Abstract
Background
ZIP5 localizes to the baso-lateral membranes of intestinal enterocytes and pancreatic acinar cells and is internalized and degraded coordinately in these cell-types during periods of dietary zinc deficiency. These cell-types are thought to control zinc excretion from the body. The baso-lateral localization and zinc-regulation of ZIP5 in these cells are unique among the 14 members of the Slc39a family and suggest that ZIP5 plays a role in zinc excretion.
Methods/Principal Findings
We created mice with floxed Zip5 genes and deleted this gene in the entire mouse or specifically in enterocytes or acinar cells and then examined the effects on zinc homeostasis. We found that ZIP5 is not essential for growth and viability but total knockout of ZIP5 led to increased zinc in the liver in mice fed a zinc-adequate (ZnA) diet but impaired accumulation of pancreatic zinc in mice fed a zinc-excess (ZnE) diet. Loss-of-function of enterocyte ZIP5, in contrast, led to increased pancreatic zinc in mice fed a ZnA diet and increased abundance of intestinal Zip4 mRNA. Finally, loss-of-function of acinar cell ZIP5 modestly reduced pancreatic zinc in mice fed a ZnA diet but did not impair zinc uptake as measured by the rapid accumulation of 67zinc. Retention of pancreatic 67zinc was impaired in these mice but the absence of pancreatic ZIP5 sensitized them to zinc-induced pancreatitis and exacerbated the formation of large cytoplasmic vacuoles containing secretory protein in acinar cells.
Conclusions
These studies demonstrate that ZIP5 participates in the control of zinc excretion in mice. Specifically, they reveal a paramount function of intestinal ZIP5 in zinc excretion but suggest a role for pancreatic ZIP5 in zinc accumulation/retention in acinar cells. ZIP5 functions in acinar cells to protect against zinc-induced acute pancreatitis and attenuate the process of zymophagy. This suggests that it may play a role in autophagy.
Introduction
Zinc homeostasis is tightly controlled which reflects the essential functions of this metal in a vast array of proteins including enzymes, transcription factors, cell surface receptors and proteins involved in signalling cascades [1], [2]. Ultimately when zinc is deficient, cell division, growth and viability are impaired. Control of zinc homeostasis is exerted predominately by three families of proteins [3]–[6]. The most abundant and ubiquitously expressed members of the cysteine-rich metallothionein family (MT-I and II in mice) are induced by zinc and function as intracellular zinc buffers which provide a biologically available pool of zinc. Over-expression of these genes in mice provides protection against dietary zinc deficiency whereas loss-of -function renders mice more sensitive to zinc deficiency [7], [8]. Uptake and efflux of zinc involve two diverse families of zinc transporters. Members of the Slc39a or Zip family (14 known genes) are thought to transport zinc into the cytoplasm of cells, either from the extracellular milieu or from the vesicular compartment [5]. Some of these family members may also transport other essential metals such as iron or cadmium, and many display cell-specific patterns of expression and regulation [9]–[12]. Members of the Slc30a or ZnT family (10 known genes) are generally thought to efflux zinc out of the cytosol and into the extracellular milieu or into the vesicular compartment [3]. As noted above, family members may also play an important role in the transport of other metals such as manganese [13] and many display cell specific patterns of expression [14]. The complexity of the protein families involved in zinc homeostasis clearly reflects the diverse functions of this essential metal.
Recent genetic studies have begun to reveal physiological roles of many of the members of these two zinc transporter families. Among the 14 members of the Zip gene family, 7 have been mutated in mice and the physiological consequences examined. Our studies of Zips1, 2 and 3, members of a highly conserved subfamily, revealed that these transporters are not essential. However, they each play unique tissue-specific roles during zinc deficiency [12], [15]. In contrast, we demonstrated that Zip4 is an essential gene in mice and expression of this gene specifically in the intestinal epithelium or yolk sac endoderm mediates the acquisition of dietary zinc in newborn and adult mice or by the early embryo, respectively [16], [17]. Loss-of-function of this gene leads to wasting unless these mice are maintained on high levels of zinc [17]. The Zip4 gene is mutated in humans with acrodermatitis enteropathica, a potentially lethal zinc deficiency disease [18], [19]. Studies of mice expressing a Zip8 hypomorphic allele revealed that active expression of this gene is essential during late fetal and early postnatal life and is important for multi-organ development [20]. This gene has also been shown to increase sensitivity to cadmium toxicity [21]. Other recent studies found that Zip13 is not essential for viability, but deletion of this gene results in impaired connective tissue development in mice [22]. This results in changes in bone, teeth and connective tissue similar to that noted in humans with Ehlers-Danlos syndrome, some of whom have mutations in this gene [22]. Finally, mice lacking Zip14 exhibit growth retardation with impaired gluconeogenesis and reduced hepatocyte proliferation during liver regeneration [23], [24].
In the current study we probed the physiological roles of Zip5 (Slc39a5) in zinc homeostasis. This zinc transporter is particularly interesting because it localizes to the baso-lateral cell membrane and is abundant specifically in intestinal enterocytes, pancreatic acinar cells and embryonic visceral endoderm cells [25]. These cell types play key roles in mammalian zinc homeostasis. ZIP5 regulation also appears to be unique in that this protein is internalized and degraded coordinately in each of these cell-types during periods of dietary zinc deficiency [25], [26]. Translation of the Zip5 mRNA is stalled during zinc deficiency in a mechanism which involves a conserved 3′-untranslated region that is predicted to form a stable stem-loop structure and to interact with specific microRNAs [26], [27]. Given its unique zinc regulation, cellular localization and active expression in organs involved in the control of zinc homeostasis in mammals, we created mice with floxed Zip5 genes and examined mice in which this gene was disabled specifically in enterocytes or acinar cells or disabled in all cells. We found that this gene is not essential for growth and viability of mice. However, our studies revealed a novel role for enterocyte ZIP5 in zinc excretion, and a role for pancreatic acinar cell ZIP5 in protection against zinc toxicity. Loss-of-function of enterocyte ZIP5 led to increased accumulation of pancreatic zinc whereas loss-of-function of acinar cell ZIP5 exacerbated the formation of large cytoplasmic granules containing secretory protein and acinar cell atrophy during zinc-induced acute-pancreatitis. These findings suggest a role for acinar cell ZIP5 and/or zinc in a process termed zymophagy, the selective autophagy of secretory granules [28], [29].
Results
Knocking out the Zip5 gene in every cell, in the intestinal epithelium, or in pancreatic acinar cells
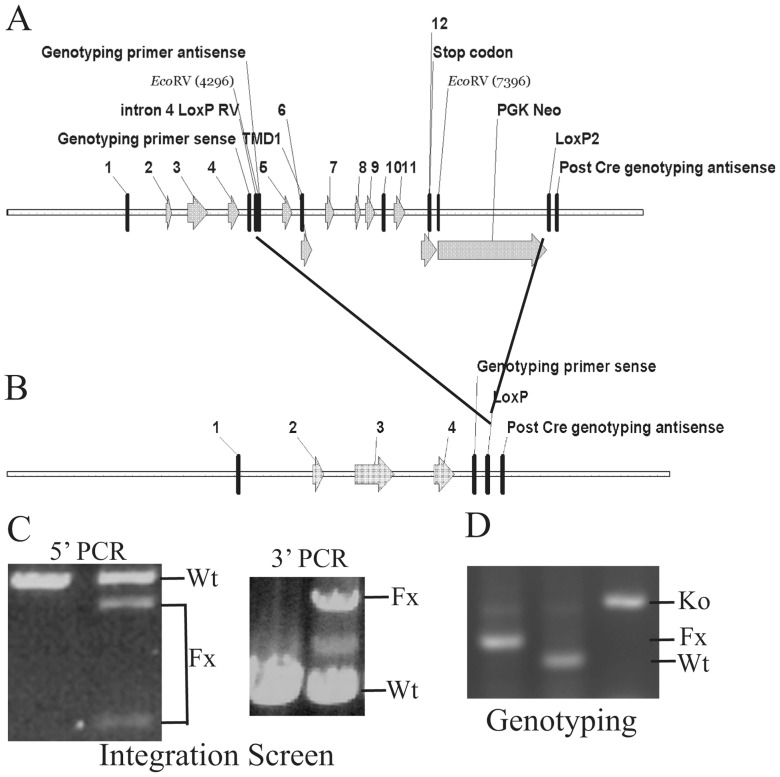
A mouse Zip5 gene targeting construct was created using BAC recombineering. A LoxP site flanked by an EcoRV restriction site was inserted into intron 4, and a second LoxP site was inserted downstream from a PGK-Neomycin cassette placed after the last exon in the Zip5 gene (Fig. 1A). Recombination of this construct results in the removal of the entire transmembrane domain of ZIP5 leaving only exons 1–4 intact (Fig. 1B). This construct was targeted in E14 embryonic stem cells and homologous integration of the floxed Zip5 gene was identified using long-range PCR and primers outside of the engineered targeting construct coupled with overlapping internal primers (Fig. 1C). The 5′ integration screen (Fig. 1C) amplified a 6.22 kb product from the wild-type and the floxed alleles and cleavage of the floxed allele with EcoRV yielded the predicted 4.5 and 1.7 kb restriction fragments indicative of homologous recombination of the floxed allele. This was confirmed by the 3′ integration screen which yielded a 3.39 kb PCR product from the floxed allele and a 5.26 kb PCR product from the wild-type allele. Targeted E14 ES cells were used to create chimeric mice by blastocyst injection and agouti offspring from the chimeric mice were genotyped using primers which flank the LoxP-EcoRV insertion site in intron 4 (Fig. 1D). Genotyping PCR yielded a 197 bp product from the floxed allele which could be cut by EcoRV, a 157 bp product from the wild-type allele, and a 275 bp product from the recombined allele after CRE excision (Fig. 1D).
