Abstract
We sought cognitive event-related potential (ERP) biomarkers of “Preclinical Alzheimer’s disease” (Pre-AD) using an incidental verbal learning paradigm with high sensitivity to prodromal AD. Seven elderly persons, with normal cognition at the time of ERP recordings, but who showed subsequent cognitive decline or AD pathology at autopsy (n=5, mean Braak stage=2.8), were compared to 12 “robust” normal elderly (RNE) who remained cognitively normal (Mfollow-up=9.0 years). EEG was recorded during a word repetition paradigm (semantically congruous (50%) and incongruous target words repeat ~10–140 seconds later). The RNE P600 congruous word repetition ERP effects (New minus Old congruous words) were significantly larger than in Pre-AD (mean amplitudes = 3.28 vs. 0.10 µV, p= 0.04). High group discrimination (84%) was achieved (by a P600 amplitude cutoff of ~1.5 µV). Abnormal P600 word repetition effects in cognitively normal elderly persons may be an important sign of synaptic dysfunction and Preclinical AD.
Search Terms: Mild Cognitive Impairment, EEG, memory, Alzheimer’s disease, Event-related potentials
INTRODUCTION
There is mounting evidence that AD may be primarily a synaptic disorder (Klein, 2006; Selkoe, 2002). Recently proposed research criteria for ‘Preclinical’ AD (Pre-AD) (Sperling, Aisen, Beckett, et al., 2011) divides this entity into 3 stages based on the presence/absence of synaptic dysfunction, cognitive symptoms, and subtle cognitive decline. Event-related brain potentials (ERPs), comprised primarily of summed excitatory and inhibitory postsynaptic potentials (Nunez & Srinivasan, 2006), may provide non-invasive measures of synaptic dysfunction underlying very early cognitive alternations in AD.
Two ERP components that are of special interest for investigation of AD are a late positive component (LPC), sometimes termed the “P600,”and the N400, as elicited in a word repetition paradigm (Olichney, Van Petten, Paller, et al., 2000). The P600 component can index both memory encoding and retrieval processes (Olichney et al., 2000; Paller & Kutas, 1992; Van Petten, Kutas, Kluender, et al., 1991) and the P600 repetition effect has been consistently found to be correlated with episodic verbal memory abilities and declarative memory of the experimental stimuli (Olichney et al. 2000, 2002, 2008). FMRI studies (Olichney, Taylor, Chan et al., 2010; Olichney, Taylor, Hillert et al., 2010) using a similar word repetition paradigm have found left hemisphere dominant New > Old effects in medial temporal lobe (MTL), cingulate gyrus, fusiform gyrus, inferior parietal lobe (IPL), superior parietal lobe, middle and inferior frontal gyri (IFG). Those putative P600 generators fit well with the medial temporal, prefrontal and parietal networks associated with both encoding and retrieval of episodic memory (see Spaniol, Davidson, Kim et al., 2009 for a review). Dale and colleagues (2009) combined fMRI and MEG to study word repetition without a recognition task and found significant New > Old activation in frontal and temporal regions around 540 ms post-stimulus onset. Intracranial studies have identified putative P600 generators in the medial temporal lobe (parahippocampal gyrus, hippocampus), paralimbic (e.g. temporal pole, cingulate, rhinal cortex) and multimodal association cortex (Guillem, Rougier, Claverie, 1999; Halgren, Baudena, Heit, et al., 1994). Therefore, the P600 elicited by word repetition and the well-known episodic LPC both reflect episodic memory, but likely index similar yet not identical cognitive processes. The episodic LPC has been found to have parietal generators (see Rugg & Curran, 2007 for a review).
The N400 is a scalp-negative component which peaks ~400 ms after verbal stimulus onset; its amplitude varies with semantic processing load (Kutas & Federmeier, 2011). The main N400 generators are believed to be in the left > right fusiform neocortex (McCarthy, Nobre, Bentin, et al., 1995). The N400 has been linked to implicit memory and semantic priming, with a right posterior maximal distribution, which differs from the mid-frontal N400 (FN400), the electrophysiological signature of familiarity-related memory processes (Rugg & Curran, 2007).
Using the ERP word repetition paradigm, we have found that the P600 repetition effect is reduced or absent in mild AD (Olichney, Iragui, Salmon, et al., 2006). Furthermore, in patients with mild cognitive impairment (MCI), reductions in either the P600 or N400 word repetition effect are associated with greater likelihood of subsequent transition to AD dementia (Olichney, Taylor, Gatherwright, et al., 2008).
We thus sought to test if abnormal P600 (or N400) repetition effects can be detected in cognitively normal elderly who later showed evidence of very early AD. We hypothesized that decrements in the P600 or N400 word repetition effect amplitude would be found in elderly who harbored Pre-AD pathology as evidenced by later cognitive decline (to MCI or AD dementia), and/or the presence of AD pathology at autopsy. To test this hypothesis, we reviewed the ERP results and clinical outcomes in cognitively normal elderly Alzheimer’s Disease Research Center (ADRC) participants on whom we have extensive longitudinal neuropsychological data, and in some cases autopsy confirmation of AD pathology. We retrospectively defined a group of 7 persons with Pre-AD and compared their ERPs to a group of 12 robust normal elderly (RNE) persons, who remained cognitively normal and stable for a minimum of 3 years and the duration of their clinical follow-up. If the P600 is a sensitive biomarker of Pre-AD, these individuals, who test within normal limits on neuropsychological tests and can perform the semantic categorization ERP task well, will nonetheless show reduced P600 repetition effects.
