Abstract
Leishmania (Viannia) braziliensis control and tissue damage relate to the effector immune response, which in turn affects clinical outcome. Leishmania reactive CD4+ and CD8+ T cells are expanded in long-term healed cutaneous leishmaniasis (hCL) patients but their functional characteristics remain to be determined. This study investigates antigen-specific recall in long-term healed CL caused by L. braziliensis infection. Healed CL subjects were grouped according to the time elapsed since the end of therapy: less than two years and two to five years. Activation phenotype (CD69+ or CD25+) and subpopulations of memory T cell phenotypes [central memory (Tcm): CD45RO+ CCR7+ or effector memory (Tem): CD45RO+ CCR7-] were quantified in ex vivo blood mononuclear cells and after Leishmania antigens stimuli. A reduction in the percentage of activated Leishmania-responder CD4+ and CD8+ T cells in hCL was associated with the time elapsed since clinical cure. Percentage of CD69+ in TCD4+ and TCD8+ cells were negatively correlated with IL-10 levels. Ex vivo analyses showed contracted Tem CD4+ and Tem CD8+ compartments from hCL with long time elapsed since clinical cure, although renewal of these compartments was observed following in vitro exposure to leishmanial stimuli. Our results show that healed L. braziliensis infected patients exhibit a recall response to Leishmania antigens with evident expansion of effector memory T cells. Regulated leishmanial-specific response seems to emerge only about two years after initial contact with the parasite antigens.
Introduction
Cutaneous leishmaniasis (CL) due to Leishmania (Viannia) braziliensis is characterized by ulcerative lesions in the skin that heal either spontaneously or following therapy. Even if healed patients develop a delayed hypersensitivity reaction to leishmanial antigens, which induce the expansion of Leishmania-reactive T lymphocytes, the development of the disease is not always prevented [1-3]. Although lesion healing is accompanied by parasite control and reduction of inflammatory cells in scars [4,5], evidence of parasite persistence [6-9] and leishmanial immune response is still found long after therapy, despite the absence of clinical symptoms [10]. Infections can recidivate not only at the original lesion sites, but also in the mucosal membranes of the upper respiratory tract, resulting in mucosal leishmaniasis (ML).
Ideally, healed CL patients should be monitored for approximately five years post-therapy to enable early detection of possible relapses or metastatic lesions, as this period is considered critical for disease resurgence [11,12]. Contributing factors to an unfavorable prognosis are, however, still a matter of debate, although evidence suggests that immunological factors are decisive. Indeed, a higher percentage of activated T CD4+CD69+ and IFN-γ+ lymphocytes has been associated with larger lesions, hence more severe disease [13]. Moreover, higher frequencies of CD4+ T cells expressing CD28-CD69+CD62Llow are seen in ML than CL cases, along with low regulatory capacity for IFN-γ secretion [14]. These data reinforce the idea of an exacerbated type 1 response in ML subjects, sustained by the replenishment of activated effector cells that may not be down-modulated appropriately, maintaining a persistent inflammatory response and tissue damage. Conversely, an appropriate balance between inflammatory and regulatory factors (ex. IFN-γ/IL-10) might favor parasite control and the stability of clinical cure [15-17].
The withdrawing of regulatory T cells (Treg), or associated molecules, results in an exacerbation of the effector immune response [18]. Although previous research has focused mostly on immune responses during the active phase of the disease, investigation following clinical cure has demonstrated a reduction in the activation of effector cells and in the amount of inflammatory mediators [5,10,17,19-23]. These results point to key roles for regulatory mechanisms on sustaining clinical cure.
Several experimental studies indicate that T-cell memory compartments lead to stronger and faster adaptive immune response against Leishmania antigens [24-26]. Indeed, this is the basis for the design of vaccines against infectious organisms [27]. However, whether anti-Leishmania memory remains after parasitological cure still needs to be determined [24-26,28-30]. Recently, Leishmania-reactive proliferating effector memory TCD8+ were identified as the most frequent subset cells in cured L. major or L. tropica leishmaniasis patients from Iran, which suggested their role in recall immune response [31]. However, the characterization of Tcm (CD45RO+CCR7+) and Tem (CD45RO+CCR7-) induced by L. (V.) braziliensis infection and the magnitude of T cell recall long term after cure are not known. These features are of crucial importance for understanding the ultimate protective immune response.