Figure 1. Structures of the pre- and post-Cre floxed mouse Zip5 gene and integration and genotyping screen designs.
(A) The mouse Zip5 gene was captured using gap-repair and manipulated using galK recombineering. Exons (1–12) and the exon encoding transmembrane domain 1 (TMD1) are indicated, as are the positions of LoxP sites (intron 4 and downstream of PGK Neo), the PGK-neomycin (PGK Neo) cassette and the locations of primers used for genotyping. The LoxP site in intron 4 is flanked by an EcoRV restriction enzyme cleavage site. (B) The structure of the Zip5 gene after Cre recombination is shown. Recombination eliminates the transmembrane domain of ZIP5. (C) The floxed Zip5 gene was targeted into E14 ES cells and properly targeted ES cells were identified by long range PCR using flanking and internal primers. PCR products from the wild-type (Wt) and floxed (Fx) alleles are indicated. EcoRV cleavage was used to differentiate between the floxed and wild-type alleles in the 5′ PCR screen whereas the 3′ PCR screen yielded the predicted larger product from the wild-type allele. Targeted ES cells were used to generate mice homozygous for the floxed Zip5 allele. (D) Mice were genotyped by PCR amplification of the intron 4 region containing the LoxP site. The PCR product from homozygous Zip5 floxed mice before Cre-induced recombination is shown in the left lane while that from control mice is shown in the center lane and that from Zip5-knockout mice (Ko) is shown in the right lane. For the intestine- and pancreas-specific knockout mice, detection of Zip5 mRNA and/or protein was employed to monitor the efficacy of recombination.
The mouse Zip5 gene is not essential but loss-of-function leads to changes in zinc accumulation in the liver, intestine and pancreas
Total knockout of the Zip5 gene was accomplished by crossing Zip5Fx/Fx mice with mice that express Cre under control of the ubiquitously expressed EIIa promoter. Mice showing complete recombination of the Zip5Fx/Fx alleles were selected to develop a working colony and age matched Zip5Fx/Fx mice were used as controls. Zip5-knockout mice displayed normal fecundity (6.6±1.8 KO vs 7.0±1.9 control pups per litter) and had no overt phenotype up to 1 year of age when they were no longer active breeders and were euthanized. Thus, the mouse Zip5 gene is not essential.
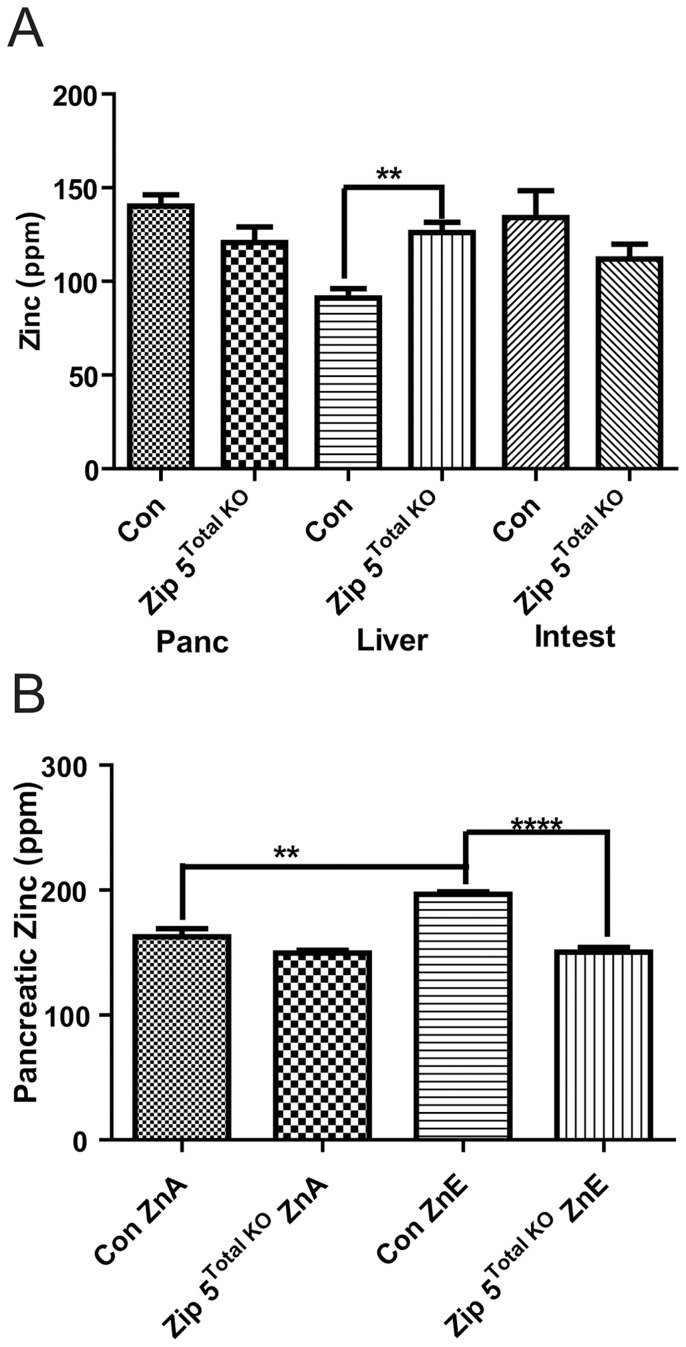
However, elemental analyses of major organs (liver, pancreas and intestine) involved in zinc homeostasis revealed a significant increase (∼38%) in liver zinc in the knockout mice and a trend toward lower levels of zinc in the intestine and pancreas in mice fed a zinc adequate diet (ZnA), although the latter did not reach statistical significance (Fig. 2A). There were no other significant differences in the multiple essential metals and other elements measured using inductively-coupled plasma mass spectrometry (ICP-MS). Mice were then challenged chronically by providing drinking water containing excess zinc (250 ppm zinc; ZnE) for 8 days. Tissues were analyzed by ICP-MS (Fig. 2B). Whereas control mice provided with drinking water containing excess zinc (ZnE) for 8 days accumulated zinc in the pancreas, these knockout mice did not (Fig. 2B). This was the only significant difference noted in all elements examined. The above results show that ZIP5 functions to modulate zinc homeostasis and suggest that the absence of this zinc transporter leads to an increase in the burden of zinc causing accumulation in the liver instead of accumulation in the pancreas which normally occurs (see below).
Figure 2. Total knockout of the Zip5 gene results in increased hepatic zinc and modestly attenuates pancreatic zinc accumulation.
Mice with homozygous floxed Zip5 genes were crossed with mice that ubiquitously express CRE recombinase under control of the EIIa promoter. Offspring with complete recombination of the Zip5 gene in tail DNA were identified and crossed to create a colony of Zip5- knockout mice (Zip5 Total KO). Zip5 Fx/Fx mice served as controls (Con). (A) Mice (n = 4 or 5) were maintained on normal diet (ZnA) diet and pancreas (Panc), liver and intestine (Intest) were harvested two weeks after weaning and from age matched Zip5 Total KO and control and tissue elements were quantified using ICP-MS and are expressed as ppm/dry weight. Zinc in the liver was the only element which differed significantly between the Zip5 Total KO and control mice (P = 0.0032). (B) Intestine, pancreas and liver were harvested from control and Zip5 Total KO mice fed normal chow (ZnA) or normal chow plus 250 ppm zinc in the drinking water (ZnE) for 8 days and tissue elements were quantified using ICP-MS. Only pancreatic zinc is shown since no other significant changes in any other element were found (**; P<0.005: ****; P<0.0001).
Loss-of-function of the intestine Zip5 gene leads to increased accumulation of zinc in the pancreas
The above results are consistent with ZIP5 playing a role, albeit subtle, specifically in zinc homeostasis, but total knockout does not address tissue-specific roles for this zinc transporter. To explore this possibility we examined the effects of conditionally deleting this gene in the intestine and pancreas, the organs which most actively express the Zip5 gene. ZIP5 protein accumulates on the basolateral surfaces of enterocytes and acinar cells, cell-types which are thought to play key roles in mammalian zinc homeostasis.
To enable tissue-specific recombination of the floxed Zip5 gene specifically in the intestinal epithelium, newly weaned Zip5Fx/Fx mice expressing an estrogen receptor-Cre recombinase fusion protein under control of the villin promoter [30] and Zip5Fx/Fx littermates that do not expresses the transgene were injected with tamoxifen to drive the Cre-ERT2 fusion protein to the nucleus and initiate recombination of the floxed gene. Northern blot analysis of RNA from the small intestine of Zip5 Intest KO mice revealed a dramatic reduction in Zip5 mRNA and an increase in the abundance of Zip4 mRNA (Fig. 3A). Efficacy of the knockout was examined in multiple mice and was estimated to be >90%.
Figure 3. Loss of function of the intestine Zip5 gene results in the accumulation of zinc in the pancreas.

Newly weaned mice were injected with tamoxifen to induce Zip5 recombination in the intestine by activation of Cre-ERT2 expressed from a vil-Cre-ERT2 transgene. (A) Northern blot detection of Zip5 and Zip4 mRNAs in the small intestine of control (Con) littermates and intestine-specific Zip5-knockout (Zip5 Intest KO) mice. Results from 2 mice per group are shown but 5 mice per group were analyzed with identical results. (B) Intestine, pancreas and liver were harvested from control and Zip5Intest KO mice fed normal chow (ZnA) or normal chow plus 250 ppm zinc in the drinking water (ZnE) for 8 days after knocking out the Zip5 gene in the intestine. Tissue elements were quantified using ICP-MS and are expressed as ppm/dry weight of tissue. Only zinc levels in the pancreas are shown because this was the only change detected (n = 5: ****; P<0.0001). (C) Control and Zip5Intest KO mice (n = 3) were given a gavage containing 30 ppm zinc and zinc levels in the pancreas, liver and intestine were monitored using ICP-MS at the indicated times (3 to 16 h) thereafter. No significant change in zinc or any other element was noted in the liver and intestine. Only zinc increased in the pancreas of the Zip5Intest KO mice. (**; P<0.006).