METHODS
Participants
Participants served as volunteers after providing informed consent according to University of California, San Diego (UCSD) human research protection program guidelines. They were recruited from the Shiley-Marcos ADRC at UCSD, where they received annual evaluations which included the CDR (Morris, 1993). Rosen modified Hachinski Ischemic Scale (Rosen, Terry, Fuld, et al., 1980), neurological examination, laboratory tests, and extensive neuropsychological testing, including global assessments [MMSE, Dementia Rating Scale (DRS)] and tests of memory [e.g. WMS-R Logical Memory, CERAD Word List, Heaton Visual Reproduction], language [Boston Naming Test, Verbal Fluency Test, ANART], executive function [Wisconsin Card Sorting Test, Trail Making Test], visuospatial function [WISC-R block design, Clock Drawing/Setting], and attention [WAIS-R digit symbol and digit span]. Exclusion criteria included a history of stroke, epilepsy, schizophrenia, or other chronic neuropsychiatric conditions known to be associated with cognitive deficits. We also excluded subjects who took CNS-active medications within 24 hours before the ERP session, severe visual or auditory impairment, left-handedness, severe chronic medical conditions (e.g., renal, hepatic, or respiratory insufficiency). All subjects were studied with ERPs when they carried an ADRC consensus diagnosis of Normal Control. In order to define a group of participants with Pre-AD, follow-up data and any available neuropathological data were reviewed.
The diagnostic criteria for amnestic MCI, as described by Petersen and colleagues (1999) were: subjective memory complaints and/or a history of memory problems according to a reliable informant, mild cognitive impairment on neuropsychological tests (with predominant deficits in the memory domain), with absence of significant functional decline, thus not meeting criteria for dementia or Probable AD by NINCDS-ADRDA criteria (i.e. requires decline in two or more cognitive domains, and functional decline).
Table 1 details the diagnostic histories of the 7 individuals classified as Pre-AD. For the 5 Pre-AD cases who underwent brain autopsy, the mean Braak neurofibrillary stage was 2.8 (range 0–6) and the amyloid letter stage ranged from A to C. Three of the six subjects who transitioned to MCI (Minterval from ERP to MCI =1.5 years) subsequently declined to Probable AD (Minterval to Probable AD =3.0 years) during life, and their autopsies confirmed AD pathology (Mean Braak stage =4.3, range=3–6). One of the MCI cases (case #5) remained with MCI (diagnosed 1.25 years post-ERP) until death (7.25 years post-ERP); brain autopsy done 12 months after the last clinical evaluation confirmed abundant senile plaques (SPs, 31.5 per low-power field, averaged across 4 regions), but minimal neurofibrillary pathology (Braak Stage 1C). The other 2 MCI cases were lost to clinical follow-up, and have not been autopsied. The seventh subject remained cognitively within normal limits throughout 5 years of post-ERP follow-up (with slight decline on WISC-R block design test only), and autopsy performed 7 years post-ERP showed numerous diffuse SPs (mean =39.5 per low-power field) and neuritic plaques (NPs) in the neocortex, but no tangles (Braak stage = 0C). The mean plaque and tangle counts, averaged across 4 brain regions (described below) for the 5 autopsied Pre-AD cases were 21.6 (SE =6.9) SPs, 8.8 (SE =4.5) NPs, and 4.0 (SE =2.2) neurofibrillary tangles (NFTs). The mean time interval from ERP testing to brain autopsy was 7.28 years (range =1.67–11.38).
Table 1.
Demographics, clinical diagnostic history, and neuropathology of the preclinical AD individuals.
| Case | Age at ERP |
Sex | MMSE | Clinical outcomes | Age at Death |
Neuropathology (diagnoses) |
SPs | NPs | NFTs | Braak Stage |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 77 | M | 30 | Remained cognitively normal, last visit at age 82. | 84 | AD changes, Infarct. | 39.5 | 13.3 | 0 | 0 |
| 2 | 80 | M | 30 | Converted to MCI at age 90. | - | - | n/a | n/a | n/a | n/a |
| 3 | 68 | M | 27 | Converted to MCI at age 69, then to probable AD at 70. | 70 | AD, multiple infarcts, Wallerian degeneration. | 23.3 | 14 | 1 | 3 |
| 4 | 76 | F | 28 | Converted to MCI at age 77, then to possible AD at age 78, probable AD at 80. | 85 | AD, CVD | 39 | 25 | 6 | 6 |
| 5 | 89 | M | 27 | Converted to MCI at age 90. | 96 | AD changes, LBV of AD. | 31.5 | 2 | 1.3 | 1 |
| 6 | 76 | M | 30 | Converted to MCI at age 82. | - | - | n/a | n/a | n/a | n/a |
| 7 | 76 | M | 29 | Converted to MCI at age 79, then to probable AD at age 81 | 87 | Tangle-predominant AD | 2 | 0.5 | 11.8 | 4 |
notes CVD = cerebrovascular disease, LBV = Lewy body variant, n/a = not assessed, SPs = senile plaques, NPs = neuritic plaques. SPs and NPs are plaque counts per 125× microscopic field, averaged across 4 brain regions. NFTs = mean neurofibrillary tangle counts per 500× field.