The present study investigates antigen-specific recall in long-term healed CL caused by infection with L. braziliensis. Such capacity was examined by ex vivo leishmanial-antigen stimulation to determine the renewal of memory T cell compartments and replenishment of phenotypically defined CD4+ and CD8+ T cells. Our previous results suggest that qualitative and quantitative changes in immune response can occur in leishmaniasis patients over time: a higher proportion of leishmanial-reactive CD4+ than CD8+ T cells were observed in long-term cured patients and, although this profile was similar to active disease [10], it was characterized by lower cell percentages. Our hypothesis is that long-term effector memory T cell compartments enable the activation of cellular immunologic pathways under L. braziliensis stimulus. Because determining the T cell compartment that is preferentially expanded to control the Leishmania infection is critical to understand protective immune responses, our results represent a step forward towards the design of an effective vaccine to control this disease, currently considered one of the priority endemic diseases of the world [32].
Materials and Methods
Ethics statement
This study was approved by the ethics committee of the Instituto de Pesquisa Clinica Evandro Chagas (IPEC), Ministério da Saúde, Brazil. It abides by the Helsinki Declaration on human subject research. Written consent was obtained from all volunteers and the study protocol was approved by the Ethical Committee of IPEC (n° 069/2008).
Study population
Twenty-six subjects (12 males, 39.7±16.9 years old) with healed cutaneous leishmaniasis (hCL), as defined by past CL diagnosis and absence of recurrent lesions, were enrolled in the study. Patients were from areas in Rio de Janeiro (Brazil) where L. braziliensis is endemic. Clinically healed lesions were defined as scars presenting a complete re-epithelialization with absence of hyperemia, oedema, and desquamation. Subjects were treated according to the guidelines of the Brazilian Ministry of Health (15-20 mg/kg/day of Sb+5 for 20-30 days) and were followed-up for five years at IPEC/FIOCRUZ. Enrolled individuals were sub-divided into two groups, depending on the time elapsed since the end of therapy: less than two years (hCL< 2y, n=13, 40.6±18.5 years old, 8 males) or from two to five years (hCL 2-5 y, n=13, 38.6±14.4 years old, 10 males). Healthy subjects (HS, n=12, 26 ± 5.7 years old, 5 males) had no clinical or epidemiological evidence of Leishmania infection, as well as negative lymphocyte proliferative responses to L. braziliensis antigens.
In vitro stimulation of peripheral blood mononuclear cells with Leishmania braziliensis antigens
Peripheral blood mononuclear cells (PBMC) were separated by centrifugation over a gradient of Ficoll-Hypaque (Histopaque 1077; Sigma Chemical Company, St Louis, MO, USA). PBMC (3 x 106 cells per mL) were cultured in vitro in 24-well flat-bottom plates (Nunc, Roskilde, Denmark) in the presence of 5 x 106 (50 µg/well) disrupted L. braziliensis (MHOM/BR75/M2903) promastigotes (Lb-Ag). Cells cultured for five days at 37°C in a humidified atmosphere of 5% CO2 in air were harvested and lymphocyte proliferation response (LPR) measured by 3H-thymidine incorporation, as described previously [10]. Culture supernatants were collected and stored at −20°C until cytokine levels were measured.
To conduct the phenotypic analysis, ex vivo PBMC and in vitro culture cells were stained with fluorescein isothiocyanate-labelled anti-CD4, phycoerythrin-Cy5-labelled anti-CD8 (Immunotech, Beckman Coulter Corporation, Marseille, France), phycoerythrin-labelled anti-CD25 or anti-CCR7, and phycoerythrin-Cy7 labelled anti-CD69 or anti-CD45RO (Becton Dickinson Bioscience Pharmingen, Franklin Lakes, NJ, USA). Forty thousand events were acquired from each sample into a lymphocyte gate using a Cyan flow cytometer (Beckman Coulter Inc., FL, USA). Surface molecules were analyzed using the Summit 4.3 software (DakoCytomation, Fort Collins, CO, USA). Lymphocyte populations were defined by gating on CD3+ cells. The frequency of positive cells was analyzed in two regions: lymphocyte gate (ex vivo PBMC) and large lymphocyte blast gate (in vitro antigen stimulated PBMC). The limits for the quadrant markers were always set based on negative populations and isotype controls. Results are expressed as percentage of positive cells.