Elemental analysis of the pancreas revealed a ∼60% increase in pancreatic zinc in the Zip5 Intest KO mice fed a zinc adequate (ZnA) diet (Fig. 3B) but no change in liver or intestine zinc (data not shown). This difference between control and Zip5 Intest KO mice was exacerbated in mice provided excess zinc in the drinking water for 8 days. However, in this experiment neither the control nor Zip5 Intest KO mice showed a statistically significant increase in pancreatic zinc in response to chronic exposure to excess dietary zinc. In contrast, figures 2B and 5A show that excess dietary zinc normally leads to significant increases in zinc in the pancreas of control mice. The reason for this discrepancy is unclear but the results in figure 3B clearly demonstrate that the Zip5Intest KO pancreas contains much higher levels of zinc than does that of control mice. After acute exposure to excess zinc by gavage, Zip5 Intest KO mice accumulated significantly more pancreatic zinc than did control mice (Fig. 3C) confirming that loss-of-function of intestine ZIP5 causes increased accumulation of pancreatic zinc. There were no significant differences between the Zip5 Intest KO and control mice in the other organs or other elements examined in these experiments.
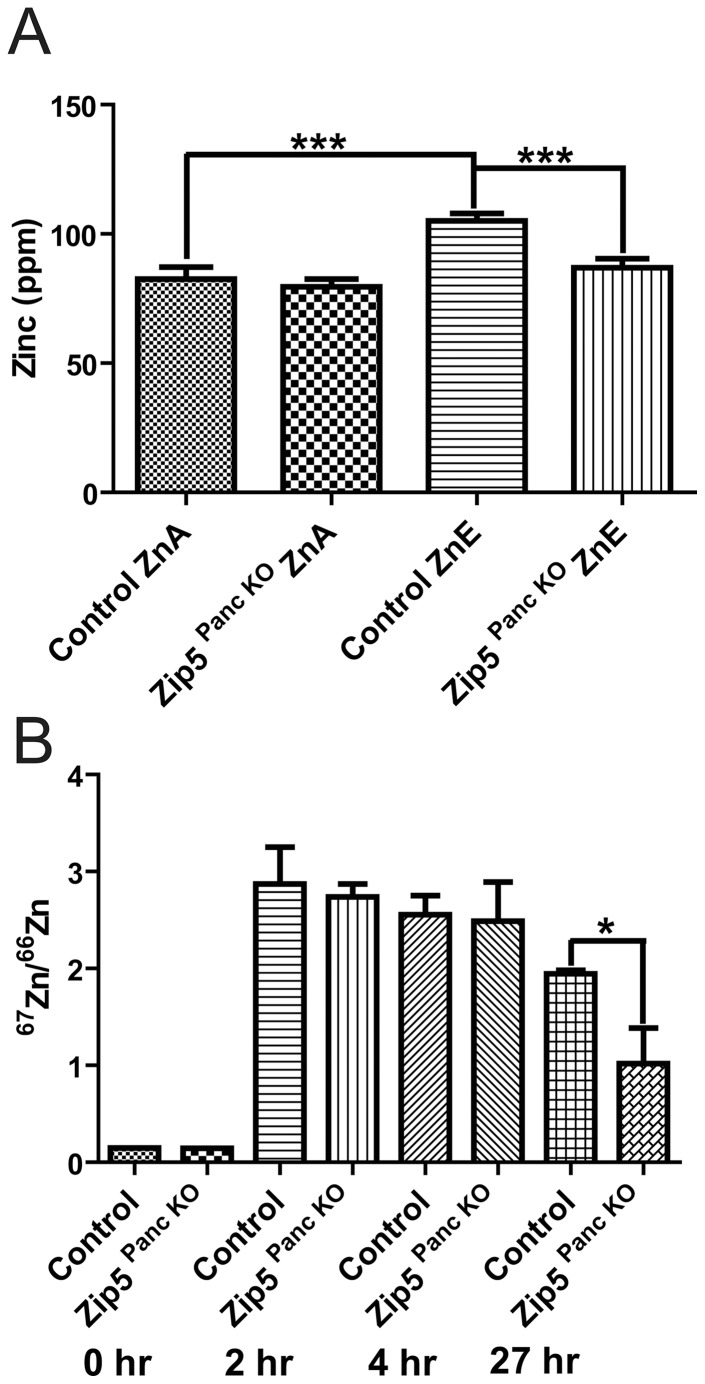
Figure 5. Knockdown of the pancreas acinar cell Zip5 gene has no effect on the rapid accumulation of zinc but apparently impairs zinc retention in the pancreas.
(A) Control littermates and pancreas-specific Zip5-knockout (Zip5 Panc KO) mice (n = 4– 5) were killed 8 days after the last tamoxifen injection. Intestine, pancreas and liver were harvested from mice fed normal chow (ZnA) or normal chow plus 250 ppm zinc in the drinking water (ZnE) during those 8 days and pancreatic zinc was quantified using ICP-MS and is expressed as ppm/dry weight of tissue. (B) Control and Zip5 Panc KO mice were injected I.P. with 6.25 mg 67Zn/kg body weight and the pancreas was harvested at 0 h, 2 h and 4 hr after the injection or (C) 27 hr after the injection. The ratio of 67Zn/66Zn was measured by ICP-MS. The natural ratio of these stable isotopes is 0.146. Only pancreatic zinc is shown since no other significant changes in any other element were found (*; P<0.019; ***; P<0.0007).
The above results reveal cross-talk between the intestine and pancreas with regard to zinc homeostasis and are consistent with the concept that ZIP5 plays a role in zinc excretion from the intestine by the uptake of zinc from the blood into the intestine.
Loss-of-function of the pancreas Zip 5 gene sensitizes mice to zinc-induced pancreatitis and reduces the accumulation but not the uptake of pancreatic zinc
To enable tissue-specific recombination of the floxed Zip5 gene in pancreatic acinar cells, newly weaned Zip5 Fx/Fx mice that express Cre-ERT2 recombinase under control of the elastase promoter [31] and Zip5Fx/Fx littermates were injected with tamoxifen to initiate recombination of the floxed gene. Northern blot analysis of pancreatic RNA from Zip5 Panc KO mice revealed that Zip5 mRNA was dramatically reduced but remained detectable in many of the samples (Fig. 4A). Detection of ZIP5 by immunohistochemistry (IHC) in sections of the pancreas revealed that the knockout was mosaic (Fig. 4B). Quantification of the extent of the knockout by counting the relative number of positive cells per field of view indicated an efficacy of 70 to 90%.
Figure 4. Knockout of the pancreas acinar cell Zip5 gene is mosaic.
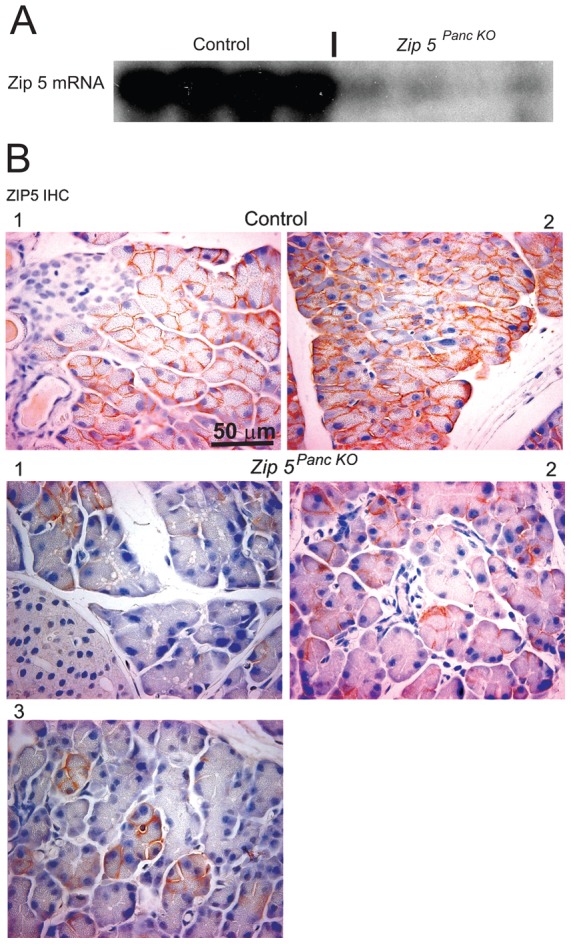
Newly weaned mice were injected with tamoxifen to induce Zip5 recombination in the pancreas by activation of Cre-ERT2 expressed from an Ela-Cre-ERT2 transgene. (A) Northern blot detection of Zip5 mRNA in the pancreas of control (Control) littermates and pancreas-specific Zip5-knockout (Zip 5 Panc KO) mice killed 5 days after the last tamoxifen injection. (B) Detection of ZIP5 protein using immunohistochemistry (ZIP5 IHC). Paraffin sections of pancreas were incubated with an anti-ZIP5 peptide antibody and specific binding was detected as a dark brown precipitate on the baso-lateral surfaces of acinar cells. Sections from 2 control mice and 3 different knockout mice are shown at 200× magnification. Quantification of the number of ZIP5 immuno-positive and negative cells per field of view in multiple sections from several mice indicated an efficiency of the knockout of ∼70 to ∼90%. In Part B number 1 knockout, the percentage of remaining positive cells was 8% (6/80) indicating a 92% efficacy of the knockout. In part B number 2 knockout, the percentage of remaining positive cells 32% (39/120) indicating an ∼68% efficacy of the knockout. I; indicates Islets of Langerhans: D; indicates a pancreatic duct.