To contrast with the Pre-AD group, a robust normative sample (Sliwinski, Lipton, Buschke, et al., 1996) from ADRC elderly Normal Controls (NC) who participated in ERP studies was defined by having a continued diagnosis of NC (with annual neuropsychological testing) for a minimum of 3 years after the ERP session (Mfollow-up =9.0 years, minimum =3.3 years), and a most recent clinical diagnosis of NC.
Procedure
Between 16 and 32 channel EEG was recorded with an electrode cap (sampling rate=250 Hz, bandpass=0.016–100 Hz, re-referenced offline to linked mastoids) while subjects performed a semantic judgment task. Category-statements (e.g., ‘A body of water’—‘river’) were read aloud to the subject, followed ~1 second later by a visual target word (duration=300 ms) presented on a computer screen. After a 3-second pause, participants stated the perceived target word followed by “yes” or “no” indicating whether or not the target word fit the preceding category-statement. Half of the target words were semantically congruous (e.g. ‘A breakfast food’—‘pancake’) and half were incongruous (e.g. ‘An emotion’—‘nectar’, and ‘Part of a watch’—‘visitor’). Also, half of the presented category statement-target word pairs were new and half were repeated. The repeats followed their initial presentations from ~10–140 seconds later (1–14 trials). The ERP recordings were performed in three blocks of 144 trials each (~20 minutes/block).
ERP analyses
The ERP data were analyzed by split-plot analysis of variance (ANOVA) with the between-subject factor of Group and within-subject factors of Electrode and Repetition (or semantic Congruity for analyses of the N400 elicited by New words). The 300–550 and 550–800 ms latency windows were selected to quantify the N400 and P600 amplitudes, respectively. The 13 scalp electrodes common to all subjects were analyzed for the ANOVAs of the N400 (to incongruous words and to all New words). A common subset of 7 centrotemporal electrodes (Cz, 41L/R, WL/R, T5/6), which reliably captured the P600 across subjects, was selected for the primary ANOVA of the P600. Follow-up ANOVAs were performed where appropriate, with the additional within-subject factors of Hemisphere and Anterior-Posterior channel location. Main effects and interaction effects with p≤ 0.05 (after Greenhouse-Geisser correction for violations of sphericity) were considered significant.
Neuropathological methods
Within 24 hours of death, brains were removed and divided sagitally. The left hemibrain was fixed in formalin, then examined externally and cut into 1-cm thick coronal sections. In a standardized protocol, blocks were sampled from 13 brain regions (Olichney, Hansen, Galasko, et al., 1996). Standardized procedures were used to quantify sections for plaques and tangles using thioflavin-stained slides of selected brain regions of the hippocampus and the neocortex in order to apply neuropathological diagnostic consensus criteria for AD and to perform Braak staging (Braak & Braak, 1991; Merdes, Hansen, Jeste, et al., 2003). Three 125× microscopic fields (field size 1.76 mm2) were counted for total NPs and SPs, and three 500× (field size 0.1 mm2) for NFTs. These single plaque and tangle counts for each of four brain regions (midfrontal, inferior parietal, superior temporal, and posterior hippocampus) were then averaged to provide overall NP, SP, and NFT scores for each autopsied case (Merdes, et al., 2003).
RESULTS
Demographic and Behavioral Data
The two groups were similar in age and education (p’s≥ 0.49), but Pre-AD had an increased proportion of males, which approached significance (χ2 =3.52, p=0.06) (Table 2). The mean MMSE score was 1.1 point higher in the RNE group, an intergroup difference that was statistically significant but not useful for clinical application or diagnosis, with most participants scoring near ceiling. There was no significant group difference (p’s> 0.19) in the prevalence of vascular risk factors (hypertension, diabetes, hyperlipidemia).
Table 2.
Demographics and mental status scores for robust normals and preclinical AD groups.
Both groups performed at ceiling on the semantic judgment task (accuracy: RNE=99.7 ±0.3%, Pre-AD=99.6 ±0.5%; t=0.85, p=0.41).
EEG artifact rejection
ERP trials contaminated by eye movements or other artifacts were rejected off-line, using a semi-automated computer algorithm. For the RNE, 31.8% of trials were rejected versus 29.5% of trials in Pre-AD (t= 0.27, p= 0.79), leaving an average of 295 ±75 trials accepted in RNE and 305 ±78 trials in Pre-AD.