IL-10 cytokine measurement
Levels of IL-10 were measured in culture supernatants by ELISA according to the manufacturer’s instructions (R&D Systems, Emeryville, CA, USA). Samples were tested in duplicate and their concentration analyzed using the SOFTmax®PRO 4.0 program (Life Sciences Edition, Molecular Devices Corporation, USA). The sensitivity of the assay ranged from 31.25 to 2,000 pg/mL.
Statistical analysis
Because the data were not normally distributed, Kruskal-Wallis with post-test Dunns, to compare selected pars of columns, and Spearman rank correlations were performed using GraphPad Prism version 5.0 for Windows (GraphPad Software, San Diego, CA, USA). Notice that sample size differences were due to differences in the number of cells obtained for the experiments.
Results
Healing duration is inversely related to T cell activation levels
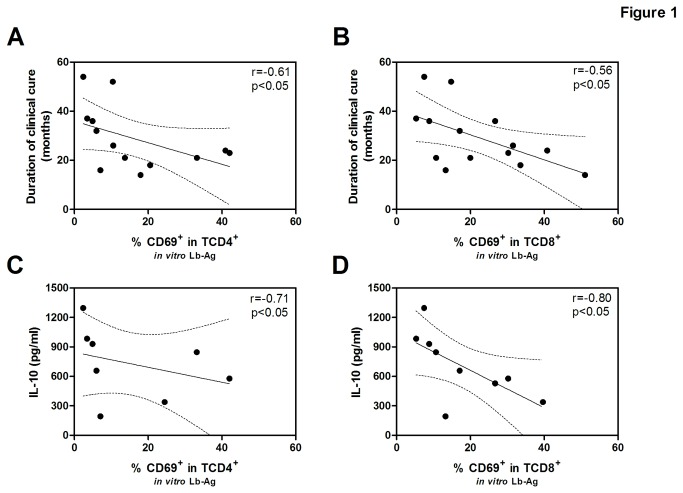
The percentage of CD69+ and CD25+ ex vivo and in Leishmania-reactive T CD4+ and TCD8+ cells in the two groups (hCL<2y and hCL>2-5y) is shown in Table 1. The ex vivo percentage of TCD4+ and TCD8+ cells presenting CD69 on the cell surface membrane was higher in recently cured patients than in healthy subjects (HS) (p<0.01 and p>0.05, respectively). In the hCL<2y group, Lb-Ag stimuli of PBMC increased the CD69+ percentage in TCD4+ (8.9-fold increase) and TCD8+ (8.1-fold increase) cells when compared to non-stimulated cells. The same was observed for, CD25+CD4+ (3.6-fold increase) and CD25+CD8+ (10-fold increase). However, CD69 levels in those patients in the hCL 2-5y group were not significantly different from those of HS, except for CD69+ in TCD8+. Accordingly, activation levels in Lb-Ag stimulated cells were inversely associated with duration of clinical cure, as observed for CD69+ in TCD4+ (r= -0.61, p<0.05, n=13), CD69+ in TCD8+ (r= -0.56, p<0.05, n=14) (Figures 1A and 1B, respectively), and CD25+ in TCD8+ (r= -0.69, p<0.001, n=22), but not for CD25+ in T CD4+ (r= -0.28, p>0.05, n=21).
Table 1. Comparative analysis of the percentage of activated T lymphocytes prior to and following Leishmania antigen stimulation in healed cutaneous leishmaniasis groups (hCL<2years: period since end of therapy lower than 2 years; hCL 2-5years: period since end of therapy from two to five years) and healthy subjects.