Elemental analysis of intestine, liver and pancreas from Zip5 Panc KO and control mice revealed a decrease in liver iron (Fig.S1A). Although the amount of pancreatic zinc in control versus Zip5 Panc KO mice fed the ZnA diet did not reach statistical significance in the data shown (Fig. 5A), increasing the number of animals in the assay revealed a small but statistically significant decrease in pancreatic zinc in the knockout mice relative to the controls when fed a ZnA diet (Fig. S1B). There were no other statistically significant changes in the abundance of the other elements examined in these organs and no overt phenotypic changes were noted in Zip5 Panc KO mice fed a ZnA diet. However, Zip5 Panc KO mice provided with zinc in the drinking water for 8 days failed to accumulate significantly more zinc in the pancreas, whereas control littermates accumulated pancreatic zinc under these experimental conditions (Fig. 5A). These results suggest that pancreatic ZIP5 might play a role in the retention of pancreatic zinc.
To further examine that possibility we studied the accumulation and retention of 67Zn, a stable isotope of zinc, in these mice. In initial experiments Zip5 Panc KO mice and Zip5 Intest KO mice were given an oral gavage containing 250 or 500 ppm 67Zn and the accumulation of 67Zn relative to 66Zn was monitored 24 hr later (Fig. S2). The natural ratio of 67Zn/66Zn is 0.146. In these initial experiments, the data showed a trend toward reduced accumulation of pancreatic zinc in the Zip5 Panc KO mice, but variability in the data was high and the results were not statistically significant. In subsequent experiments, mice were given an intraperitoneal (I.P.) injection of 67Zn (6.25 mg/kg body weight) and pancreatic accumulation of this stable isotope was monitored (Fig. 5B & C). The results showed that both control and Zip5 Panc KO mice accumulated similar amounts of pancreatic 67Zn at 2 and 4 hr after the injection (Fig. 5B). Thus, ZIP5 is not essential for the acute uptake of zinc in the pancreas under these conditions. In contrast, by 27 hr after the injection of 67Zn (Fig. 5B), the control mice had retained significantly more pancreatic 67Zn than had the Zip5 Panc KO mice (13.2 fold increase vs 7.07 fold increase over control in 67Zn, respectively). These results are consistent with the concept that ZIP5 might function in the pancreas to aid in the retention of zinc but not in the uptake of zinc.
The pancreas is sensitive to zinc-induced pancreatitis [32]. Therefore we investigated the effects of knocking out the pancreatic Zip5 gene on the development of pancreatitis in response to an I.P. injection of zinc. In an initial experiment, mice were injected with 6.25 mg zinc/kg body weight and 24 hr later the pancreas was harvested, fixed and sectioned. Sections were examined blindly by a pathologist (Table 1). The results revealed that the Zip5 Panc KO mice developed a more severe peri-pancreatic inflammation (8/9 of the mice shown in Table 1), as judged by the increased presence of immune cells around the pancreas, than did control mice, but these mice showed no overt signs of toxicity. In addition, remarkably large cytoplasmic vacuoles were noted in pancreatic acinar cells in the majority of the Zip5 Panc KO mice treated with zinc (6/9 of the mice shown in Table 1) but were very rare or absent in control mice treated with zinc and absent in untreated control and Zip5 Panc KO mice. These vacuoles were often much larger than nuclei. Acinar cell atrophy was present in 2 of nine Zip5 Panc KO mice but not in any of the control mice after this zinc treatment. These results suggest that pancreatic injury in response to injected zinc may have also contributed to the diminished retention of pancreatic zinc in the Zip5 Panc KO mice (Fig. 5B) and also reveal that pancreatic ZIP5 plays a role in protecting the pancreas against zinc toxicity.
Table 1. Zinc-induced pancreatic pathology in control and Zip5 Panc KO mice.
| Genotype | Large Cytoplasmic Vacuoles | Peripancreatic Inflammation |
| Zip5panc KO | No | severe |
| Yes | moderate | |
| Yes | severe | |
| Yes (prominent) | severe | |
| Yes (prominent) | severe | |
| Yes (prominent) | severe | |
| Yes | severe | |
| No (atrophy of acinar cells) | severe | |
| No (atrophy of acinar cells) | severe | |
| Control | No | mild to moderate |
| No | moderate | |
| No | moderate | |
| No | moderate |
Newly weaned mice were injected with tamoxifen to induce Zip5 recombination in pancreatic acinar cells by activation of Cre:ERT2 expressed from an ela-Cre-ERT2 transgene. Two weeks after the last tamoxifen injection, control (Control) littermates and pancreas-specific Zip5-knockout (Zip5 Panc KO) were given an I.P. injection of zinc sulfate (6.25 mg zinc/kg body weight) and 24 h later pancreata were harvested and paraffin sections were prepared, stained with hematoxylin and eosin and evaluated blind by a pathologist. Each row represents an individual animal.
To further probe this finding, mice were injected with a larger dosage of zinc (12.5 mg/kg) and the pancreas was examined by histology and IHC (Fig. 6 and Fig. 7; Table 2). Those mice showing signs of acute toxicity were euthanized within a few hours of the injection. Mice were killed at 24 to 48 hr after the zinc injection and showed no overt signs of toxicity. Control mice, Zip5Total KO mice and Zip5 Panc KO mice were compared (Table 2). Histological examination of the pancreas 48 hr after the zinc injection revealed more severe atrophy of acinar cells in both types of knockout mice (pancreas or total knockout) than in control mice (Table 2). The severity of acinar cell atrophy was greatest in the total knockout mice (7/8 mice shown in Table 2 had severe atrophy) followed by the Zip5 Panc KO mice (2/7 mice showed severe atrophy) whereas the majority of control mice exhibited mild atrophy of acinar cells (4/6 mice displayed mild atrophy) (see Fig. 6: panels A and B are control mice whereas panels C and D are Zip5 Panc KO mice-). Histological examination of the pancreas revealed an abundance of large cytoplasmic vacuoles in many Zip5 Panc KO mice (3/7) as noted above (Table 2; Figs. 6F and 7). Acinar cells with these large vacuoles were also abundant in the majority of Zip5Total KO mice (5/7 of the mice shown in Table 2) after zinc injection (Table 2) but were very rare in control mice. These large cytoplasmic vacuoles were generally noted only in acinar cells which lacked ZIP5 (Fig. 6F) although there were a few exceptions. Interestingly, these large vacuoles were present within 24 hr after the injection and contained α-amylase (Fig. 7) which is normally found in secretory vesicles. Formation of similar large vacuoles in acinar cells in other models of acute pancreatitis has been described recently [33]. The above results demonstrate that ZIP5 functions to protect the pancreas from zinc toxicity and suggest a function of this zinc transporter in the autophagy of secretory vesicles during acute pancreatitis.
Figure 6. Knockdown of the pancreas acinar cell Zip5 gene sensitizes mice to zinc-induced acute pancreatitis.
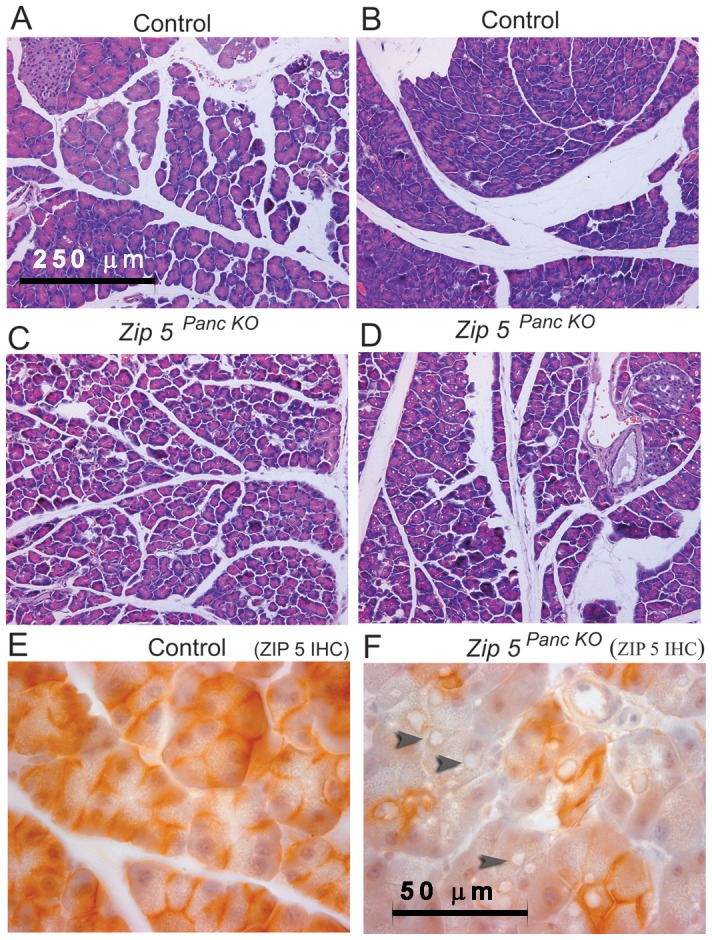
Two weeks after the last tamoxifen injection, control (Control) littermates and pancreas-specific Zip5-knockout (Zip5 Panc KO) (n = 5) were given an I.P. injection of zinc sulfate (12.5 mg zinc/kg body weight) and 48 hr later pancreata were harvested and paraffin sections were prepared and stained with hematoxylin and eosin (panels A–D) or stained for ZIP5 using immunohistochemistry (panels E and F). Panels A and B represent control mice whereas panels C and D represent ZIP5- knockout mice (Zip5 Panc KO). Dark brown deposits on the basolateral surfaces of acinar cells indicate positive staining. Black arrowheads in panel F demarcate large cytoplasmic vaculoles found in acinar cells of Zip5 Panc KO mice in response to zinc. Panels A–D are photographed at 200× magnification and panels E and F are photographed at 1000× magnification.