ERP Results
N400 congruity effects of new words
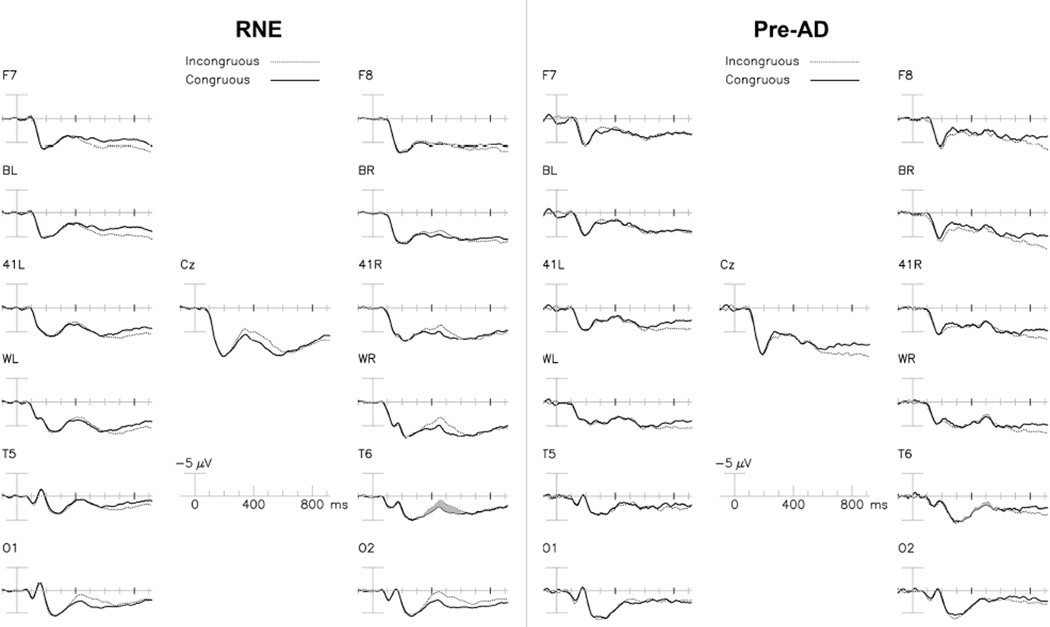
Figure 1 shows the grand–averaged ERPs to new congruous and incongruous words for the two groups (Figure 1). The N400 congruity effect (ERPs to initial presentations of congruous words vs. incongruous words) is normally maximal in right posterior channels, as in the RNE group, and is less evident in the Pre-AD group’s grand-averaged waveforms. In the RNE group, the congruity effect began approximately 300 ms post-stimulus onset and peaked at ~450 ms. In the Pre-AD grand-average, the N400 congruity effect has a slightly later onset, starting well after 400 ms.
Figure 1. New words.
Grand-averaged ERPs to initial presentation of congruous (solid line) and incongruous (dotted line) words in RNE (left) and Pre-AD (right) participants. The N400 congruity effect is shaded at channel T6 (300–600 ms).
While the mean N400 congruity effect amplitude tended to be smaller in Pre-AD, the Group × Congruity interaction [F= 2.00, p= 0.175] was not significant. ANOVA of the N400 amplitude to new words showed a nearly significant Group × Congruity × Electrode interaction [F= 2.56, ε = 0.29, p= 0.056], suggesting a possible difference in the topography of the N400 congruity effect in Pre-AD.
N400 repetition effects of incongruous words
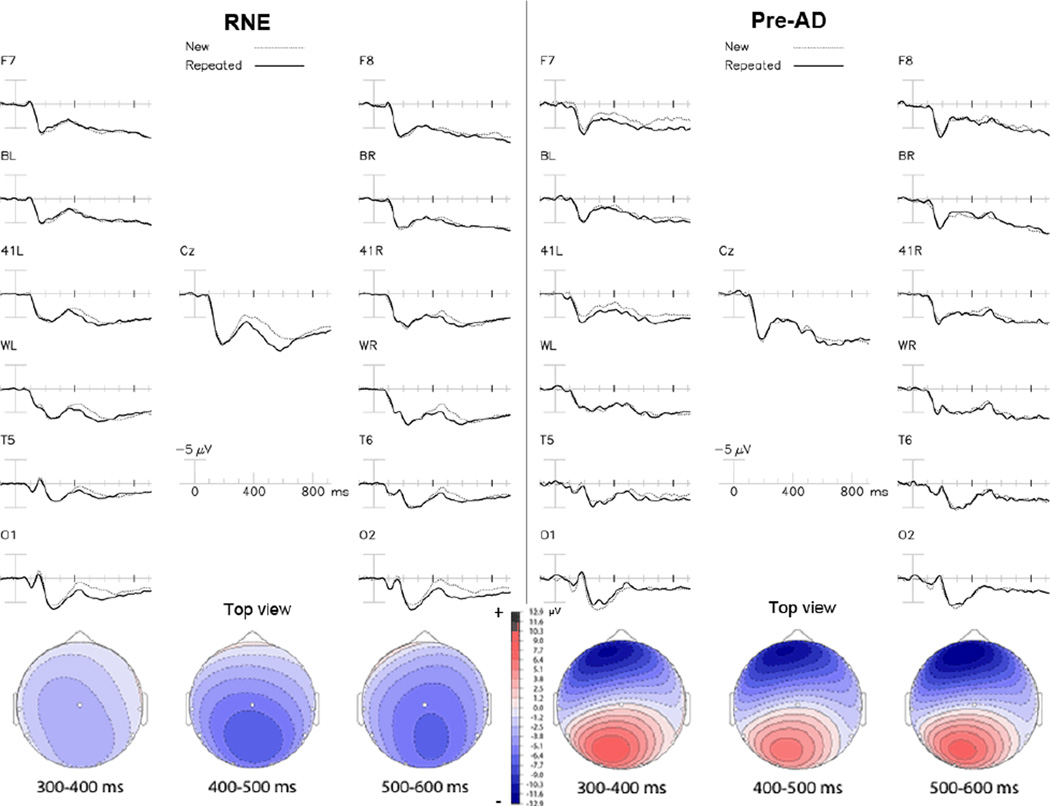
Both groups had larger N400s to initial presentations of incongruous words than to repeated presentations (Figure 2), but the size of this repetition effect tended to be larger in RNE (mean amplitude at Cz = −2.49 µV in RNE vs. −0.16 µV in Pre-AD). However the Group × Repetition interaction was non-significant [F= 1.39, p= 0.26]. Also, Figure 2 shows the N400 word repetition effect had its usual posterior scalp distribution in the RNE group, but showed an atypical anterior > posterior scalp topography in the Pre-AD group. There was a significant 3-way Group × Repetition × Electrode interaction [F= 3.30, ε = 0.25, p= 0.027], supporting the different scalp