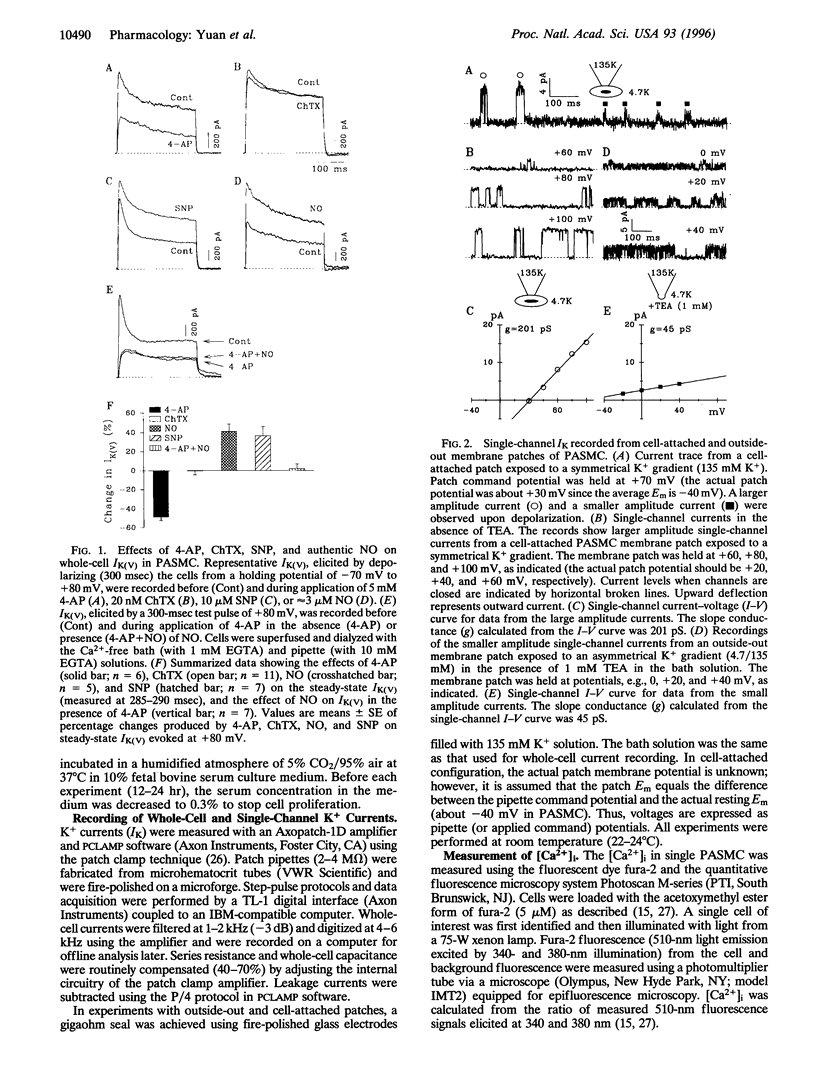
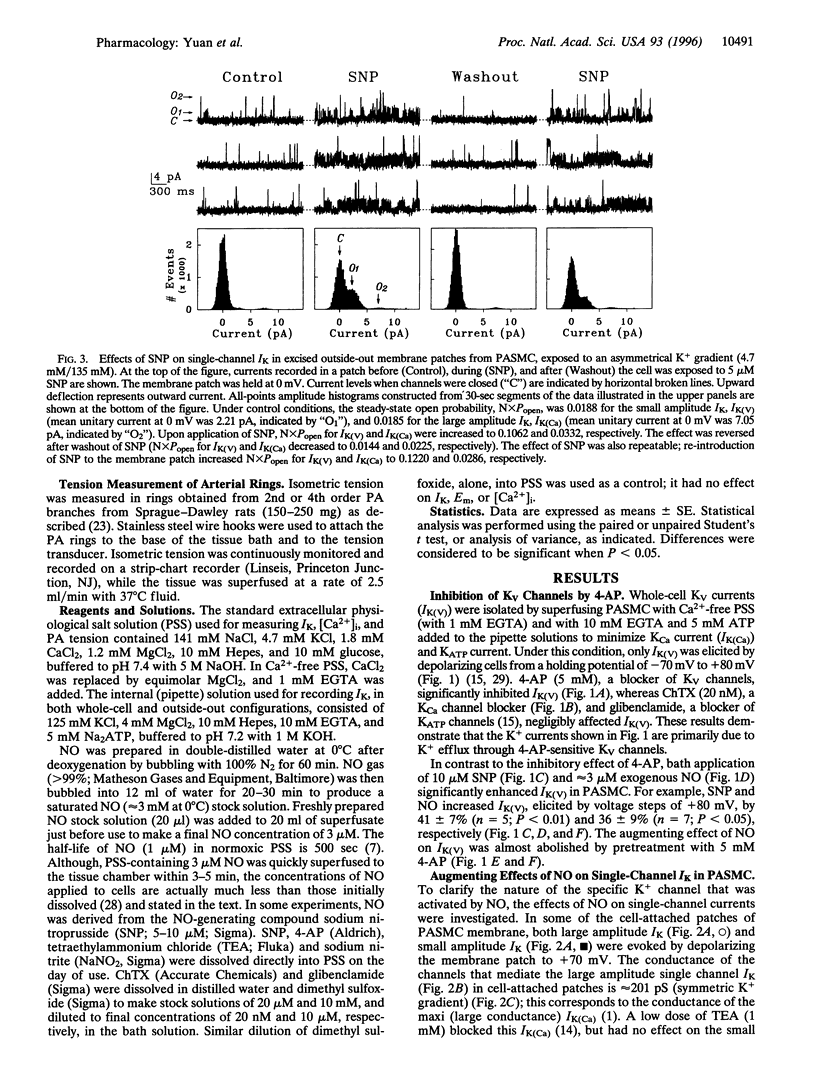
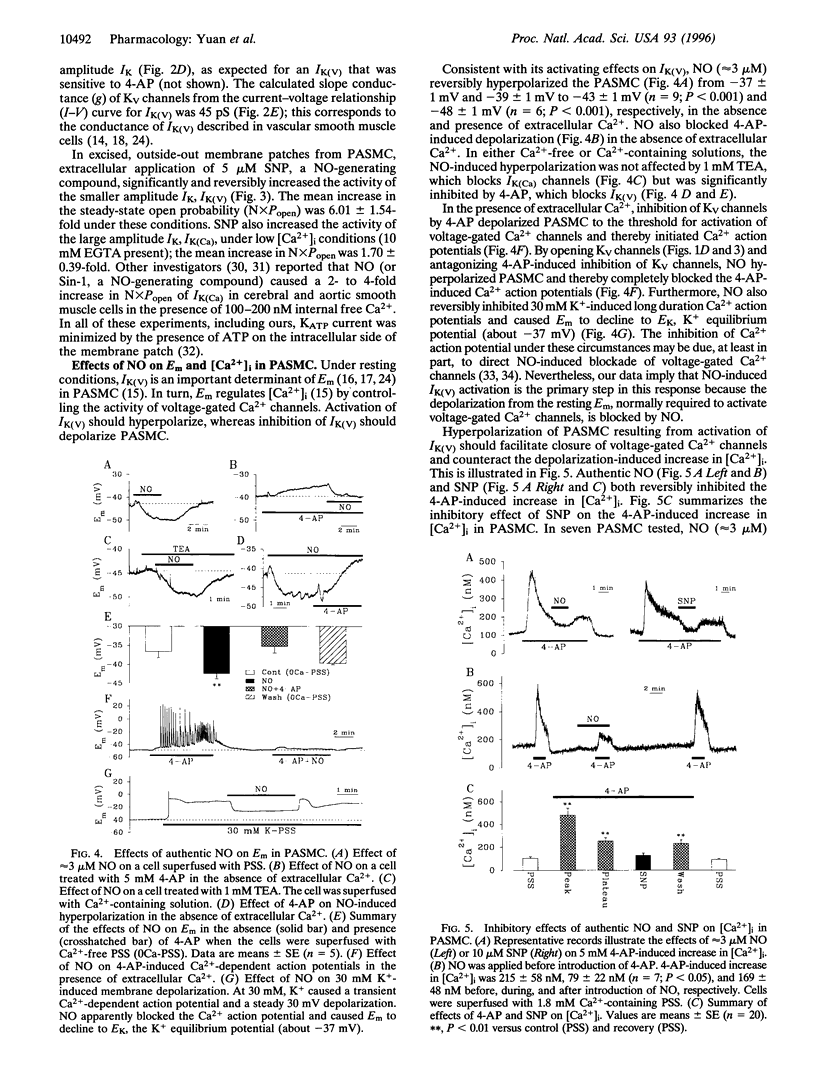
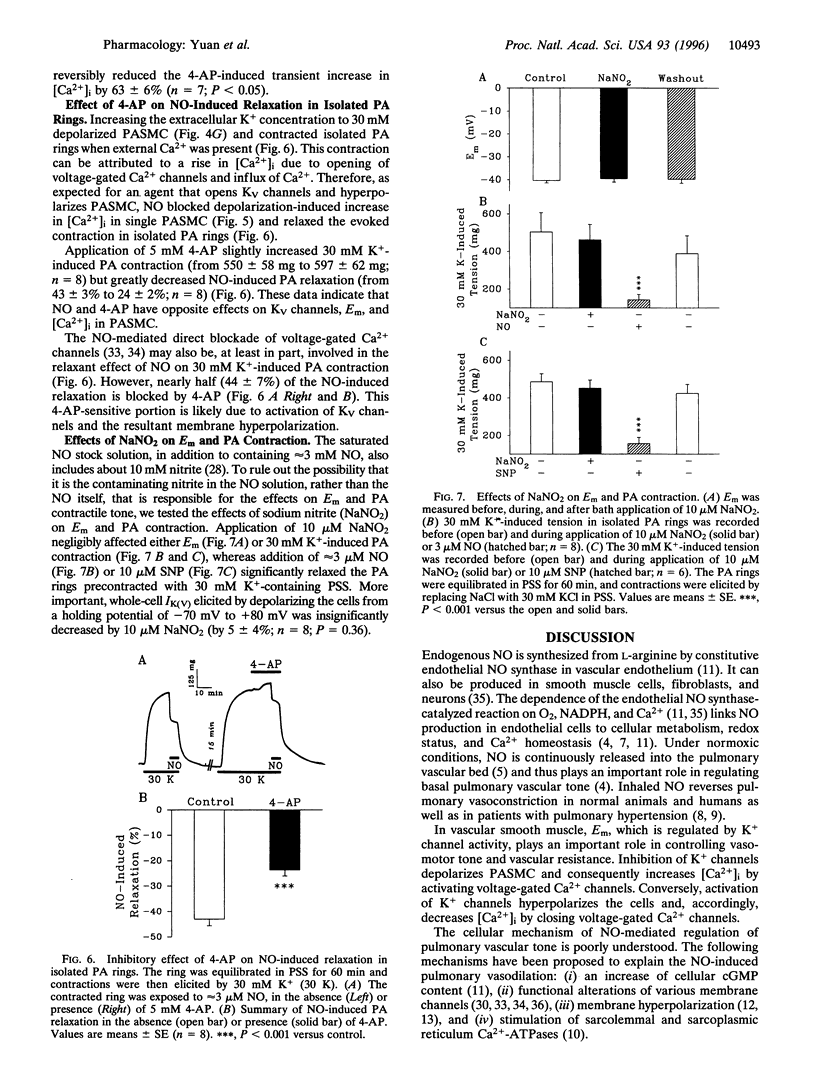
Abstract
NO causes pulmonary vasodilation in patients with pulmonary hypertension. In pulmonary arterial smooth muscle cells, the activity of voltage-gated K+ (Kv) channels controls resting membrane potential. In turn, membrane potential is an important regulator of the intracellular free calcium concentration ([Ca2+]i) and pulmonary vascular tone. We used patch clamp methods to determine whether the NO-induced pulmonary vasodilation is mediated by activation of Kv channels. Quantitative fluorescence microscopy was employed to test the effect of NO on the depolarization-induced rise in [Ca2+]i. Blockade of Kv channels by 4-aminopyridine (5 mM) depolarized pulmonary artery myocytes to threshold for initiation of Ca2+ action potentials, and thereby increased [Ca2+]i. NO (approximately 3 microM) and the NO-generating compound sodium nitroprusside (5-10 microM) opened Kv channels in rat pulmonary artery smooth muscle cells. The enhanced K+ currents then hyperpolarized the cells, and blocked Ca(2+)-dependent action potentials, thereby preventing the evoked increases in [Ca2+]i. Nitroprusside also increased the probability of Kv channel opening in excised, outside-out membrane patches. This raises the possibility that NO may act either directly on the channel protein or on a closely associated molecule rather than via soluble guanylate cyclase. In isolated pulmonary arteries, 4-aminopyridine significantly inhibited NO-induced relaxation. We conclude that NO promotes the opening of Kv channels in pulmonary arterial smooth muscle cells. The resulting membrane hyperpolarization, which lowers [Ca2+]i, is apparently one of the