Figure 7. Detection of α-amylase in zinc-induced large cytoplasmic vaculoles in acinar cells of Zip5 Panc KOmice.
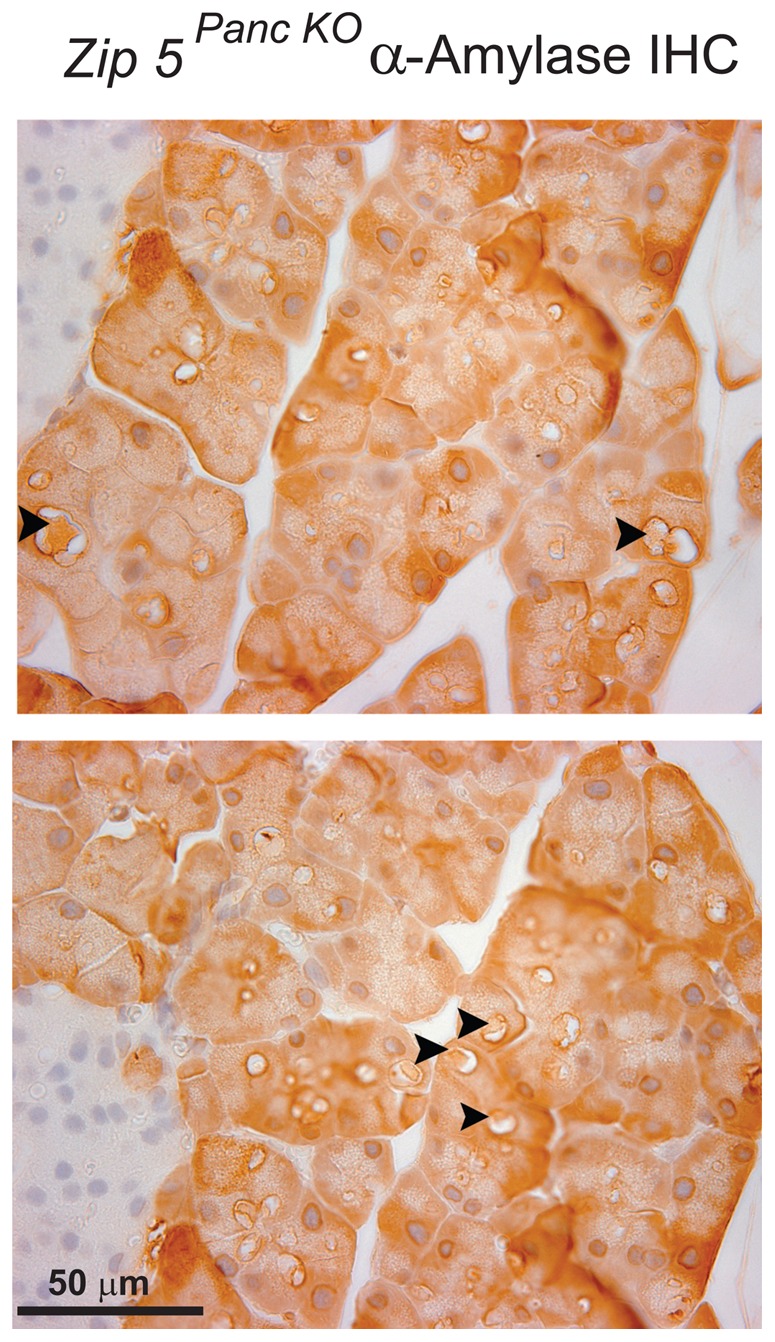
Two weeks after the last tamoxifen injection pancreas-specific Zip5-knockout (Zip5 Panc KO) mice were given an I.P. injection of zinc sulfate (12.5 mg zinc/kg body weight) and 24 hr later pancreata were harvested and paraffin sections were prepared and stained for α-amylase using immunohistochemistry. Sections from two different mice are shown. Dark brown deposits in acinar cells indicate positive staining. Black arrowheads demarcate large cytoplasmic vacuoles containing α-amylase. Sections were photographed at 630× magnification. I; indicates an Islet of Langerhans.
Table 2. Zinc-induced pancreatic pathology in Zip5 Panc KO mice, Zip5 Total KO and control mice.
| Genotype | Large Cytoplasmic Vacuoles | Acinar Cell Atrophy |
| Zip5 Panc KO | Yes | moderate > severe |
| No | moderate > severe | |
| Yes | severe > moderate | |
| No | moderate > severe | |
| Yes | severe > moderate | |
| Yes (rare) | moderate > severe | |
| No | moderate > severe | |
| Zip5 Total KO | No | severe > moderate |
| Yes | severe > moderate | |
| Yes | severe > moderate | |
| Yes | severe > moderate | |
| Yes | severe > moderate | |
| Yes | severe > moderate | |
| Yes (rare) | severe > moderate | |
| Yes | moderate > severe | |
| Control | No | mild > moderate |
| No | mild > moderate | |
| No | mild > moderate | |
| No | mild > moderate | |
| Yes (rare) | moderate > severe | |
| Yes (rare) | moderate > severe |
Zip5 recombination in pancreatic acinar cells was induced by activation of CRE:ERT2 expressed from an ela-Cre-ERT2 transgene and Zip5 recombination in all cells was created using a ubiquitously expressed CRE recombinase. Two to three weeks after weaning, control (Control), pancreas-specific Zip5-knockout (Zip5 Panc KO) and Zip5-total knockout mice (Zip5 Total KO) were given an I.P. injection of zinc sulfate (12.5 mg zinc/kg body weight) and 48 h later pancreata were harvested and paraffin sections were prepared, stained with hematoxylin and eosin and evaluated for the presence of zinc-induced vacuoles in acinar cells and for acinar cell atrophy. Sections were evaluated by the authors in this table. Each row represents an individual animal.
Discussion
The zinc transporter ZIP5 (Slc39a5) localizes to the baso-lateral surface when transfected into polarized cells as well as in situ in intestinal enterocytes and pancreatic acinar cells in mice [25], [34]. This protein is regulated by zinc and accumulates when zinc is replete but is internalized and degraded when zinc is deficient [26], [35]. These findings lead us to hypothesize that ZIP5 may function in the removal of zinc from the body. We tested this hypothesis by deleting the Zip5 gene specifically in intestinal enterocytes or pancreatic acinar cells or in every cell in mice and then examining the effects on zinc homeostasis.
Although several members of the Slc39a family are known to be important for proper development of the embryo or neonate, only Zip4 has been shown to be essential throughout the life of mice from the peri-implantation period to adulthood[16], [17]. In contrast, Zip5 is a non-essential gene since mice lacking this gene reproduce normally and no overt problems with development are noted. However, mice lacking ZIP5 accumulate more zinc in the liver and fail to accumulate excess zinc in the pancreas. This suggests that zinc homeostasis is disturbed in these knockout mice. The accumulation of hepatic zinc is consistent with the possibility that the excretion of zinc from the body is impaired in the absence of ZIP5.
Zinc homeostasis in mammals is not well understood at the molecular level. However, our recent studies proved that ZIP4 (Slc39a4) is essential for the uptake of sufficient dietary zinc by the intestine [17]. Uptake of zinc is enhanced when dietary zinc is deficient and repressed when dietary zinc is adequate. However, when zinc is replete the majority of zinc taken up from the diet is excreted [36]–[38] and isotopic tracer studies indicate that intestinal excretion of zinc into the gut lumen plays a critical role in maintaining zinc homeostasis [37], [39]. Furthermore, a significant amount of zinc is found in pancreatic secretions which empty into the gut lumen, and this organ is thought to also play a role in zinc homeostasis [40]–[42]. When zinc is in excess, pancreatic metallothioneins expression is augmented and the organ accumulates a labile pool of zinc that is available during periods of zinc deficiency [43].
Our studies reveal that ZIP5 plays an important role in the intestinal excretion of zinc. The loss-of-function of this gene in the intestinal enterocytes is accompanied by increased accumulation of zinc in the pancreas. This strongly suggests that an increased body burden of zinc occurs in the absence of enterocyte ZIP5. We also noted that enterocyte Zip4 mRNA is more abundant in these mice. Enterocyte Zip4 mRNA is normally induced in response to zinc deficiency [44]. Therefore, these findings are consistent with the concept that ZIP5 functions to move zinc from the blood into the enterocyte for subsequent excretion into the intestinal lumen. In the absence of ZIP5 intestinal enterocytes may be mildly zinc deficient, which stabilizes Zip4 mRNA, and the pancreas accumulates excess zinc that would normally be excreted via the intestine. However, it is clear that mice can adapt to the absence of intestinal ZIP5 under normal dietary conditions.
Our studies confirm that ZIP5 in pancreatic acinar cells also functions in zinc homeostasis. Deletion of this gene in these cells led to diminished steady state levels of pancreatic zinc similar to what was found in mice with total knockout of ZIP5. In addition, chronic excess dietary zinc failed to accumulate in pancreatic acinar cells which lack ZIP5. This effect did not appear to reflect a lack of acute uptake of zinc into acinar cells indicating that ZIP5 is not essential for zinc uptake by the pancreas. Instead our studies suggest that ZIP5 might be involved in the retention of zinc. A compartmental model of zinc kinetics in mice suggested that zinc loss from the pancreas could be explained in part by secretion into the plasma [45]. Therefore, ZIP5 is localized on acinar cells in a position to function in the reuptake of zinc from plasma. Many zinc transporters are expressed in the pancreas [38]. With regard to the expression of members of the Slc39a family, it was recently reported that ZIP14 localizes to the plasma membrane of acinar cells [46] suggesting that this transporter could play a dominant role in the acute uptake of zinc. In contrast, the expression of Zip1 and Zip3 genes is most active in ductal epithelia cells [47] which lead us to hypothesize that these transporters might also function in the retention of pancreatic zinc by scavenging zinc from the exocrine secretions. However, zinc released into the pancreatic ductal system which feeds into the intestine is thought to be largely associated with secretory proteins and metallothioneins [48]. It remains to be determined how zinc is rapidly taken up by acinar cells and the mechanism by which ZIP5 impacts zinc retention.