topographies of the repetition effects to incongruous words in the two groups. Follow-up ANOVA of all lateral channels showed no significant effects of Hemisphere, but there was a significant Group × Repetition × Anterior-Posterior interaction [F= 3.66, ε = 0.31, p= 0.049]. Follow-up t-tests showed significant intergroup differences at three posterior electrodes (O1, O2, WL) with an absent or reversed N400 repetition effect in Pre-AD at these electrodes (e.g. at O1: Pre-AD = 0.84 ± 2 µV, RNE = −1.52 ± 1.5; t = 2.9, p = 0.01), but no significant differences in any of the frontal channels (all p’s > 0.10). Overall, the Pre-AD group had reduced N400 repetition effect over posterior regions compared to the RNE group (Figure 2).
Figure 2. Incongruous words.
The incongruous word repetition effect. Top: Grand-averaged ERPs to initial (dotted line) and repeated (solid line) presentations of incongruous category words, collapsed across repetition lag in RNE (left) and Pre-AD (right) participants. Bottom: Topographical maps of the repetition effect (new – old words) with Pre-AD on the right.
P600 repetition effects of congruous words
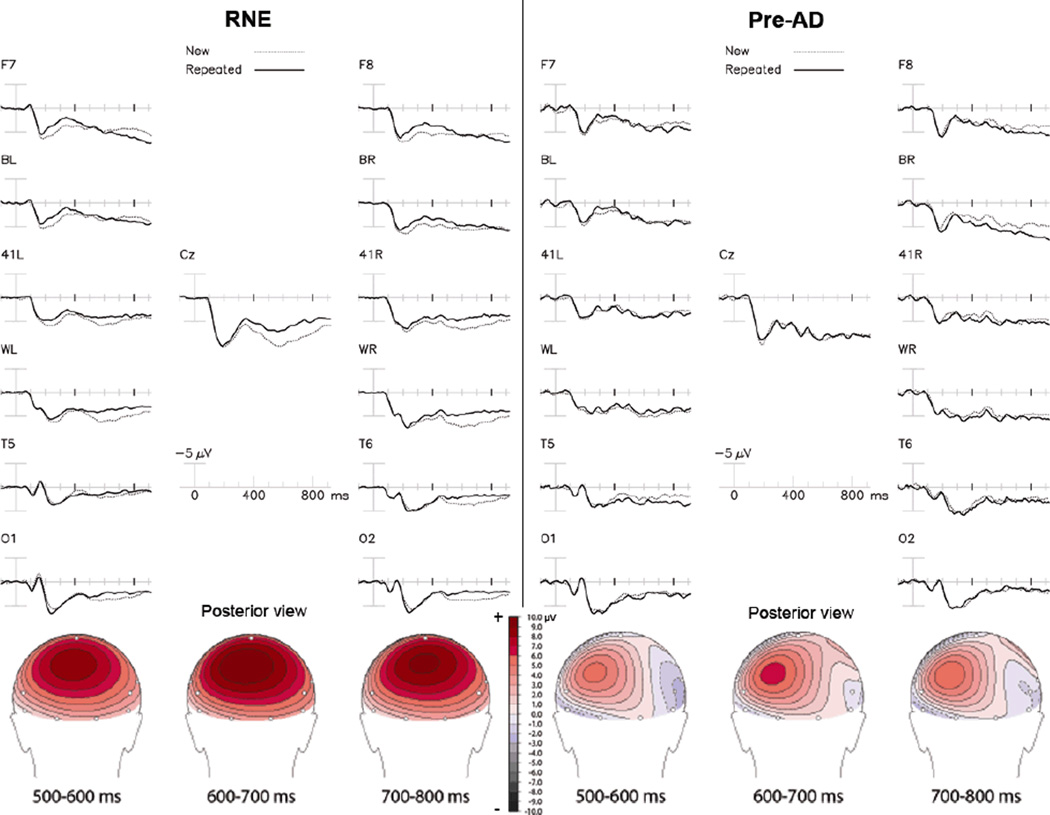
In RNE, the initial presentations of congruous target words elicited typical P600 potentials, with a centro-parietal peak, which became smaller with repeated presentations (Figure 3). This decline in P600 amplitude resulting from the repetition of congruous words (i.e., the P600 effect) was larger in the RNE group than in the Pre-AD group. ANOVA indicated a significant Group × Repetition interaction [F= 5.99, p= 0.026 across centro-temporal channels; F= 4.72, p= 0.04 across all 13 common scalp channels], substantiating that the RNE group had larger P600 repetition effects than the Pre-AD group (e.g. mean amplitudes at Cz =3.28 ±3.70 vs 0.10±0.89 µV). Note the absence of the P600 congruous word repetition effect in most channels of the Pre-AD group data. The topography of each group’s P600 repetition effect was maximal over parietal scalp.