mechanisms by which NO induces pulmonary vasodilation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albarwani S., Robertson B. E., Nye P. C., Kozlowski R. Z. Biophysical properties of Ca(2+)- and Mg-ATP-activated K+ channels in pulmonary arterial smooth muscle cells isolated from the rat. Pflugers Arch. 1994 Oct;428(5-6):446–454. doi: 10.1007/BF00374564. [DOI] [PubMed] [Google Scholar]
- Archer S. L., Huang J. M., Hampl V., Nelson D. P., Shultz P. J., Weir E. K. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7583–7587. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter L. A., Wier W. G. Nitric oxide decreases [Ca2+]i in vascular smooth muscle by inhibition of the calcium current. Cell Calcium. 1994 Feb;15(2):122–131. doi: 10.1016/0143-4160(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Bolotina V. M., Najibi S., Palacino J. J., Pagano P. J., Cohen R. A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994 Apr 28;368(6474):850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Clapp L. H., Gurney A. M. ATP-sensitive K+ channels regulate resting potential of pulmonary arterial smooth muscle cells. Am J Physiol. 1992 Mar;262(3 Pt 2):H916–H920. doi: 10.1152/ajpheart.1992.262.3.H916. [DOI] [PubMed] [Google Scholar]
- Clapp L. H., Gurney A. M. Modulation of calcium movements by nitroprusside in isolated vascular smooth muscle cells. Pflugers Arch. 1991 Jun;418(5):462–470. doi: 10.1007/BF00497774. [DOI] [PubMed] [Google Scholar]
- Cornfield D. N., Stevens T., McMurtry I. F., Abman S. H., Rodman D. M. Acute hypoxia increases cytosolic calcium in fetal pulmonary artery smooth muscle cells. Am J Physiol. 1993 Jul;265(1 Pt 1):L53–L56. doi: 10.1152/ajplung.1993.265.1.L53. [DOI] [PubMed] [Google Scholar]