|
hCL < 2 years
|
hCL 2-5 years
|
Health Subjects
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T | Ex vivo | In vitro | Fold | P – value c | Ex vivo | In vitro | Fold | P – value c | Ex vivo | In vitro | Fold |
| lymphocyts | PBMC | Lb-Ag | increase b | PBMC | Lb-Ag | increase b | PBMC | Lb-Ag | increase b | ||
| stimulated | stimulated | stimulated | |||||||||
| %CD69+ | 6.3 a | 20.5 | 8.9±2.9 | <0.05 | 1.3 | 5.4 | 2.7±2.4 | n.s. | 1.1 | 1.4 | 1.5±1.1 |
| in T CD4+ | 3.7 - 30.7 | 13.7 - 40.9 | 0.8 - 2.5 | 3.2 - 10.4 | 0.6 - 2.9 | 1.1 - 5.6 | |||||
| n=7 | n=7 | n=8 | n=6 | n=8 | n=7 | ||||||
| %CD69+ | 5.9 | 30.2 | 8.1±6.3 | <0.01 | 1.9 | 14.7 | 2.6±2.3 | <0.05 | 2.5 | 2.9 | 1.5±1.4 |
| in T CD8+ | 2.0 - 8.3 | 13.3 - 40.8 | 0.9 - 3.0 | 7.5 - 26.7 | 1.7 - 3.6 | 2.1 - 5.1 | |||||
| n=7 | n=7 | n=8 | n=7 | n=8 | n=7 | ||||||
| %CD25+ | 9.4 | 40.9 | 3.6±1.7 | <0.05 | 8.1 | 17.2 | 3.0±1.7 | n.s. | 9.5 | 10.2 | 1.4±0.7 |
| in T CD4+ | 8.0 - 15.3 | 19.4 - 50.4 | 6.3 - 9.5 | 10.6 - 43.4 | 6.1 - 13.7 | 7.3 - 12.3 | |||||
| n=10 | n=11 | n=13 | n=10 | n=12 | n=10 | ||||||
| %CD25+ | 2.9 | 19.5 | 10.0±6.9 | <0.01 | 1.4 | 5.5 | 1.9±1.7 | n.s. | 1.2 | 1.6 | 2.6±1.8 |
| in T CD8+ | 1.2 - 10.0 | 10.5 - 55.4 | 0.7 - 2.5 | 3.7 - 8.7 | 0.8 - 2.3 | 1.2 -8.5 | |||||
| n=10 | n=11 | n=13 | n=11 | n=12 | n=10 | ||||||
Data expressed as median, 25th and 75th percentile and number of subjects evaluated.
Fold increase between in vitro Ag-Lb stimulated and non-stimulated cells. Values indicate the mean ± standard deviation.
Statistical significance of the difference in the percentage of activation of in vitro Leishmania braziliensis antigens (Lb-Ag) stimulated cells between the hCL groups (hCL<2years or hCL 2-5years) and healthy controls (HS), as determined by Kruskal-Wallis with post-test Dunns to compare selected pars of columns.
n.s. - not significant.
Figure 1. Correlation analysis of clinical and/or immunological parameters from healed cutaneous leishmaniasis subjects.
Correlation between the percentage of recently activated CD4+ (A, n=13) or CD8+ (B, n=14) T lymphocytes from PBMC after in vitro Lb-Ag stimulation and the duration of clinical cure; Correlation between the percentage of activated CD4+ (C, n=8) or CD8+ (D, n=9) T lymphocytes and the concentration of IL-10 from cell culture supernatant; Each point represents one subject. The graphs show the best fitted lines with 95% confidence intervals. r= correlation coefficient; p= significance level, (Spearman test).
Leishmania-activated T cell levels may be down-modulated by regulatory functions
As the percentage of CD69+ T cells induced by leishmanial antigens is lower in long-term healed CL patients, we examined whether regulatory factors could be associated with reduced lymphocyte activation. The results show a negative correlation between IL-10 levels following Lb-Ag stimuli in vitro and the percentage of T cells activated in terms of CD69 expression both in TCD4+ (r= -0.71, p<0.05, n=08) and TCD8+ cells (r= -0.80, p<0.05, n=09) (Figures 1C and 1D, respectively), indicating that IL-10 may play a role in controlling cell activation status.