Figure 3. Congruous words.
The congruous word repetition effect. Top: Grand average ERPs to initial (dotted line) and repeated (solid line) presentations of congruous category words, collapsed across repetition lag in RNE (left) and Pre-AD (right) participants. Bottom: Topographical maps of the repetition effect (new – old words) with Pre-AD on the right.
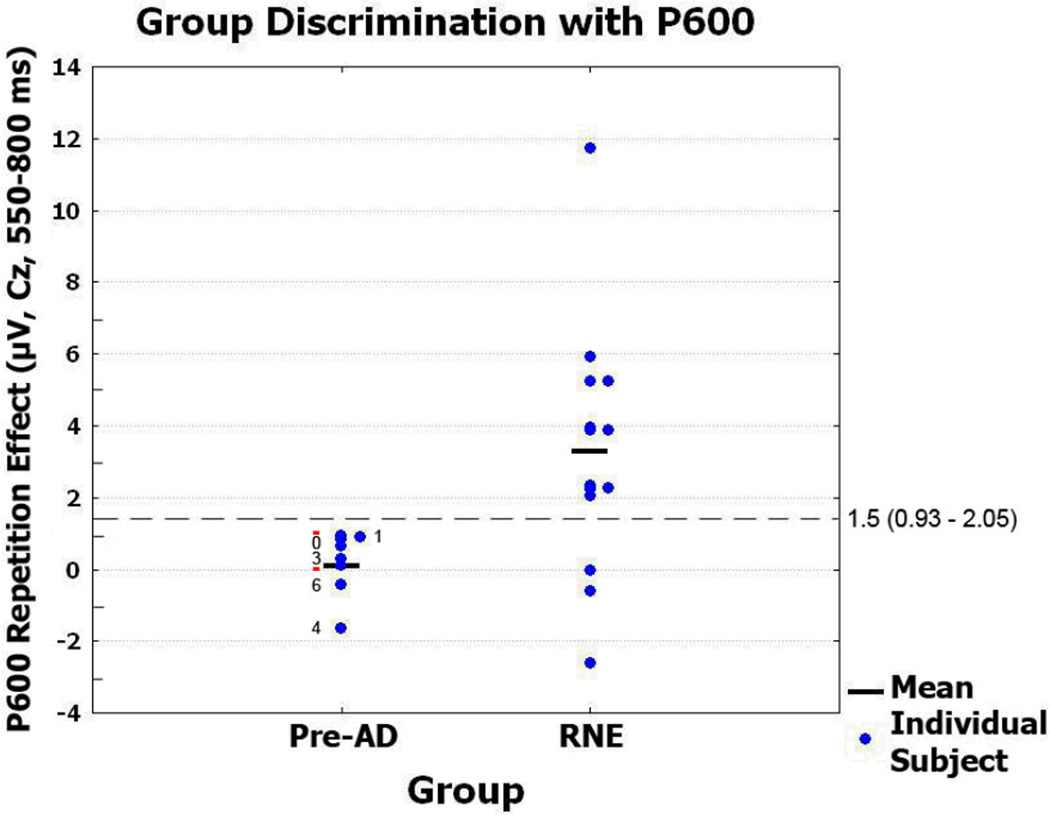
Group discrimination
Very good group discrimination (16/19 = 84%) with 100% sensitivity (7/7 Pre-AD) and 75% specificity (9/12 RNE) was achieved by a P600 repetition effect cutoff of ~ 1.5 µV (same result for cutoffs in 0.93–2.05 µV range) at channel Cz (Figure 4). A N400 repetition effect cutoff of 0.75 µV (0.70–0.88 µV range) at channel Cz achieved slightly poorer group discrimination (15/19 = 79%) with 71% sensitivity (5/7 Pre-AD) and 83% specificity (10/12 RNE).
Figure 4. P600 effect group discrimination with Braak staging of Pre-AD subjects.
Group discrimination with P600 repetition effect at Cz. The dash line indicates the cutoff value with 100% sensitivity and 75% specificity (the range given had the same results). Braak staging of neurofibrillary AD pathology is shown for all autopsied subjects (n = 5), red dashes indicating Pre-AD cases (n = 2) not autopsied.