- Cremona G., Wood A. M., Hall L. W., Bower E. A., Higenbottam T. Effect of inhibitors of nitric oxide release and action on vascular tone in isolated lungs of pig, sheep, dog and man. J Physiol. 1994 Nov 15;481(Pt 1):185–195. doi: 10.1113/jphysiol.1994.sp020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. W., Standen N. B., Stanfield P. R. ATP-dependent potassium channels of muscle cells: their properties, regulation, and possible functions. J Bioenerg Biomembr. 1991 Aug;23(4):509–535. doi: 10.1007/BF00785809. [DOI] [PubMed] [Google Scholar]
- Fleischmann B. K., Washabau R. J., Kotlikoff M. I. Control of resting membrane potential by delayed rectifier potassium currents in ferret airway smooth muscle cells. J Physiol. 1993 Sep;469:625–638. doi: 10.1113/jphysiol.1993.sp019834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gaston B., Drazen J. M., Loscalzo J., Stamler J. S. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med. 1994 Feb;149(2 Pt 1):538–551. doi: 10.1164/ajrccm.149.2.7508323. [DOI] [PubMed] [Google Scholar]
- Gelband C. H., Hume J. R. [Ca2+]i inhibition of K+ channels in canine renal artery. Novel mechanism for agonist-induced membrane depolarization. Circ Res. 1995 Jul;77(1):121–130. doi: 10.1161/01.res.77.1.121. [DOI] [PubMed] [Google Scholar]
- Goldman W. F., Bova S., Blaustein M. P. Measurement of intracellular Ca2+ in cultured arterial smooth muscle cells using Fura-2 and digital imaging microscopy. Cell Calcium. 1990 Feb-Mar;11(2-3):221–231. doi: 10.1016/0143-4160(90)90073-4. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janigro D., West G. A., Nguyen T. S., Winn H. R. Regulation of blood-brain barrier endothelial cells by nitric oxide. Circ Res. 1994 Sep;75(3):528–538. doi: 10.1161/01.res.75.3.528. [DOI] [PubMed] [Google Scholar]
