute myeloid leukemia is characterized by chromosomal reorganization at 5q31 and 11q23 bands [31-33]. Our findings show that there are a few chromosomal bands especially prone to breakage in oil exposure that could induce chromosomal instability, which could explain the increased risk of cancer (leukemia and lymphomas) reported in chronically benzene-exposed individuals [22-24]. Nevertheless, future genotoxic studies using the new microarray technologies applied to the genome itself (duplications, deletions, epigenetic changes) and to mRNA translation and its control mechanisms through miRNA are necessary to elucidate the role of genomic instability in the formation of cancer-specific CAs, and impact of environmental factors like oil exposure on this instability.
Table 3. Chromosomal bands most affected in present study, genes and recurrent chromosomal reorganization present in hematopoietic malignances, and comparising with chronic benzene exposure.
| Chromosomal Bands | Cellular cycle control | DNA repair | Oncogenes | Tumor suppressors | Apoptosis | Type of Cancer | Individuals exposed chronically to benzene | References |
|---|---|---|---|---|---|---|---|---|
| 2q21 | CCNT2 | ERCC3 | CH-Ras | CXCR4 | - | T-Cell lymphoma | No | [31] |
| NMTC1, TP21 | Chronic lymphocytic leukemia (CLL) | No | [40] | |||||
| 3q27 | - | - | - | BCL6 | - | T-Cell lymphoma | No | [31] |
| Non-Hodgkin lymphoma (NHL) | No | [41] | ||||||
| 5q31 | CDC23 | - | TGFBI | IRF1 | DND1 | Acute lymphoblastic leukemia (ALL) | No | [32] |
| CDC25 | EGR1 | Myelodysplastic Syndrome (MDS) | Yes | [42] | ||||
| CDKL3 | Chronic myelomonocytic leukemia (CMML) | No | [43] | |||||
| Myelodysplastic Syndrome | No | [44] | ||||||
| Acute myeloid leukemia (AML) and myelodysplastic Syndrome (MDS) | No | [45] | ||||||
| Acute lymphoblastic leukemia (ALL) | No | [46] | ||||||
| 11q23 | - | - | - | - | - | Leukemogenesis (ALL and AML) | No | [47] |
| Acute lymphoblastic leukemia (ALL) | No | [32] | ||||||
| Acute lymphoblastic leukemia (ALL) | Yes | [23] | ||||||
| Acute lymphoblastic leukemia (ALL) | No | [30] | ||||||
| 5q31/11q23 | - | - | - | - | - | Acute myeloid leukemia (AML) | Yes | [33] |
The toxic and carcinogenic compounds of the oil, such as aromatic hydrocarbons, may induce CAs directly or indirectly, by affecting the DNA repair mechanisms, in a process that, if persistent, might predispose cells to the development of cancer. To determine whether the increase in structural CAs previously detected [10] in exposed participants could be a consequence of dysfunctions in the DNA repair mechanisms, aphidicolin was added to the culture media. Aphidicolin is an inhibitor of the DNA polymerases α, δ and ε. These polymerases are involved in DNA replication and repair. The presence of aphidicolin produces breaks in DNA by stopping replication or by causing dysfunctions in DNA repair (nucleotide excision repair and base excision repair) [34]. The breakages induced by aphidicolin could be analysed using several biomarkers such as comet assay, micronucleus testing or chromosomal lesions/structural alterations analysis. The most useful biomarker is chromosomal lesions because the increase of lesions is associated with a mutagenic agent effect [35]. Our results, in 14 exposed and 14 non-exposed individuals, show that the chromosomal damage, expressed mainly as chromosomal lesions, is statistically significant and higher in exposed than in non-exposed participants, confirming the existence of dysfunction in DNA repair mechanisms due to oil exposure. Previous studies using ionizing radiation instead of aphidicolin [36-38] reported that chronically benzene-exposed individuals show a lower DNA repair efficiency. These findings along with the present study suggest that chronic and acute exposure to benzene/oil could affect DNA repair. In this regard, it has recently been published [39] that individuals who have chronic exposure to toxic substances will develop DNA repair deficiency, suggesting that this functional biomarker can be used to predict genetic risk of cancer.