DISCUSSION
Individuals with Preclinical AD showed a severe reduction in the P600 repetition effect amplitude (smaller positivity between 550–800 ms in response to the repetition of category exemplars) compared to robust normal elderly controls. As reviewed in the Introduction section, the P600 effect has been linked to episodic/declarative memory processes and appears particularly sensitive to incipient AD. The P600 may provide an objective measure of memory dysfunction for the early prediction or diagnosis of AD. Although the present study is limited by small sample sizes, the results suggest that cognitive ERPs could serve as a sensitive tool for identifying synaptic dysfunction in very early AD. There is a now recognized need for reliable markers of the synaptic dysfunction in Pre-AD. Indeed, the recently proposed research criteria for staging of ‘Preclinical’ AD requires signs of synaptic dysfunction for Stages 2 and 3, with the difference between these stages being the presence of subtle cognitive decline on neuropsychological testing (Sperling et al., 2011). Abnormal P600 word repetition effects in apparently normal elderly may be an important sign of synaptic dysfunction and ‘Preclinical’ AD.
Although the size of the N400 repetition effect did not significantly differentiate the two groups, there was a significant group difference in the scalp distribution of this effect. The Pre-AD participants showed an atypically anterior N400 distribution, similar to what we have reported for MCI converters (Olichney et al., 2008) and mild AD patients (Olichney et al., 2006). The N400 is an index of semantic activation, and these abnormalities may reflect subtle changes in semantic/linguistic processing and/or dysfunction of the N400 neural generators (Kutas & Federmeier, 2011). Prior intracranial studies have identified N400 generators in the bilateral anterior fusiform gyri (McCarthy et al., 1995). The atypically anterior N400 effect in our Pre-AD group more closely resembles the ‘familiarity’ FN400, rather than the ‘classic’ posterior N400 related to semantic analysis or priming, and other implicit conceptual memory processes. However, considering our small sample size, the N400 topographic group differences we found should be considered preliminary until an independent replication is confirmed.
ERPs have limited spatial resolution, but they offer superb temporal resolution and several practical advantages (e.g. inexpensive, noninvasive, direct measure of summated synaptic currents, high signal-to-noise ratio) in the characterization of brain disorders. The well-established sensitivity of ERPs to cognitive processes supports their potential use as a cost-effective index for early AD and other cognitive disorders. Paradigms such as this one are sensitive to two cardinal features of AD (i.e. episodic and semantic memory deficits), and are particularly well-suited for the characterization and identification of the earliest cognitive deficits which emerge in later stage Pre-AD.
There is a scarcity of published ERP studies with neuropathological correlations (Grunwald, Beck, Lehnertz, et al., 1999). We are not aware of any prior ERP studies of AD or MCI cohorts with neuropathological confirmation. Interestingly, even cases with minimal neurofibrillary pathology exhibited reduced P600 effects, suggesting that amyloid deposition or pre-amyloid (oligomers or other soluble forms) may be sufficient to disrupt communication among the P600 generators. Consistent with this possibility, Lesné and colleagues (2006) demonstrated that specific oligomeric forms of amyloid can disrupt LTP and synaptic plasticity.
Recently, in vivo amyloid imaging techniques (e.g. PiB-PET) that are highly sensitive to AD dementia have emerged (Rowe, Ng, Ackermann et al., 2007). However, PiB-PET imaging does not stage the degree of AD neurofibrillary pathology or neuronal loss. Also, amyloid PET imaging is an important prognostic marker in MCI, but PiB-PET sensitivity to MCI is somewhat lower (~55–70%) (Mintun, Larossa, Sheline, et al., 2006; Rowe et al., 2007) than the percentage of patients with amnestic MCI who later develop AD dementia (~80–85%) (Morris, Storandt, Miller, et al., 2001). Work in AD animal models has shown that synaptic dysfunction often occurs earlier than the deposition of extracellular amyloid plaques (Rowan, Klyubin, Cullen, et al., 2003). Therefore, it will be important to determine the extent to which combining amyloid biomarkers with electrophysiological biomarkers of AD, or other biomarkers of AD stage, might yield a more refined estimate of dementia risk and prognosis in Pre-AD.
Acknowledgements
Study Funding: Supported by NIH grants R01 AG18442, R01 AG08313, P30 AG10129, P50 AG05131, the US Department of Veterans Affairs, and the California Department of Public Health Alzheimer’s Disease Program. We would also like to thank the UC Davis ADC and the Shiley-Marcos UCSD ADRC.
Footnotes
Disclosures: Dr. Olichney has served as a consultant for Lundbeck Pharmaceuticals.
Dr. Salmon is a consultant for Bristol Myer Squibb.
Dr. Galasko is a consultant for United BioSource Corporation, Elan Pharmaceuticals and Wyeth pharmaceuticals (DSMB), and reports research support from Pfizer Pharmaceuticals and Eli Lilly, Inc. for site participation in clinical trials.
