Abstract
The nucleotide sequence of nifD, the structural gene for the α subunit of dinitrogenase from Anabaena 7120, has been determined. The coding sequence contains 1,440 nucleotides, which predict an amino acid sequence of 480 residues and Mr of 54,283. The predicted sequence contains eight cysteines, of which five are conserved with respect to adjoining sequences and position relative to the α subunits of dinitrogenase from Azotobacter, Clostridium, and Klebsiella. Because there are also five conserved cysteines in the β subunit of Anabaena dinitrogenase [Mazur, B. J. & Chiu, C.-F. (1982) Proc. Natl. Acad. Sci. USA 79, 6782-6786], the number of cysteine residues participating as ligands to FeS clusters is likely to be 20 per α2β2 tetramer. This number is sufficient to accommodate the known four Fe4S4 clusters, leaving at least four cysteines to be shared among the two FeMo cofactors and the more poorly characterized two-iron center. Although the α- and β-subunit gene sequences are not recognizably homologous, their secondary structures, predicted from the sequences, indicate similar domains around three of the conserved cysteine residues.
Keywords: nitrogen fixation, blue-green algae, protein sequence conservation
Full text
PDF



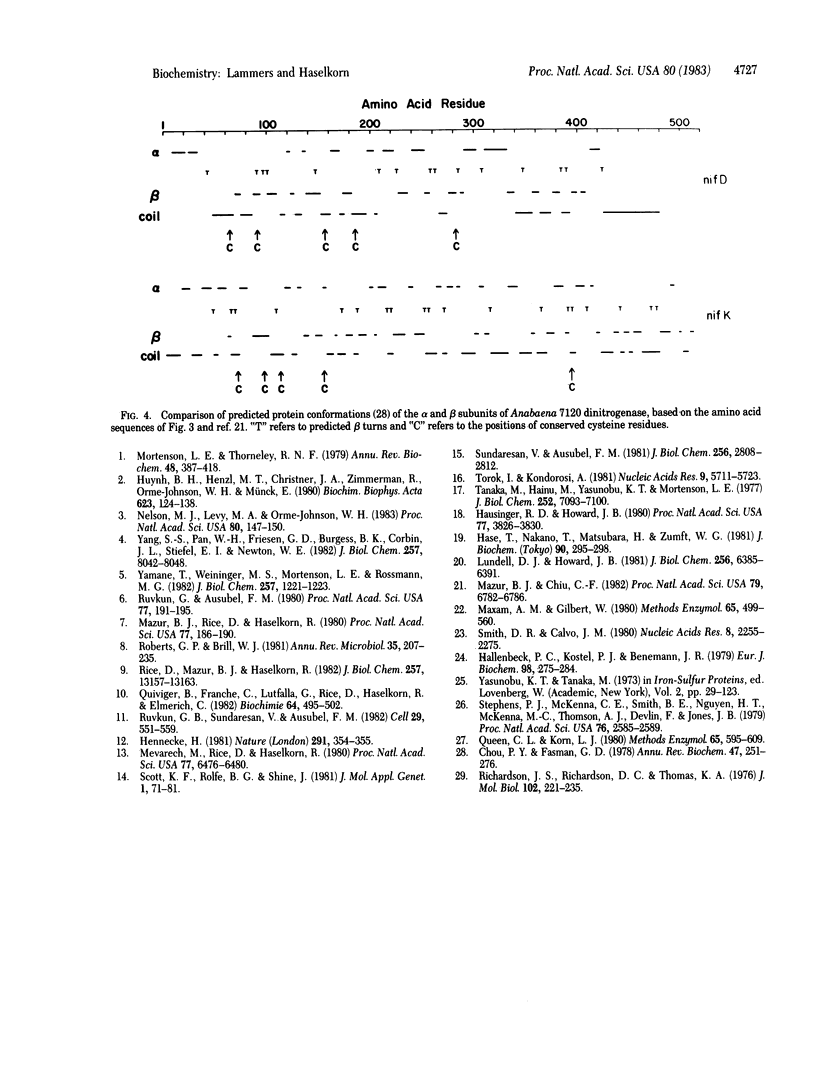
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Hallenbeck P. C., Kostel P. J., Benemann Purification and properties of nitrogenase from the cyanobacterium, Anabaena cylindrica. Eur J Biochem. 1979 Jul;98(1):275–284. doi: 10.1111/j.1432-1033.1979.tb13186.x. [DOI] [PubMed] [Google Scholar]
- Hase T., Nakano T., Matsubara H., Zumft W. G. Correspondence of the larger subunit of the MoFe-protein in clostridial nitrogenase to the nif D gene products of other N2-fixing organisms. J Biochem. 1981 Jul;90(1):295–298. doi: 10.1093/oxfordjournals.jbchem.a133466. [DOI] [PubMed] [Google Scholar]
- Hausinger R. P., Howard J. B. Comparison of the iron proteins from the nitrogen fixation complexes of Azotobacter vinelandii, Clostridium pasteurianum, and Klebsiella pneumoniae. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3826–3830. doi: 10.1073/pnas.77.7.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh B. H., Henzl M. T., Christner J. A., Zimmermann R., Orme-Johnson W. H., Münck E. Nitrogenase XII. Mössbauer studies of the MoFe protein from Clostridium pasteurianum W5. Biochim Biophys Acta. 1980 May 29;623(1):124–138. doi: 10.1016/0005-2795(80)90015-x. [DOI] [PubMed] [Google Scholar]
- Lundell D. J., Howard J. B. Isolation and sequences of the cysteinyl tryptic peptides from the MoFe-protein of Azotobacter vinelandii nitrogenase. J Biol Chem. 1981 Jun 25;256(12):6385–6391. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F. Sequence of the gene coding for the beta-subunit of dinitrogenase from the blue-green alga Anabaena. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6782–6786. doi: 10.1073/pnas.79.22.6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur B. J., Rice D., Haselkorn R. Identification of blue-green algal nitrogen fixation genes by using heterologous DNA hybridization probes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):186–190. doi: 10.1073/pnas.77.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevarech M., Rice D., Haselkorn R. Nucleotide sequence of a cyanobacterial nifH gene coding for nitrogenase reductase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6476–6480. doi: 10.1073/pnas.77.11.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortenson L. E., Thorneley R. N. Structure and function of nitrogenase. Annu Rev Biochem. 1979;48:387–418. doi: 10.1146/annurev.bi.48.070179.002131. [DOI] [PubMed] [Google Scholar]