The ability to renewal effector memory T cells is maintained long after cutaneous leishmaniasis healing
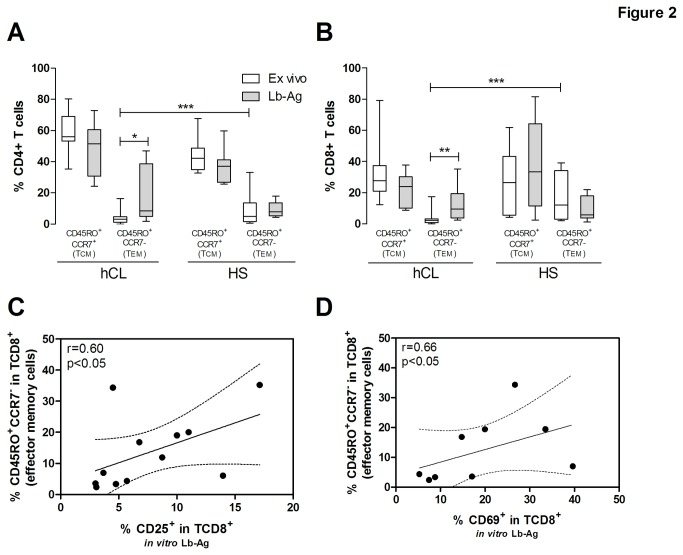
To determine if the progressive reduction of Leishmania-activated T cells was due to a putative loss of T cells able to recognize these antigens, central (Tcm) and effector (Tem) memory compartments were investigated. Tcm (CD45RO+CCR7+) and Tem (CD45RO+CCR7-) frequency from hCL with long time elapsed since clinical cure were analyzed ex vivo and after in vitro Lb-Ag stimuli. Ex vivo analyses show that these patients exhibit much lower percentages of Tem CD4+ (4.4%±4.4%, median=3.1%, n=15, p<0.001) and Tem CD8+ (3.3%±4.2%, median=2.3%, n=15, p<0.001) than HS (Figures 2A and 2B, respectively). However, a significant increase in the percentage of Tem CD4+ (18.6%±17.6%, median=8.4%, n=12, p<0.05; Figure 2A) and Tem CD8+ cells (13.7%±11.7%, median=9.4%, n=12, p<0.01; Figure 2B) was observed following exposure to leishmanial antigen. The results also show that renewal capacity was highly heterogeneous for hCL subjects, with two patients presenting much higher levels of Tem CD8+ (34.4% and 35.2%) than the median (9.4%).
Figure 2. Analysis of renewal capacity of memory compartments and activated T cell replenishment of healed cutaneous leishmaniasis subjects.
Renewal capacity of CD4+ (A) and CD8+ (B) T cells phenotipically characterized by central memory (Tcm, CD45RO+CCR7+) or effector memory (Tem, CD45RO+CCR7-). The white box and whiskers represent those cells evaluated immediately after PBMC (ex vivo) and the gray boxes represent those cells after in vitro stimuli with Lb-Ag. The central line represents median values. hCL= healed cutaneous leishmaniasis; HS= healthy subjects; *p<0.05, **p<0.01, ***p<0.001 (Kruskal-Wallis with post-test Dunns). Correlation analysis between the percentage of activated CD25+ (C, n=9) or CD69+ (D, n=12) in CD8+ T cells after in vitro Lb-Ag stimuli and the percentage of effector memory (CD45RO+CCR7-) CD8+ T cells after in vitro Lb-Ag stimuli. Each point represents one subject. The graphs show the best fitted lines with 95% confidence intervals. r= correlation coefficient; p= significance level, (Spearman test).
The percentage of Tem CD8+ renewed in response to leishmanial stimuli was positively associated with the level of antigen-activated CD8+ T cells (%CD25+ in TCD8+: r= 0.60, p<0.05, Figure 2C and %CD69+ in TCD8+: r= 0.66, p<0.05, Figure 2D), indicating that Tem CD8+ cells contribute to the generation of activated CD8+ T cells, with a possible effector function.
Discussion
T cell recall response to Leishmania antigens developed by long-term healed CL patients can mimic, at least partially, a possible re-exposure to this parasite due to re-infection or persistent parasite replication. In both cases, activation of specific memory clones is induced. Although previous studies have shown that Leishmania reactive CD4+ and CD8+ T cells are expanded in long-term cured patients, the functional characteristics of these cells remain to be determined. The present results indicate a reduction in the percentage of activated Leishmania-responder CD4+ and CD8+ T cells in healed CL patients, which depends on the time elapsed since clinical cure. Additionally, we have shown that such reduction might be associated with an increased participation of regulatory mechanisms in this process. Surprisingly, ex vivo analyses of peripheral blood show contracted Tem CD4+ and Tem CD8+ compartments in hCL patients, although renewal of these compartments was also observed following in vitro exposure to Leishmania stimuli. Finally, this increase of Tem CD8+ under Lb-Ag stimuli was positively correlated with the frequency of activated TCD8+ cells, but was not associated with cytotoxity phenotype.