References
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26:55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- Grunwald T, Beck H, Lehnertz K, Blumcke I, Pezer N, Kurthen M, et al. Evidence relating human verbal memory to hippocampal N-methyl-D-aspartate receptors. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:12085–12089. doi: 10.1073/pnas.96.21.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem F, Rougier A, Claverie B. Short- and long-delay intracranial ERP repetition effects dissociate memory systems in the human brain. Journal of Cognitive Neuroscience. 1999;11:437–458. doi: 10.1162/089892999563526. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke M, Marinkovic K, Chauvel P. Spatio-temporal stages in face and word processing. 2. Depth-recorded potentials in the human frontal and Rolandic cortices. Journal of Physiology Paris. 1994;88:51–80. doi: 10.1016/0928-4257(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Klein WL. Synaptic targeting by A beta oligomers (ADDLS) as a basis for memory loss in early Alzheimer's disease. Alzheimers Dementia. 2006;2:43–55. doi: 10.1016/j.jalz.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP) Annual Review of Psychology. 2011;62:621–647. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Nobre AC, Bentin S, Spencer DD. Language-related field potentials in the anterior-medial temporal lobe: I. Intracranial distribution and neural generators. Journal of Neuroscience. 1995;15:1080–1089. doi: 10.1523/JNEUROSCI.15-02-01080.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes AR, Hansen LA, Jeste DV, Galasko D, Hofstetter CR, Ho GJ, Thal LJ, Corey-Bloom J. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology. 2003;60:1586–1590. doi: 10.1212/01.wnl.0000065889.42856.f2. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Archives of Neurology. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric fields of the brain: the neurophysics of EEG. 2nd ed. New York: Oxford University Press; 2006. pp. 163–166. [Google Scholar]
- Olichney JM, Hansen LA, Galasko D, Saitoh T, Hofstetter CR, Katzman R, Thal LJ. The apolipoprotein E epsilon 4 allele is associated with increased neuritic plaques and cerebral amyloid angiopathy in Alzheimer’s disease and Lewy body variant. Neurology. 1996;47:190–196. doi: 10.1212/wnl.47.1.190. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Iragui VJ, Salmon DP, Riggins BR, Morris SK, Kutas M. Absent event-related potential (ERP) word repetition effects in mild Alzheimer’s disease. Clinical Neurophysiology. 2006;117:1319–1330. doi: 10.1016/j.clinph.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Morris SK, Ochoa C, Salmon DP, Thal LJ, Kutas M, Iragui VJ. Abnormal verbal event-related potentials in mild cognitive impairment and incipient AD. Journal of neurology, neurosurgery, and psychiatry. 2002;73:377–384. doi: 10.1136/jnnp.73.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Taylor JR, Chan S, Yang JC, Stringfellow A, Hillert DG, Simmons AL, Salmon DP, Iragui-Madoz V, Kutas M. fMRI responses to words repeated in a congruous semantic context are abnormal in mild Alzheimer’s disease. Neuropsychologia. 2010;48:2476–2487. doi: 10.1016/j.neuropsychologia.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Taylor JR, Gatherwright J, Salmon DP, Bressler AJ, Kutas M, Iragui-Madoz VJ. Patients with MCI and N400 or P600 abnormalities are at very high risk for conversion to dementia. Neurology. 2008;70:1763–1770. doi: 10.1212/01.wnl.0000281689.28759.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Taylor JR, Hillert DG, Chan SH, Salmon DP, Gatherwright J, Iragui VJ, Kutas M. fMRI congruous word repetition effects reflect memory variability in normal elderly. Neurobiology of Aging. 2010;31:1975–1990. doi: 10.1016/j.neurobiolaging.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Van Petten C, Paller KA, Salmon DP, Iragui VJ, Kutas M. Word repetition in amnesia: Electrophysiological measures of impaired and spared memory. Brain. 2000;123:1948–1963. doi: 10.1093/brain/123.9.1948. [DOI] [PubMed] [Google Scholar]
- Paller KA, Kutas M. Brain potentials during memory retrieval provide neurophysiological support for the distinction between conscious recollection and priming. Journal of Cognitive Neuroscience. 1992;4:375–391. doi: 10.1162/jocn.1992.4.4.375. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Annals of Neurology. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- Rowan MJ, Klyubin I, Cullen WK, Anwyl R. Synaptic plasticity in animal models of early Alzheimer's disease. Philosophical Transactions of the Royal Society London B: Biological Sciences. 2003;358:821–828. doi: 10.1098/rstb.2002.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, Cowie TF, Dickinson KL, Maruff P, Darby D, Smith C, Woodward M, Merory J, Tochon-Danguy H, O'Keefe G, Klunk WE, Mathis CA, Price JC, Masters CL, Villemagne VL. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- Rugg M, Curran T. Event-related potentials and recognition memory. Trends in Cognitive Science. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Sliwinski M, Lipton RB, Buschke H, Stewart W. The effects of preclinical dementia on estimates of normal cognitive functioning in aging. Journals of Gerontology B Series Psychological Sciences. 1996;51:217–225. doi: 10.1093/geronb/51b.4.p217. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH .Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dementia. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petten C, Kutas M, Kluender R, Mitchiner M, McIsaac H. Fractionating the word repetition effect with event-related potentials. Journal of Cognitive Neuroscience. 1991;3:131–150. doi: 10.1162/jocn.1991.3.2.131. [DOI] [PubMed] [Google Scholar]