- Leblanc N., Wan X., Leung P. M. Physiological role of Ca(2+)-activated and voltage-dependent K+ currents in rabbit coronary myocytes. Am J Physiol. 1994 Jun;266(6 Pt 1):C1523–C1537. doi: 10.1152/ajpcell.1994.266.6.C1523. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M., Cornwell T. L. Intracellular cyclic GMP receptor proteins. FASEB J. 1993 Feb 1;7(2):328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- Meisheri K. D., Cipkus-Dubray L. A., Hosner J. M., Khan S. A. Nicorandil-induced vasorelaxation: functional evidence for K+ channel-dependent and cyclic GMP-dependent components in a single vascular preparation. J Cardiovasc Pharmacol. 1991 Jun;17(6):903–912. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Murray T. R., Chen L., Marshall B. E., Macarak E. J. Hypoxic contraction of cultured pulmonary vascular smooth muscle cells. Am J Respir Cell Mol Biol. 1990 Nov;3(5):457–465. doi: 10.1165/ajrcmb/3.5.457. [DOI] [PubMed] [Google Scholar]
- Nagao T., Vanhoutte P. M. Endothelium-derived hyperpolarizing factor and endothelium-dependent relaxations. Am J Respir Cell Mol Biol. 1993 Jan;8(1):1–6. doi: 10.1165/ajrcmb/8.1.1. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Quayle J. M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995 Apr;268(4 Pt 1):C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pearl R. G. Inhaled nitric oxide. The past, the present, and the future. Anesthesiology. 1993 Mar;78(3):413–416. [PubMed] [Google Scholar]
- Pepke-Zaba J., Higenbottam T. W., Dinh-Xuan A. T., Stone D., Wallwork J. Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet. 1991 Nov 9;338(8776):1173–1174. doi: 10.1016/0140-6736(91)92033-x. [DOI] [PubMed] [Google Scholar]
- Post J. M., Gelband C. H., Hume J. R. [Ca2+]i inhibition of K+ channels in canine pulmonary artery. Novel mechanism for hypoxia-induced membrane depolarization. Circ Res. 1995 Jul;77(1):131–139. doi: 10.1161/01.res.77.1.131. [DOI] [PubMed] [Google Scholar]