Previous research has shown that L. braziliensis infection affects the activation of circulating T lymphocyte subtypes [13,14,33]. Here we show that healed CL patients maintain high levels of ex vivo activated TCD4+ and TCD8+ cells compared to HS controls. T cell activation levels only return to baseline (control) values approximately two years after the end of therapy. Indeed, except for the percentage of CD25+ in CD4+ T cells, CD4+ and CD8+ T cell activation levels are negatively associated with the duration of clinical cure. These data show that although disease is apparently limited to the skin, cells from other compartments (such as blood and lymphoid organs) are also affected, as similarly observed in non-human primates [23]. Additionally, the low frequency of Leishmania responder lymphocytes in the blood compared to that in lesions [34,35] suggests that both specific and nonspecific cells may be systemically activated, possibly by soluble factors released from Leishmania stimulated cells [36,37]. Our findings show that these phenotypic alterations take longer to return to normal status. Similarly, discrete to moderate inflammatory infiltrates are still detected in 1- and 3-year-old CL scars, reinforcing the idea that complete resolution of pathological damage also takes longer to occur [5].
An alternative possibility is that reminiscent parasites in the lymphonodes act as a source of antigenic stimuli, as observed in HIV infection [38]. We examined this possibility by investigating how circulating T cells responded to Leishmania antigens. Although a significant increase in CD69+ T cell subsets was observed for long-term hCL patients compared to healthy individuals, the increase of antigen-activated T cells was lower in the latter group than in recently cured individuals. This reduced T cell activation was to be expected, as high activation levels are associated with greater disease severity, including the occurrence of larger cutaneous lesions [13] and mucosal disease [14]. The reduction in parasite burden after therapy and consequent decrease of Leishmania-antigen cell stimulation has been indeed suggested to contribute to a homeostatic immune ambiance.
The magnitude of effector T cell responses can be controlled by regulatory T cells at the lesion site by suppressing lymphocyte proliferation [39]. For example, antigen-induced FoxP3 and IL-10 have been associated with an increased risk of cutaneous lesions in populations exposed to L. braziliensis [40]. Conversely, low levels of IL-10 and absence of IL-10 receptor are related to exacerbated immune responses in mucosal leishmaniasis [16,41,42], suggesting that a failure in the regulatory mechanism that down-modulates effector responses may lead to mucosal damage. Our results show higher IL-10 levels in cured patients presenting lower activation levels. Accordingly, the recruitment of Treg cells (CD4+CD25+IL-10+) has been reported in healing tissues of non-human primate models infected with L. braziliensis [23].
Memory T cell responses guarantee a rapid secondary reaction in re-exposure to Leishmania infection either by reinfection or persistent Leishmania replication [27,29]. This is the basis of immune protection against infectious diseases. In this study, although blood Tem compartment size in HS was consistent with that previously reported [43,44], blood Tem from long-term hCL was contracted, even more than two years following therapy. This is an unexpected result, especially considering the normal tendency of Tem expansion in successively experienced immune response [31,45]. As this phenomenon was seen in blood lymphocytes, it indicates that those cells that are specific for all other antigens, not Leishmania-specific, are globally affected. In contrast, patients cleared from filarial infection presented blood Tem levels similar to those of healthy subjects [44]. In addition, there were no age differences between early and late hCL individuals (a factor that could potentially affect these memory compartments) [45]. Further research is therefore needed to examine putative mechanisms that would enable Leishmania infection to affect the size of the effector memory compartment even long after the infection has been controlled.
Lymphocytes from long-term healed CL recognize leishmanial stimuli and proliferate upon exposure. Although blood Tem CD4+ and Tem CD8+ compartments were contracted, our results show that they can be renewed after a secondary challenge with parasite antigens, as suggested by the association between the increase in Tem and the levels of activated TCD8+. Moreover, leishmanial induced IFN-γ production is maintained in long-term healed CL [10,16]. However, it is interesting to take account that in murine model memory T cells can be maintained even in the absence of persistent L. major infection [24]. Altogether, these results indicate a capacity to generate anti-Leishmania immune effector mechanisms. Maintenance of this protective immune response along with regulatory effects is of paramount importance, since long-term hCL individuals cannot achieve sterile cure, once parasite persistence was detected in the blood [7,8], scars [6] and in lymph nodes of clinically cured subjects [46]. Two patients have also presented high levels of induction of antigen reactive Tem CD8+, pointing to a possible generation of exacerbated effector responses predisposing to mucosal disease [47]. While constant parasite stimulus leads to benefic induction of immunological memory, it can also contribute to chronic maintenance of activated effector cells. In addition to a poor regulatory response, exacerbated TCD8+ activity could, however, underlie an unfavorable fate with regard to ML [16,17,41,42,47,48].