These findings suggest that, in the same way that benzene may induce hematopoietic malignancies, acute oil exposure may be involved in the origin of cancer caused by chromosomal damage. Nonetheless, taking into account the wide inter-individual genetic susceptibility to carcinogens in the general population, more genetic research is necessary to clarify the existence of a relationship between acute oil exposure and subsequent cancer development. Finally, if this relationship is confirmed, this cancer risk cannot be extrapolated to the approximately 300,000 individuals that participated occasionally in oil clean-up tasks because, in our study, exposed individuals were strictly selected on the basis of intense exposure. Additionally, limitations of the present study include the small sample size, and the possibility of some kind of selection bias should be considered.
Conclusion
Our findings show an increase of chromosomal breakage at 2q21, 3q27 and 5q31 bands in PB lymphocytes two years after exposure. These chromosomal bands, which are commonly involved in hematological cancer, could be considered as useful genotoxic oil biomarkers. Moreover, breakages in these bands could induce chromosomal instability, which can explain the increased risk of cancer (leukemia and lymphomas) reported in chronically benzene-exposed individuals. In addition, our results suggest that the increase in chromosomal damage in these individuals could be a consequence of the reduction of DNA repair efficiency.
Acknowledgments
The kind participation of the fishermen’s cooperatives and the efforts made by Antonio Devesa are gratefully acknowledged. The authors wish to thank Yolanda Torralba (SEPAR), Ana Souto Alonso, Marisa Rodríguez Valcárcel, Luisa Vázquez Rey, Emma Rodríguez (Complexo Hospitalario Universitario A Coruña), Maria Angels Rigola, Ana Utrabo, Angels Niubó (Universitat Autònoma de Barcelona). The investigators are greatly indebted to Dr. J. Ancochea and Dr. J.L. Alvarez-Sala, current and past presidents of SEPAR for their initiative and support.
Funding Statement
For this study was provided by grants from the Health Institute Carlos III FEDER/ERDF (PI03/1685), Sociedad Española de Neumología y Cirugía Torácica (SEPAR), Comissionat per a Universitats i Recerca from Generalitat de Catalunya (SGR09-1107), Centro de Investigación en Red de Enfermedades Respiratorias and Universidad Autónoma de Barcelona (PS-456-01/08). The different sponsors were not involved in the design of the study, sample collection, cytogenetic analysis, and interpretation of the data, and preparation/revision of the manuscript.
References
- 1. IARC (1998). International Agency for Research on Cancer. Monographs on the Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42. IRAC Monographs Carcinogenic risk to Human (Supplement 7). Lyon: International Agency for Research on Cancer. [PubMed] [Google Scholar]
- 2. Clare MG, Yardley-Jones A, Maclean AC, Dean BJ (1984) Chromosome analysis from peripheral blood lymphocytes of workers after an acute exposure to benzene. Br J Ind Med 41: 249-253. PubMed: 6722051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cole J, Beare DM, Waugh AP, Capulas E, Aldridge KE et al. (1997) Biomonitoring of possible human exposure to environmental genotoxic chemicals: lessons from a study following the wreck of the oil tanker Braer. Environ Mol Mutagen 30: 97-111. doi: 10.1002/(SICI)1098-2280(1997)30:2. PubMed: 9329634. [DOI] [PubMed] [Google Scholar]
- 4. Laffon B, Fraga-Iriso R, Pérez-Cadahía B, Méndez J (2006) Genotoxicity associated to exposure to Prestige oil during autopsies and cleaning of oil-contaminated birds. Food Chem Toxicol 44: 1714-1723. doi: 10.1016/j.fct.2006.05.010. PubMed: 16814914. [DOI] [PubMed] [Google Scholar]