- Nelson M. J., Levy M. A., Orme-Johnson W. H. Metal and sulfur composition of iron-molybdenum cofactor of nitrogenase. Proc Natl Acad Sci U S A. 1983 Jan;80(1):147–150. doi: 10.1073/pnas.80.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C. L., Korn L. J. Computer analysis of nucleic acids and proteins. Methods Enzymol. 1980;65(1):595–609. doi: 10.1016/s0076-6879(80)65062-9. [DOI] [PubMed] [Google Scholar]
- Quiviger B., Franche C., Lutfalla G., Rice D., Haselkorn R., Elmerich C. Cloning of a nitrogen fixation (nif) gene cluster of Azospirillum brasilense. Biochimie. 1982 Jul;64(7):495–502. doi: 10.1016/s0300-9084(82)80165-x. [DOI] [PubMed] [Google Scholar]
- Rice D., Mazur B. J., Haselkorn R. Isolation and physical mapping of nitrogen fixation genes from the cyanobacterium Anabaena 7120. J Biol Chem. 1982 Nov 10;257(21):13157–13163. [PubMed] [Google Scholar]
- Richardson J. S., Richardson D. C., Thomas K. A., Silverton E. W., Davies D. R. Similarity of three-dimensional structure between the immunoglobulin domain and the copper, zinc superoxide dismutase subunit. J Mol Biol. 1976 Apr 5;102(2):221–235. doi: 10.1016/s0022-2836(76)80050-2. [DOI] [PubMed] [Google Scholar]
- Roberts G. P., Brill W. J. Genetics and regulation of nitrogen fixation. Annu Rev Microbiol. 1981;35:207–235. doi: 10.1146/annurev.mi.35.100181.001231. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. Interspecies homology of nitrogenase genes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):191–195. doi: 10.1073/pnas.77.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Sundaresan V., Ausubel F. M. Directed transposon Tn5 mutagenesis and complementation analysis of Rhizobium meliloti symbiotic nitrogen fixation genes. Cell. 1982 Jun;29(2):551–559. doi: 10.1016/0092-8674(82)90171-4. [DOI] [PubMed] [Google Scholar]
- Scott K. F., Rolfe B. G., Shine J. Biological nitrogen fixation: primary structure of the Klebsiella pneumoniae nifH and nifD genes. J Mol Appl Genet. 1981;1(1):71–81. [PubMed] [Google Scholar]
- Smith D. R., Calvo J. M. Nucleotide sequence of the E coli gene coding for dihydrofolate reductase. Nucleic Acids Res. 1980 May 24;8(10):2255–2274. doi: 10.1093/nar/8.10.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens P. J., McKenna C. E., Smith B. E., Nguyen H. T., McKenna M. C., Thomson A. J., Devlin F., Jones J. B. Circular dichroism and magnetic circular dichroism of nitrogenase proteins. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2585–2589. doi: 10.1073/pnas.76.6.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan V., Ausubel F. M. Nucleotide sequence of the gene coding for the nitrogenase iron protein from Klebsiella pneumoniae. J Biol Chem. 1981 Mar 25;256(6):2808–2812. [PubMed] [Google Scholar]
- Tanaka M., Haniu M., Yasunobu K. T. The amino acid sequence of Clostridium pasteurianum iron protein, a component of nitrogenase. III. The NH2-terminal and COOH-terminal sequences, tryptic peptides of large cyanogen bromide peptides, and the complete sequence. J Biol Chem. 1977 Oct 25;252(20):7093–7100. [PubMed] [Google Scholar]
- Török I., Kondorosi A. Nucleotide sequence of the R.meliloti nitrogenase reductase (nifH) gene. Nucleic Acids Res. 1981 Nov 11;9(21):5711–5723. doi: 10.1093/nar/9.21.5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane T., Weininger M. S., Mortenson L. E., Rossmann M. G. Molecular symmetry of the MoFe protein of nitrogenase. Structural homology/nitrogen fixation/x-ray crystallography. J Biol Chem. 1982 Feb 10;257(3):1221–1223. [PubMed] [Google Scholar]
- Yang S. S., Pan W. H., Friesen G. D., Burgess B. K., Corbin J. L., Stiefel E. I., Newton W. E. Iron-molybdenum cofactor from nitrogenase. Modified extraction methods as probes for composition. J Biol Chem. 1982 Jul 25;257(14):8042–8048. [PubMed] [Google Scholar]