One limitation of this study derives from the analysis of cells responsive to several Leishmania antigens, as opposed to the analysis of lymphocytes specific to dominant epitopes in viral infections [49,50]. Regardless, our results clearly showed that after stimuli with leishmanial antigens it was observed a frequency augment of activated lymphocytes or effector memory T cells derived from hCL. However, this study provides evidence of the presence of memory compartments in human leishmaniasis resulting from infection by L. braziliensis.
In conclusion, our results show that healed L. braziliensis infected patients exhibit a recall response to Leishmania antigens with evident expansion of effector memory T cells. They also indicate that regulatory mechanisms favor a down-modulation of activation status, which can prevent exacerbated immune responses without the loss of protective immunity. Which memory cell compartment should be preferentially expanded by a vaccine candidate is a crucial matter for discussion. The fact that we still do not have a vaccine effective against parasitic infections that rely on cellular immune response, and the finding that regulated leishmanial-specific response will emerge only about two years after initial contact with the parasite antigens, lead to the question: are clinical trials aimed at determining vaccine efficacy being conducted too early?
Acknowledgments
The authors thank Flow Cytometry Core of Oswaldo Cruz Institute, FIOCRUZ and Alessandro Marins dos Santos for flow cytometry acquisition. We also thank Mr. Ricardo dos Santos Nogueira for helping us with the experimental procedures and for helpful discussions. We are also grateful to Ms Rosangela Pellegrino for secretarial assistance.
This manuscript was reviewed by a professional science editor and by a native English-speaking copy editor to improve readability.
Funding Statement
This work was funded by IOC/FIOCRUZ internal funds, PAPESIV/VPPDT/FIOCRUZ (www.ioc.fiocruz.br), and by FAPERJ APQ-1 (grant number E-26/170•844/2003) (www.faperj.br). AG-S is a PhD student sponsored by CNPq (www.cnpq.br). COM-A received a postdoctoral scholarship from CAPES/FAPERJ (PAPDRJ - E-26/102.457/2010). AMD-C is a CNPq and FAPERJ (JCNE) fellowship researcher. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Carvalho EM, Johnson WD, Barreto E, Marsden PD, Costa JLM et al. (1985) Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J Immunol 135: 4144-4148. PubMed: 4067312Available: . PubMed: 4067312. [PubMed] [Google Scholar]
- 2. Mendonça SC, Coutinho SG, Amendoeira RR, Marzochi MC, Pirmez C (1986) Human American cutaneous leishmaniasis (Leishmania b. braziliensis) in Brazil: lymphoproliferative responses and influence of therapy. Clin Exp Immunol 64: 269-276. PubMed: 3742876Available: . PubMed: 3742876. [PMC free article] [PubMed] [Google Scholar]
- 3. Saravia NG, Valderrama L, Labrada M, Holguín AF, Navas C et al. (1989) The relationship of Leishmania braziliensis subspecies and immune response to disease expression in New World leishmaniasis. J Infect Dis 159: 725-735. doi: 10.1093/infdis/159.4.725. PubMed: 2647862Available: . PubMed: 2647862. [DOI] [PubMed] [Google Scholar]
- 4. Amaral V, Pirmez C, Gonçalves A, Ferreira V, Grimaldi G Jr (2000) Cell populations in lesions of cutaneous leishmaniasis of Leishmania (L.) amazonensis-infected Rhesus macaques, Macaca mulatta . Mem Inst Oswaldo Cruz 95: 209-216. doi: 10.1590/S0074-02762000000200012. PubMed: 10733740Available: . PubMed: 10733740. [DOI] [PubMed] [Google Scholar]