- 5. Perez-Cadahia B, Laffon B, Pasaro E, Méndez J (2006) Genetic damage induced by accidental environmental pollutants. Scientific World J 6: 1221-1237. doi: 10.1100/tsw.2006.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pérez-Cadahía B, Lafuente A, Cabaleiro T, Pásaro E, Méndez J et al. (2007) Initial study on the effects of Prestige oil on human health. Environ Int 33: 176-185. doi: 10.1016/j.envint.2006.09.006. PubMed: 17055056. [DOI] [PubMed] [Google Scholar]
- 7. Pérez-Cadahía B, Laffon B, Porta M (2008a) Relationship between blood concentrations of heavy metals and cytogenetic and endocrine parameters among subjects involved in cleaning coastal areas affected by the Prestige tanker oil spill. Chemosphere 7: 447-455. [DOI] [PubMed] [Google Scholar]
- 8. Pérez-Cadahía B, Laffon B, Valdiglesias V, Pásaro E, Méndez J (2008b) Cytogenetic effects induced by Prestige oil on human populations: the role of polymorphisms in genes involved in metabolism and DNA repair. Mutat Res 653: 117-123. doi: 10.1016/j.mrgentox.2008.04.002. PubMed: 18495522. [DOI] [PubMed] [Google Scholar]
- 9. Pérez-Cadahía B, Méndez J, Pásaro E, Lafuente A, Cabaleiro T et al. (2008c) Biomonitoring of human exposure to Prestige oil: Effects on DNA and endocrine parameters. Environ Health Insights 2: 83-92. PubMed: 21572833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodríguez-Trigo G, Zock JP, Pozo-Rodríguez F, Gómez FP, Monyarch G et al. (2010) Health changes in fishermen 2 years after clean-up of the Prestige oil spill. Ann Intern Med 153: 489-498. doi: 10.7326/0003-4819-153-8-201010190-00279. PubMed: 20733177. [DOI] [PubMed] [Google Scholar]
- 11. Norppa H, Bonassi S, Hansteen IL, Hagmar L, Strömberg U et al. (2006) Chromosomal aberrations and SCEs as biomarkers of cancer risk. Mutat Res 600: 37-45. doi: 10.1016/j.mrfmmm.2006.05.030. PubMed: 16814813. [DOI] [PubMed] [Google Scholar]
- 12. Stallings RL (2007) Are chromosomal imbalances important in cancer? Trends Genet 23: 278-283. doi: 10.1016/j.tig.2007.03.009. PubMed: 17400327. [DOI] [PubMed] [Google Scholar]
- 13. Au WW (2007) Usefulness of biomarkers in population studies: from exposure to susceptibility and to prediction of cancer. Int J Hyg Environ Health 210: 239-246. doi: 10.1016/j.ijheh.2006.11.001. PubMed: 17174154. [DOI] [PubMed] [Google Scholar]
- 14. Bonassi S, Norppa H, Ceppi M, Strömberg U, Vermeulen R et al. (2008) Chromosomal aberration frequency in lymphocytes predicts the risk of cancer: results from a pooled cohort study of 22,358 subjects in 11 countries. Carcinogenesis 29: 1178-1183. doi: 10.1093/carcin/bgn075. PubMed: 18356148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fröhling S, Döhner H (2008) Chromosomal abnormalities in cancer. N Engl J Med 359: 722-734. doi: 10.1056/NEJMra0803109. PubMed: 18703475. [DOI] [PubMed] [Google Scholar]
- 16. Zock JP, Rodríguez-Trigo G, Pozo-Rodríguez F, Barberà JA, Bouso L et al. (2007) Prolonged respiratory symptoms in clean-up workers of the prestige oil spill. Am J Respir Crit Care Med 176: 610-616. doi: 10.1164/rccm.200701-016OC. PubMed: 17556713. [DOI] [PubMed] [Google Scholar]
- 17. ISCN (2013). An International System for Human Cytogenetic Nomenclature. Shafer NLG, McGowan-Jordan J, Schmid M. S. Karger; Basel. [Google Scholar]
- 18. Böhm U, Dahm PF, McAllister BF, Greenbaum IF (1995) Identifying chromosomal fragile sites from individual: a multinomial statistical model. Hum Genet 95: 249-256. PubMed: 7868115. [DOI] [PubMed] [Google Scholar]
- 19. Greenbaum IF, Fulton JK, White ED, Dahm PF (1997) Minimum sample sizes for identifying chromosomal fragile sites from indviduals: Monte Carlo estimation. Hum Genet 101: 109-112. doi: 10.1007/s004390050596. PubMed: 9385380. [DOI] [PubMed] [Google Scholar]