A novel and interesting finding in our studies is that acinar cell ZIP5 functions to attenuate zinc-induced pancreatitis. This is consistent with an important function of ZIP5 in pancreatic zinc homeostasis. However, the mechanism behind this protection from zinc toxicity is unclear. Our previous studies showed that metallothioneins, which are zinc-bound predominately, protect against caerulein-induced pancreatitis [49]. This suggests the possibility that the decreased zinc-content of acinar cells lacking ZIP5 predisposes them to a subsequent challenge with a toxic dosage of zinc. Previous studies have indicated that zinc deficiency exacerbates pancreatitis [50], [51]. Pancreatitis involves increased oxidative stress and inflammation [52] and in our studies inflammation was increased in mice lacking acinar cell ZIP5. Increased peri-pancreatic inflammation in these mice indicates that extra-pancreatic zinc toxicity was exacerbated. This could reflect an increased body burden of zinc, consistent with a role of pancreatic ZIP5 in the excretion of zinc. Surprisingly the acute uptake of zinc did not differ between control and Zip5Panc KO mice. Therefore, it is not simply increased cytoplasmic zinc content that dictates the toxicity of zinc in this model.
Another novel finding in our studies is that in the absence of ZIP5 many acinar cells become filled with large cytoplasmic vacuoles containing secretory protein when challenged with a toxic dosage of zinc. Impaired autophagy has been shown to mediate acinar cell vacuole formation in rodent models of acute pancreatitis [29], [33], [53]. Vaccaro [28] has termed this process “zymophagy” indicating the autophagy of zymogen granules. These vacuoles can be larger than the nucleus in some models of pancreatitis; for example in LAMP-2 deficient mice [54]and N-acetylglucosamine-1-phostransferase deficient mice (see Fig. 5 in [53]). Our results reveal that this process of zymophagy is exacerbated during zinc-induced pancreatitis in mice lacking acinar cell ZIP5. Autophagic, lysosomal and mitochondrial dysfunctions have been proposed to be keys to the pathogenesis of pancreatitis [29]. Thus, our studies quite unexpectedly suggest that ZIP5 may also have important functions in autophagy. ZIP5 undergoes internalization and degradation in response to zinc deficiency [26], [27] and can therefore be associated with components of the vesicular compartment. The intriguing possibility of a function of ZIP5 in autophagy warrants further investigation.
Materials and Methods
Animals
Experiments involving mice were performed in accordance with the guidelines from the National Institutes of Health for the care and use of animals and were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee (Protocol #2012-2057).
BAC Recombineering of a floxed Zip5 (Slc39a5) gene
We previously described the structure of the mouse Zip5 (Slc39a5) gene in detail [25]. The final structure of the floxed Zip5 (Zip5Fx) gene targeting vector and of the targeted chromosomal locus is shown in Fig. 1. BAC recombineering [55] using galK selection was employed to manipulate the Zip5 gene [56]. An 8910 bp region containing the Zip5 gene was captured from BAC ct7-575j15 and gap-repaired in a conditionally amplifiable [57] BAC-based vector (P[acman]-M-KO) [58] that allows for negative selection (HSV-TK) in ES cells and positive selection in bacteria (ampicillin). A LoxP site engineered to contain an EcoRV restriction site was inserted into a poorly conserved region in intron 4 and a PGK-neomycin cassette was inserted about 32 bp downstream of exon 12. A second LoxP site was inserted 40 bp downstream of PGK-neomycin. The targeting vector's structure was confirmed for the entire gene including the neomycin cassette by DNA sequencing and the ends of the captured regions were sequenced to verify that no rearrangements or mutations had occurred. In addition, the functionality of the LoxP sites was confirmed by transformation of the final targeting vector into bacteria (EL 350) that express Cre recombinase under control of an arabinose inducible promoter [55].
Targeted insertion of the Zip5Fx gene in embryonic stem cells
The Transgenic and Gene-Targeting Institutional Facility at the KU Medical Center generated targeted ES cell clones and performed blastocyst injections. The Zip5 Fx targeting vector was electroporated into E14 embryonic stem (ES) cells and colonies were screened using long range PCR with LA-Taq (TaKaRa Bio, Inc.) and primers outside the captured Zip5 locus paired with primers within the Zip5 gene itself (Fig. 1). Primers flanking the LoxP site in intron 4 were used for genotyping the targeted allele in mice (Fig. 1). Homologous recombination of the targeting vector into the endogenous locus resulted in the insertion of an EcoRV site in intron 4 which aided in identifying the targeted alleles. The sequences of oligonucleotides for integration screen and genotyping are shown in Table SI.
Generation of mice with Zip5Fx/Fx alleles
Chimeric mice were generated by microinjection of two independent Zip5Fx/Wt ES cell clones into d4 C57BL/6 blastocysts, followed by transfer to pseudopregnant CD-1 foster mothers. Resulting chimeric mice were crossed with C57BL/6 females (Harlan labs). Germline transmission was confirmed by PCR from tail DNA of agouti offspring (Fig. 1). Zip5Fx/Wt mice were crossed and Zip5Fx/Fx offspring were identified by PCR amplification of the LoxP:EcoRV insertion in intron 4.
Generation of mice for inducible recombination of Zip5Fx/Fx alleles in the intestinal epithelium or pancreatic acinar cells
Zip5Fx/Fx mice were crossed to create a working colony of mice homozygous for floxed Zip5 genes. These mice were then crossed with transgenic mice bearing a tamoxifen-dependent Cre recombinase (vil-Cre-ERT2) expressed under the control of the villin promoter to allow for inducible recombination of the Zip5 gene specifically in the intestinal epithelium [30] or they were crossed with transgenic mice bearing a tamoxifen-dependent Cre recombinase (Ela-Cre-ERT2) expressed under the control of the elastase promoter to allow for inducible recombination of the Zip5 gene specifically in pancreatic acinar cells [31]. Offspring were backcrossed to yield mice heterozygous for Cre-ERT2 and homozygous Zip5Fx/Fx. These mice were then crossed with Zip5Fx/Fx mice to yield 50% offspring with Zip5Fx/Fx: Cre-ERT2 alleles and 50% with Zip5Fx/Fx alleles. The latter provided age and genetically matched controls for our experiments and were labeled as control (Con) in all the figures.
Generation of Zip5 (Slc39a5) knockout mice
Zip5Fx/Fx mice were mated with transgenic mice (strain name: B6.FVB-TgN (EIIa-Cre) C5379 Lmgd from JAX.org) which express Cre ubiquitously driven by the EIIa promoter. The extent of the knockout was monitored by genotyping DNA from tail snips. Mice with apparently complete recombination of this gene in tail DNA were crossed and offspring were genotyped to confirm that the knockout was complete. A colony of homozygous Zip5- knockout mice was then established. Zip5Fx/Fx mice served as the control strain for these studies.
Tamoxifen induction of recombination
A tamoxifen stock solution was prepared and injected as described in detail previously [17], [59]. Recently weaned mice (5 to 8 days post-weaning) were injected I.P. with 100 µl (1 mg tamoxifen) daily for 5 consecutive days.
Experimental designs
Diets were purchased from Harlan Teklad (teklad.com) and zinc levels in the diets were as follows: zinc-deficient (ZnD), <1 ppm zinc; zinc-adequate (ZnA), 50 ppm zinc. Recently weaned mice were maintained on ZnA chow and the liver, pancreas and small intestine were taken 8 to 12 days after initiation of the tissue-specific knockout or tissues were harvested from total knockout mice of the same age. To monitor zinc accumulation mice were provided with drinking water containing 250 ppm ZnSO4 (zinc excess: ZnE) for 8 days before harvest. Where indicated, some mice were gavaged (100 µl) with a slurry containing ZnD feed in deionized water (1g feed in 2 ml water) to which was added 30 ppm zinc or to which was added 67Zn (250 ppm or 500 ppm). To look at the rapid accumulation and retention of zinc, where indicated mice were injected I.P. with 67Zn (>95% enriched, 67 zinc oxide) from Trace Sciences International Corporation (isotopetrace.com). 67Zn stock was dissolved in 3 drops of concentrated HCl and then diluted to 5 mg/ml in distilled water. This stock was diluted in water to a final concentration of 125 µg 67Zn/100 µl and injected I.P. at a dosage of 6.25 mg zinc/kg body weight. Pancreas was harvested from 2 to 24 hr after injection of 67Zn and subjected to elemental analysis. Eight to ten mice per group were analyzed in this experiment.
To induce acute pancreatitis, mice were injected I.P. with ZnSO4 dissolved in acidified water (100 µl) at a dosages of 6.25 mg zinc/kg body weight or 12.5 mg zinc/kg, as indicated in Results and Figure legends. The pancreas, liver and intestine were harvested (4 to 9 mice per group) at 24 or 48 hr after the zinc injection. Sections of pancreas were scored independently by a pathologist where indicated or by a member of the laboratory. Peri-pancreatic inflammation refers to inflammation around the pancreas as judged by the infiltration of immune cells.
Northern blot hybridization
Total RNA (3 - 6 µg), isolated using Trizol reagent (Invitrogen), was size-fractionated by agarose-formaldehyde gel electrophoresis, transferred and UV cross-linked to a Zeta Probe GT nylon membrane (BioRad). Membranes were hybridized, washed and exposed to film as described previously [17]. Riboprobes for mouse Zip4 and Zip5 were described previously [25]. Probes were used at 2×106 cpm/ml of hybridization solution.