- Robertson B. E., Nelson M. T. Aminopyridine inhibition and voltage dependence of K+ currents in smooth muscle cells from cerebral arteries. Am J Physiol. 1994 Dec;267(6 Pt 1):C1589–C1597. doi: 10.1152/ajpcell.1994.267.6.C1589. [DOI] [PubMed] [Google Scholar]
- Robertson B. E., Schubert R., Hescheler J., Nelson M. T. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol. 1993 Jul;265(1 Pt 1):C299–C303. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- Smirnov S. V., Aaronson P. I. Alteration of the transmembrane K+ gradient during development of delayed rectifier in isolated rat pulmonary arterial cells. J Gen Physiol. 1994 Aug;104(2):241–264. doi: 10.1085/jgp.104.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov S. V., Robertson T. P., Ward J. P., Aaronson P. I. Chronic hypoxia is associated with reduced delayed rectifier K+ current in rat pulmonary artery muscle cells. Am J Physiol. 1994 Jan;266(1 Pt 2):H365–H370. doi: 10.1152/ajpheart.1994.266.1.H365. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Loh E., Roddy M. A., Currie K. E., Creager M. A. Nitric oxide regulates basal systemic and pulmonary vascular resistance in healthy humans. Circulation. 1994 May;89(5):2035–2040. doi: 10.1161/01.cir.89.5.2035. [DOI] [PubMed] [Google Scholar]
- Tare M., Parkington H. C., Coleman H. A., Neild T. O., Dusting G. J. Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature. 1990 Jul 5;346(6279):69–71. doi: 10.1038/346069a0. [DOI] [PubMed] [Google Scholar]