- 20. de la Chica RA, Ribas I, Giraldo J, Egozcue J, Fuster C (2005) Chromosomal instability in amniocytes from fetuses of mothers who smoke. JAMA 293: 1212-1222. doi: 10.1001/jama.293.10.1212. PubMed: 15755944. [DOI] [PubMed] [Google Scholar]
- 21. Liang KY, Zeger SL (1986) Longitudinal data analysis using generalized linear models. Biometrika 73: 13-22. doi: 10.1093/biomet/73.1.13. [DOI] [Google Scholar]
- 22. Smith MT, Zhang L (1998) Biomarkers of leukemia risk: benzene as a model. Environ Health Perspect 106: 937-946. doi: 10.2307/3434135. PubMed: 9703476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang L, Eastmond DA, Smith MT (2002) The nature of chromosomal aberrations detected in humans exposed to benzene. Crit Rev Toxicol 32: 1-42. doi: 10.1080/20024091064165. PubMed: 11846214. [DOI] [PubMed] [Google Scholar]
- 24. Barregard L, Holmberg E, Sallsten G (2009) Leukemia incidence in people living close to an oil refinery. Environ Res 109: 985-990. doi: 10.1016/j.envres.2009.09.001. PubMed: 19781695. [DOI] [PubMed] [Google Scholar]
- 25. Sasiadek M (1992) Nonrandom distribution of breakpoints in the karyotypes of workers occupationally exposed to benzene. Environ Health Perspect 97: 255-257. doi: 10.1289/ehp.9297255. PubMed: 1396464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang L, Wang Y, Shang N, Smith MT (1998) Benzene metabolites induce the loss and long arm deletion of chromosomes 5 and 7 in human lymphocytes. Leuk Res 22: 105-113. doi: 10.1016/S0145-2126(97)00157-4. PubMed: 9593466. [DOI] [PubMed] [Google Scholar]
- 27. Dillon LA, Burrow AA, Wang YH (2010) DNA instability at chromosomal fragile sites in cancer. Curr Genomics 11: 326-337. doi: 10.2174/138920210791616699. PubMed: 21286310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lukusa T, Fryns JP (2008) Human chromosome fragility. Biochim Biophys Acta 1779: 3-16. doi: 10.1016/j.bbagrm.2007.10.005. PubMed: 18078840. [DOI] [PubMed] [Google Scholar]
- 29. Lin C, Smith ER, Takahashi H, Lai KC, Martin-Brown S et al. (2010) AFF4, a component of the ELL/P-TEFb elongation complex and a shared subunit of MLL chimeras, can link transcription elongation to leukemia. Mol Cell 37: 429-437. doi: 10.1016/j.molcel.2010.01.026. PubMed: 20159561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bas Suárez MP, López Brito J, Santana Reyes C, Gresa-Muñoz M, Diaz Pulido R et al. (2011) Congenital acute lymphoblastic leukemia: a two-case report and a review of the literature. Eur J Pediatr 170: 531-534. doi: 10.1007/s00431-010-1339-8. PubMed: 21046414. [DOI] [PubMed] [Google Scholar]
- 31. Levine EG, Arthur DC, Gajl-Peczalska KJ, LeBien TW, Peterson BA et al. (1986) Correlations between immunological phenotype and karyotype in malignant lymphoma. Cancer Res 46: 6481-6488. PubMed: 3096564. [PubMed] [Google Scholar]
- 32. Borkhardt A, Bojesen S, Haas OA, Fuchs U, Bartelheimer D et al. (2000) The human GRAF gene is fused to MLL in a unique t(5;11)(q31;q23) and both alleles are disrupted in three cases of myelodysplastic syndrome/acute myeloid leukemia with a deletion 5q. Proc Natl Acad Sci U S A 97: 9168-9173. doi: 10.1073/pnas.150079597. PubMed: 10908648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Escobar PA, Smith MT, Vasishta A, Hubbard AE, Zhang L (2007) Leukemia-specific chromosome damage detected by comet with fluorescence in situ hybridization (comet-FISH). Mutagenesis 22: 321-327. doi: 10.1093/mutage/gem020. PubMed: 17575318. [DOI] [PubMed] [Google Scholar]
- 34. Speit G, Leibiger C, Kuehner S, Högel J (2013) Further investigations on the modified comet assay for measuring aphidicolin-block nucleotide excision repair. Mutagenesis 28: 145-151. doi: 10.1093/mutage/ges063. PubMed: 23221037. [DOI] [PubMed] [Google Scholar]