Histology/IHC
The small intestine and pancreas (3 to 5 mice per group) were collected, washed with cold PBS, cut into small pieces and fixed in Bouin's fixative or 4% paraformaldehyde in PBS overnight at 4°C. Fixed tissues were embedded in paraffin and sections (1 µm) were prepared by Histo-Scientific Research Laboratories (HSRL) or the KU Medical Center histology core facility. Bouin's fixed sections were deparaffinized, rehydrated and stained with hematoxylin-eosin for examination of gross morphology by a pathologist.
Paraformaldehyde fixed sections from pancreas were processed using the Histostain Plus LAB SA Detection System (Invitrogen) according to the manufacturer's instructions. Sections were incubated with antisera against ZIP5 (1∶200), as described previously [25], [26] after antigen retrieval in citrate or sections were incubated with antisera against α-amylase (1∶300; Cell Signaling). Stained slides were counterstained briefly in Mayer's hematoxylin (Sigma) and photographed using a Leica DM 4000B microscope (Leica-microsystems) with Adobe Photoshop image capture software (Adobe).
Elemental and essential metal determination
Elemental profiling via ICP-MS was performed at the Donald Danforth Plant Science Center Ionomics Core Facility in St. Louis, Missouri [60] and at the Purdue University Ionomics Facility, in West Lafayette, Indiana [61]. Mouse tissues (n = 3 to 5) were dried at 95°C in a vacuum oven, digested in concentrated HNO3 and the following elements were measured: Na, Mg, P, K, S, Ca, Fe, Co, Cu, Zn, Mn, Mg, As, Se, and Mo as described previously [12], [61]. In addition, the stable isotopes of zinc, 67Zn and 66 Zn were measured when indicated. The natural ratio of these zinc isotopes is 0.146. Tissue concentrations were determined as µg g−1 dry weight (ppm) of each element.
Statistical analyses
Graphs were generated and statistical analyses performed using GraphPad Prism5 software (GraphPad Software). Statistical significance was determined using the Unpaired T-test (two-tailed) and values were considered different if P<0.05. Data are expressed as the mean ± S.E.M. *indicates P<0.05; *** indicates P<0.001; **** indicates P<0.0001
Supporting Information
Liver iron and pancreatic zinc are reduced in Zip5 Panc KO mice. Control (Con) littermates and pancreas-specific Zip5-knockout (Zip5 Panc KO) mice were killed 8 days after the last tamoxifen injection. Intestine, pancreas and liver were harvested from mice fed normal chow (ZnA) during those 8 days and elements were quantified using ICP-MS and are expressed as ppm/dry weight of tissue. (A) Liver iron (n = 4 –5 mice per group). (B) Pancreatic zinc (n = 8 –10 mice per group) is expressed as the ratio of 66Zn to sulfur (S). This was done to normalize the values for zinc and reduce variability. Two separate groups of mice were analyzed thus there are two sets of data on this bar graph. 66Zn represents ∼28% of total zinc. There were no apparent changes in any of the other elements analyzed in these tissues.
(TIF)
Zip5 Panc KO mice appear to display reduced retention of zinc. (A) Intestine-specific Zip5-knockout (Zip5 Intest KO) mice and pancreas-specific Zip5-knockout (Zip5 Panc KO) mice were given an oral gavage containing 250 ppm or 500 ppm 67Zn and the pancreas was harvested 24 hr after the gavage (n = 4 mice per group. The ratio of 67Zn/66Zn was measured by ICP-MS. The natural ratio of these stable isotopes is 0.146. (B) Mice were given an oral gavage containing 500 ppm 67Zn and the pancreas and intestine were harvested 24 hr after the gavage. None of the values shown reached statistical significance but the data suggest a trend toward reduced retention of pancreatic zinc in the Zip5 Panc KO mice.
(TIF)
List of oligonucleotides used for integration screening in embryonic stem cells and genotyping of Zip5 alleles in mice.
(DOCX)
Acknowledgments
We thank the members of the Transgenic and Gene-Targeting Institutional Facility at the KU Medical Center for the generation of targeted ES cell clones and blastocyst injection and the members of the Biotechnology Support Facility for DNA sequencing. We also thank Ms. Jing Huang, histology manager at the Mental Retardation Research Center, KU Medical Center, for preparing tissue sections and Dr. Ossama Tawfik, Department of Pathology, KU Medical Center for evaluating pancreatitis. We owe a special debt of gratitude to Brett Lahner and David E. Salt at the Purdue University, Ionomics Core, Department of Horticulture,West Lafayette, Indiana and to Greg Ziegler and Ivan Baxter at the Danforth Ionomics Core Facility in St. Louis for ICP-MS analyses. We thank Dr. S. Robine at the Cellular Morphogenesis and Signalisation, Institut Curie, Paris, and Dr. L. Li at the Stower's Institute for Medical Research, Kansas City, Missouri, for providing vil-Cre-ERT2 transgenic mice and Dr. Craig Logsdon, M.D. Anderson Cancer Center, Houston, Texas for providing Ela-Cre-ERT2 mice. Mice with floxed Zip5 genes are available through the Mutant Mouse Regional Resource Center, University of Missouri, Columbia, Missouri.
Funding Statement
This work was supported by NIH grant R01 DK 63975. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Maret W (2013) Zinc and the zinc proteome. Met Ions Life Sci 12: 479–501. [DOI] [PubMed] [Google Scholar]
- 2. Maret W (2011) Metals on the move: zinc ions in cellular regulation and in the coordination dynamics of zinc proteins. Biometals 24: 411–418. [DOI] [PubMed] [Google Scholar]
- 3. Kambe T (2012) Molecular architecture and function of ZnT transporters. Curr Top Membr 69: 199–220. [DOI] [PubMed] [Google Scholar]
- 4. Fukada T, Yamasaki S, Nishida K, Murakami M, Hirano T (2011) Zinc homeostasis and signaling in health and diseases: Zinc signaling. J Biol Inorg Chem 16: 1123–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jeong J, Eide DJ (2013) The SLC39 family of zinc transporters. Mol Aspects Med 34: 612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang L, Tepaamorndech S (2013) The SLC30 family of zinc transporters - A review of current understanding of their biological and pathophysiological roles. Mol Aspects Med 34: 548–560. [DOI] [PubMed] [Google Scholar]
- 7. Dalton TP, Fu K, Palmiter RD, Andrews GK (1996) Transgenic mice that over-express metallothionein-I resist dietary zinc deficiency. J Nutr 126: 825–833. [DOI] [PubMed] [Google Scholar]
- 8. Andrews GK, Geiser J (1999) Expression of metallothionein-I and -II genes provides a reproductive advantage during maternal dietary zinc deficiency. J Nutr 129: 1643–1648. [DOI] [PubMed] [Google Scholar]
- 9. Dempski RE (2012) The cation selectivity of the ZIP transporters. Curr Top Membr 69: 221–245. [DOI] [PubMed] [Google Scholar]
- 10. Wang B, Schneider SN, Dragin N, Girijashanker K, Dalton TP et al (2007) Enhanced cadmium-induced testicular necrosis and renal proximal tubule damage caused by gene-dose increase in a Slc39a8-transgenic mouse line. Am J Physiol Cell Physiol 292: C1523–C1535. [DOI] [PubMed] [Google Scholar]
- 11. Pinilla-Tenas JJ, Sparkman BK, Shawki A, Illing AC, Mitchell CJ et al (2011) Zip14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. Am J Physiol Cell Physiol 301: C862–C871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kambe T, Geiser J, Lahner B, Salt DE, Andrews GK (2008) Slc39a1 to 3 (subfamily II) Zip genes in mice have unique cell-specific functions during adaptation to zinc deficiency. Am J Physiol Regul Integr Comp Physiol 294: R1474–R1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quadri M, Federico A, Zhao T, Breedveld GJ, Battisti C et al (2012) Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am J Hum Genet 90: 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seok SH, Cho WS, Park JS, Na Y, Jang A et al (2013) Rat pancreatitis produced by 13-week administration of zinc oxide nanoparticles: biopersistence of nanoparticles and possible solutions. J Appl Toxicol 33: 1089–1096. [DOI] [PubMed] [Google Scholar]
- 15. Kelleher SL, Lopez V, Lonnerdal B, Dufner-Beattie J, Andrews GK (2009) Zip3 (Slc39a3) functions in zinc reuptake from the alveolar lumen in lactating mammary gland. Am J Physiol Regul Integr Comp Physiol 297: R194–R201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dufner-Beattie J, Weaver BP, Geiser J, Bilgen M, Larson M et al (2007) The mouse acrodermatitis gene Slc39a4 (ZIP4) is essential for development and heterozygosity causes hypersensitivity to zinc deficiency. Hum Mol Genet 16: 1391–1399. [DOI] [PubMed] [Google Scholar]
- 17. Geiser J, Venken KJ, De Lisle RC, Andrews GK (2012) A Mouse Model of Acrodermatitis Enteropathica: Loss of Intestine Zinc Transporter ZIP4 (Slc39a4) Disrupts the Stem Cell Niche and Intestine Integrity. PLoS Genet 8: e1002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kury S, Dreno B, Bezieau S, Giraudet S, Kharfi M et al (2002) Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet 31: 239–240. [DOI] [PubMed] [Google Scholar]