- Tsuura Y., Ishida H., Hayashi S., Sakamoto K., Horie M., Seino Y. Nitric oxide opens ATP-sensitive K+ channels through suppression of phosphofructokinase activity and inhibits glucose-induced insulin release in pancreatic beta cells. J Gen Physiol. 1994 Dec;104(6):1079–1098. doi: 10.1085/jgp.104.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk K. A., Matsuda J. J., Shibata E. F. A voltage-dependent potassium current in rabbit coronary artery smooth muscle cells. J Physiol. 1991 Aug;439:751–768. doi: 10.1113/jphysiol.1991.sp018691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X. J., Goldman W. F., Tod M. L., Rubin L. J., Blaustein M. P. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am J Physiol. 1993 Feb;264(2 Pt 1):L116–L123. doi: 10.1152/ajplung.1993.264.2.L116. [DOI] [PubMed] [Google Scholar]
- Yuan X. J., Goldman W. F., Tod M. L., Rubin L. J., Blaustein M. P. Ionic currents in rat pulmonary and mesenteric arterial myocytes in primary culture and subculture. Am J Physiol. 1993 Feb;264(2 Pt 1):L107–L115. doi: 10.1152/ajplung.1993.264.2.L107. [DOI] [PubMed] [Google Scholar]
- Yuan X. J., Tod M. L., Rubin L. J., Blaustein M. P. Contrasting effects of hypoxia on tension in rat pulmonary and mesenteric arteries. Am J Physiol. 1990 Aug;259(2 Pt 2):H281–H289. doi: 10.1152/ajpheart.1990.259.2.H281. [DOI] [PubMed] [Google Scholar]
- Yuan X. J., Tod M. L., Rubin L. J., Blaustein M. P. Deoxyglucose and reduced glutathione mimic effects of hypoxia on K+ and Ca2+ conductances in pulmonary artery cells. Am J Physiol. 1994 Jul;267(1 Pt 1):L52–L63. doi: 10.1152/ajplung.1994.267.1.L52. [DOI] [PubMed] [Google Scholar]
- Yuan X. J. Voltage-gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary arterial myocytes. Circ Res. 1995 Aug;77(2):370–378. doi: 10.1161/01.res.77.2.370. [DOI] [PubMed] [Google Scholar]
- Zapol W. M., Rimar S., Gillis N., Marletta M., Bosken C. H. Nitric oxide and the lung. Am J Respir Crit Care Med. 1994 May;149(5):1375–1380. doi: 10.1164/ajrccm.149.5.8173780. [DOI] [PubMed] [Google Scholar]