- 35. Tedeschi B, Cicchetti R, Argentin G, Caporossi D, Pittaluga M et al. (2004) Aphidicolin and bleomycin induced chromosome damage as biomarker of mutagen sensitivity: a twin study. Mutat Res 546: 55-64. doi: 10.1016/j.mrfmmm.2003.10.006. PubMed: 14757193. [DOI] [PubMed] [Google Scholar]
- 36. Hallberg LM, Bechtold WE, Grady J, Legator MS, Au WW (1997) Abnormal DNA repair activities in lymphocytes of workers exposed to 1,3-butadiene. Mutat Res 383: 213-221. doi: 10.1016/S0921-8777(97)00004-9. PubMed: 9164482. [DOI] [PubMed] [Google Scholar]
- 37. Navasumrit P, Arayasiri M, Hiang OM, Leechawengwongs M, Promvijit J et al. (2008) Potential health effects of exposure to carcinogenic compounds in incense smoke in temple workers. Chem Biol Interact 173: 19-31. doi: 10.1016/j.cbi.2008.02.004. PubMed: 18359011. [DOI] [PubMed] [Google Scholar]
- 38. Chanvaivit S, Navasumrit P, Hunsonti P, Autrup H, Ruchirawat M (2007) Exposure assessment of benzene in Thai workers, DNA-repair capacity and influence of genetic polymorphisms. Mutat Res 626: 79-87. doi: 10.1016/j.mrgentox.2006.09.007. PubMed: 17095285. [DOI] [PubMed] [Google Scholar]
- 39. Ruchirawat M, Cebulska-Wasilewska A, Au WW (2013) Evidence for exposure-induced DNA repair abnormality is indicative of health and genetic risk. Int J Hyg Environ Health 216: 566-573. doi: 10.1016/j.ijheh.2013.03.003. PubMed: 23545294. [DOI] [PubMed] [Google Scholar]
- 40. Crowther-Swanepoel D, Mansouri M, Enjuanes A, Vega A, Smedby KE et al. (2010) Verification that common variation at 2q37.1, 6p25.3, 11q24.1, 15q23, and 19q13.32 influences chronic lymphocytic leukaemia risk. Br J Haematol 150: 473-479. PubMed: 20553269. [DOI] [PubMed] [Google Scholar]
- 41. Baron BW, Zeleznik-Le N, Baron MJ, Theisler C, Huo D et al. (2007) Repression of the PDCD2 gene by BCL6 and the implications for the pathogenesis of human B and T cell lymphomas. Proc Natl Acad Sci U S A 104: 7449-7454. doi: 10.1073/pnas.0701770104. PubMed: 17468402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stillman WS, Varella-Garcia M, Irons RD (2000) The benzene metabolite, hydroquinone, selectively induces 5q31- and -7 in human CD34+CD19- bone marrow cells. Exp Hematol 28: 169-176. doi: 10.1016/S0301-472X(99)00144-7. PubMed: 10706073. [DOI] [PubMed] [Google Scholar]
- 43. Apperley JF, Gardembas M, Melo JV, Russell-Jones R, Bain BJ et al. (2002) Response to imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet-derived growth factor receptor beta. N Engl J Med 347: 481-487. doi: 10.1056/NEJMoa020150. PubMed: 12181402. [DOI] [PubMed] [Google Scholar]
- 44. Nimer SD (2006) Clinical management of myelodysplastic syndromes with interstitial deletion of chromosome 5q. J Clin Oncol 24: 2576-2582. doi: 10.1200/JCO.2005.03.6715. PubMed: 16735711. [DOI] [PubMed] [Google Scholar]
- 45. Pinheiro RF, Serio FM, Silva MR, Briones MR, Chauffaille ML (2008) Association of loss of heterozygosity with cytogenetic abnormalities in acute myeloid leukemia and myelodysplastic syndrome. Braz J Med Biol Res 41: 610-614. doi: 10.1590/S0100-879X2008000700010. PubMed: 18719743. [DOI] [PubMed] [Google Scholar]
- 46. Wang Y, Xue Y, Chen S, Wu Y, Pan J et al. (2010) A novel t(5;11)(q31;p15) involving the NUP98 gene on 11p15 is associated with a loss of the EGR1 gene on 5q31 in a patient with acute myeloid leukemia. Cancer Gene Cytogenet 199: 9-14. doi: 10.1016/j.cancergencyto.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 47. Rowley JD (1999) The role of chromosome translocations in leukemogenesis. Semin Hematol 36: 59-72. PubMed: 10595755. [PubMed] [Google Scholar]