- 19. Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J (2002) A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am J Hum Genet 71: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galvez-Peralta M, He L, Jorge-Nebert LF, Wang B, Miller ML et al (2012) ZIP8 Zinc Transporter: Indispensable Role for Both Multiple-Organ Organogenesis and Hematopoiesis In Utero. PLoS One 7: e36055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dalton TP, He L, Wang B, Miller ML, Jin L et al (2005) Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proc Natl Acad Sci U S A 102: 3401–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fukada T, Civic N, Furuichi T, Shimoda S, Mishima K et al (2008) The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-beta signaling pathways. PLoS ONE 3: e3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hojyo S, Fukada T, Shimoda S, Ohashi W, Bin BH et al (2011) The zinc transporter SLC39A14/ZIP14 controls G-protein coupled receptor-mediated signaling required for systemic growth. PLoS ONE 6: e18059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aydemir TB, Sitren HS, Cousins RJ (2012) The zinc transporter Zip14 influences c-Met phosphorylation and hepatocyte proliferation during liver regeneration in mice. Gastroenterology 142: 1536–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dufner-Beattie J, Kuo YM, Gitschier J, Andrews GK (2004) The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc-regulation of the zinc transporters ZIP4 and ZIP5. J Biol Chem 279: 49082–49090. [DOI] [PubMed] [Google Scholar]
- 26. Weaver BP, Dufner-Beattie J, Kambe T, Andrews GK (2007) Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5). Biol Chem 388: 1301–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weaver BP, Andrews GK (2012) Regulation of zinc-responsive Slc39a5 (Zip5) translation is mediated by conserved elements in the 3′-untranslated region. Biometals 25: 319–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vaccaro MI (2012) Zymophagy: selective autophagy of secretory granules. Int J Cell Biol 2012: 396705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gukovsky I, Pandol SJ, Mareninova OA, Shalbueva N, Jia W et al (2012) Impaired autophagy and organellar dysfunction in pancreatitis. J Gastroenterol Hepatol 27 Suppl 227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. el Marjou F, Janssen KP, Chang BH, Li M, Hindie V et al (2004) Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39: 186–193. [DOI] [PubMed] [Google Scholar]
- 31. Ji B, Song J, Tsou L, Bi Y, Gaiser S et al (2008) Robust acinar cell transgene expression of CreErT via BAC recombineering. Genesis 46: 390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Onosaka S, Tetsuchikawahara N, Min KS (2002) Paradigm shift in zinc: metal pathology. Tohoku J Exp Med 196: 1–7. [DOI] [PubMed] [Google Scholar]
- 33. Mareninova OA, Hermann K, French SW, O'Konski MS, Pandol SJ et al (2009) Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Invest 119: 3340–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang F, Kim BE, Petris MJ, Eide DJ (2004) The mammalian ZIP5 protein is a zinc transporter that localizes to the basolateral surface of polarized cells. J Biol Chem 279: 51433–51441. [DOI] [PubMed] [Google Scholar]
- 35. Kelly P, Feakins R, Domizio P, Murphy J, Bevins C et al (2004) Paneth cell granule depletion in the human small intestine under infective and nutritional stress. Clin Exp Immunol 135: 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hambidge M, Krebs NF (2001) Interrelationships of key variables of human zinc homeostasis: Relevance to dietary zinc requirements. Annu Rev Nutr 21: 429–452. [DOI] [PubMed] [Google Scholar]
- 37. Krebs NE, Hambidge KM (2001) Zinc metabolism and homeostasis: the application of tracer techniques to human zinc physiology. Biometals 14: 397–412. [DOI] [PubMed] [Google Scholar]
- 38. Kelleher SL, McCormick NH, Velasquez V, Lopez V (2011) Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland. Adv Nutr 2: 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Methfessel AH, Spencer H (1973) Zinc metabolism in the rat. II. Secretion of zinc into intestine. J Appl Physiol 34: 63–67. [DOI] [PubMed] [Google Scholar]
- 40. Van Wouwe JP, Uijlenbroek JJM (1994) The role of the pancreas in the regulation of zinc status. Biol Trace Elem Res 42: 143–150. [DOI] [PubMed] [Google Scholar]
- 41. Oberleas D (1996) Mechanism of zinc homeostasis. J Inorg Biochem 62: 231–241. [DOI] [PubMed] [Google Scholar]
- 42. Ishihara N, Yoshida A, Koizumi M (1987) Metal concentrations in human pancreatic juice. Arch Environ Health 42: 356–360. [DOI] [PubMed] [Google Scholar]
- 43. Lee DK, Geiser J, Dufner-Beattie J, Andrews GK (2003) Pancreatic metallothionein-l may play a role in zinc homeostasis during maternal dietary zinc deficiency in mice. J Nutr 133: 45–50. [DOI] [PubMed] [Google Scholar]
- 44. Dufner-Beattie J, Wang F, Kuo YM, Gitschier J, Eide D et al (2003) The Acrodermatitis Enteropathica Gene ZIP4 Encodes a Tissue-specific, Zinc-regulated Zinc Transporter in Mice. J Biol Chem 278: 33474–33481. [DOI] [PubMed] [Google Scholar]
- 45. Wastney ME, House WA (2008) Development of a compartmental model of zinc kinetics in mice. J Nutr 138: 2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nam H, Wang CY, Zhang L, Zhang W, Hojyo S et al (2013) ZIP14 and DMT1 in the liver, pancreas, and heart are differentially regulated by iron deficiency and overload: implications for tissue iron uptake in iron-related disorders. Haematologica 98: 1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dufner-Beattie J, Huang ZL, Geiser J, Xu W, Andrews GK (2006) Mouse ZIP1 and ZIP3 genes together are essential for adaptation to dietary zinc deficiency during pregnancy. Genesis 44: 239–251. [DOI] [PubMed] [Google Scholar]
- 48. De Lisle RC, Sarras MP Jr, Hidalgo J, Andrews GK (1996) Metallothionein is a component of exocrine pancreas secretion: Implications for zinc homeostasis. Am J Physiol Cell Physiol 271: C1103–C1110. [DOI] [PubMed] [Google Scholar]
- 49. Fu K, Tomita T, Sarras MP Jr, De Lisle RC, Andrews GK (1998) Metallothionein protects against cerulein-induced acute pancreatitis: Analysis using transgenic mice. Pancreas 17: 238–246. [DOI] [PubMed] [Google Scholar]
- 50. Girish BN, Rajesh G, Vaidyanathan K, Balakrishnan V (2011) Assessment of oxidative status in chronic pancreatitis and its relation with zinc status. Indian J Gastroenterol 30: 84–88. [DOI] [PubMed] [Google Scholar]
- 51. Girish BN, Vaidyanathan K, Rajesh G, Balakrishnan V (2012) Effects of micronutrient status on oxidative stress and exocrine pancreatic function in patients with chronic pancreatitis. Indian J Biochem Biophys 49: 386–391. [PubMed] [Google Scholar]
- 52. Fu K, Sarras MP Jr, De Lisle RC, Andrews GK (1997) Expression of oxidative stress-responsive genes and cytokine genes during caerulein-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 273: G696–G705. [DOI] [PubMed] [Google Scholar]
- 53. Gukovskaya AS, Gukovsky I (2012) Autophagy and pancreatitis. Am J Physiol Gastrointest Liver Physiol 303: G993–G1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D et al (2000) Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature 406: 902–906. [DOI] [PubMed] [Google Scholar]
- 55. Copeland NG, Jenkins NA, Court DL (2001) Recombineering: a powerful new tool for mouse functional genomics. Nat Rev Genet 2: 769–779. [DOI] [PubMed] [Google Scholar]
- 56. Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG (2005) Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res 33: e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wild J, Hradecna Z, Szybalski W (2002) Conditionally amplifiable BACs: switching from single-copy to high-copy vectors and genomic clones. Genome Res 12: 1434–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Venken KJ, He Y, Hoskins RA, Bellen HJ (2006) P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science 314: 1747–1751. [DOI] [PubMed] [Google Scholar]
- 59. Metzger D, Chambon P (2001) Site- and time-specific gene targeting in the mouse. Methods 24: 71–80. [DOI] [PubMed] [Google Scholar]
- 60. Baxter I, Ouzzani M, Orcun S, Kennedy B, Jandhyala SS et al (2007) Purdue ionomics information management system. An integrated functional genomics platform. Plant Physiol 143: 600–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Peters JL, Dufner-Beattie J, Xu W, Geiser J, Lahner B et al (2007) Targeting of the mouse Slc39a2 (Zip2) gene reveals highly cell-specific patterns of expression, and unique functions in zinc, iron and calcium homeostasis. Genesis 45: 339–352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Liver iron and pancreatic zinc are reduced in Zip5 Panc KO mice. Control (Con) littermates and pancreas-specific Zip5-knockout (Zip5 Panc KO) mice were killed 8 days after the last tamoxifen injection. Intestine, pancreas and liver were harvested from mice fed normal chow (ZnA) during those 8 days and elements were quantified using ICP-MS and are expressed as ppm/dry weight of tissue. (A) Liver iron (n = 4 –5 mice per group). (B) Pancreatic zinc (n = 8 –10 mice per group) is expressed as the ratio of 66Zn to sulfur (S). This was done to normalize the values for zinc and reduce variability. Two separate groups of mice were analyzed thus there are two sets of data on this bar graph. 66Zn represents ∼28% of total zinc. There were no apparent changes in any of the other elements analyzed in these tissues.
(TIF)
Zip5 Panc KO mice appear to display reduced retention of zinc. (A) Intestine-specific Zip5-knockout (Zip5 Intest KO) mice and pancreas-specific Zip5-knockout (Zip5 Panc KO) mice were given an oral gavage containing 250 ppm or 500 ppm 67Zn and the pancreas was harvested 24 hr after the gavage (n = 4 mice per group. The ratio of 67Zn/66Zn was measured by ICP-MS. The natural ratio of these stable isotopes is 0.146. (B) Mice were given an oral gavage containing 500 ppm 67Zn and the pancreas and intestine were harvested 24 hr after the gavage. None of the values shown reached statistical significance but the data suggest a trend toward reduced retention of pancreatic zinc in the Zip5 Panc KO mice.
(TIF)
List of oligonucleotides used for integration screening in embryonic stem cells and genotyping of Zip5 alleles in mice.
(DOCX)