- 5. Morgado FN, Schubach A, Vasconcellos E, Azeredo-Coutinho RB, Valete-Rosalino CM et al. (2010) Signs of an in situ inflammatory reaction in scars of human American tegumentary leishmaniasis. Parasite Immunol 32: 285-295. doi: 10.1111/j.1365-3024.2009.01188.x. PubMed: 20398229Available: . PubMed: 20398229. [DOI] [PubMed] [Google Scholar]
- 6. Schubach A, Marzochi MC, Cuzzi-Maya T, Oliveira AV, Araujo ML et al. (1998) Cutaneous scars in American tegumentary leishmaniasis patients: a site of Leishmania (Viannia) braziliensis persistence and viability eleven years after antimonial therapy and clinical cure. Am J Trop Med Hyg 58: 824-827. PubMed: 9660473Available: . PubMed: 9660473. [DOI] [PubMed] [Google Scholar]
- 7. Vergel C, Palacios R, Cadena H, Posso CJ, Valderrama L et al. (2006) Evidence for Leishmania (Viannia) parasites in the skin and blood of patients before and after treatment. J Infect Dis 194: 503-511. doi: 10.1086/505583. PubMed: 16845635Available: . PubMed: 16845635. [DOI] [PubMed] [Google Scholar]
- 8. Oliveira-Camera P, Junger J, do Espirito Santo SPF, Mattos M, Oliveira-Neto MP et al. (2006) Haematogenous dissemination of Leishmania (Viannia) braziliensis in human American tegumentary leishmaniasis. Trans R Soc Trop Med Hyg 100: 1112-1117. doi: 10.1016/j.trstmh.2006.02.014. PubMed: 16765391Available: . PubMed: 16765391. [DOI] [PubMed] [Google Scholar]
- 9. Mendonça MG, de Brito ME, Rodrigues EH, Bandeira V, Jardim ML et al. (2004) Persistence of Leishmania parasites in scars after clinical cure of American cutaneous leishmaniasis: is there a sterile cure? J Infect Dis 189: 1018-1023. doi: 10.1086/382135. PubMed: 14999605Available: . PubMed: 14999605. [DOI] [PubMed] [Google Scholar]
- 10. Da-Cruz AM, Bittar R, Mattos M, Oliveira-Neto MP, Nogueira R et al. (2002) T-cell-mediated immune responses in patients with cutaneous or mucosal leishmaniasis: long-term evaluation after therapy. Clin Diagn Lab Immunol 9: 251-256. PubMed: 11874860Available: . PubMed: 11874860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zajtchuk JT, Casler JD, Netto EM, Grogl M, Neafie RC et al. (1989) Mucosal leishmaniasis in Brazil. Laryngoscope 99: 925-939. PubMed: 2671555Available: . PubMed: 2671555. [DOI] [PubMed] [Google Scholar]
- 12. Oliveira MR, Macêdo VO, Carvalho EM, Barral A, Marotti JG, et al. (1995) An evolutionary study of mucosal leishmaniasis (a 7- to 17-year follow-up) due to Leishmania (Viannia) braziliensis in Três Braços, Bahia. Rev Soc Bras Med Trop 28: 325-332Available online at: PMID: 8668831] [DOI] [PubMed]
- 13. Antonelli LR, Dutra WO, Almeida RP, Bacellar O, Carvalho EM et al. (2005) Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol Lett 101: 226-230. doi: 10.1016/j.imlet.2005.06.004. PubMed: 16083969Available: . PubMed: 16083969. [DOI] [PubMed] [Google Scholar]
- 14. Carvalho LP, Passos S, Bacellar O, Lessa M, Almeida RP et al. (2007) Differential immune regulation of activated T cells between cutaneous and mucosal leishmaniasis as a model for pathogenesis. Parasite Immunol 29: 251-258. doi: 10.1111/j.1365-3024.2007.00940.x. PubMed: 17430548Available: . PubMed: 17430548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bittar RC, Nogueira RS, Vieira-Gonçalves R, Pinho-Ribeiro V, Mattos MS et al. (2007) T-cell responses associated with resistance to Leishmania infection in individuals from endemic areas for Leishmania (Viannia) braziliensis . Mem Inst Oswaldo Cruz. 102: 625-630. doi: 10.1590/S0074-02762007005000069. PubMed: 17710308Available: . PubMed: 17710308. [DOI] [PubMed] [Google Scholar]
- 16. Gomes-Silva A, Bittar RC, Nogueira RS, Amato VS, Mattos MS et al. (2007) Can interferon-gamma and interleukin-10 balance be associated with severity of human Leishmania (Viannia) braziliensis infection? Clin Exp Immunol 149: 440-444. doi: 10.1111/j.1365-2249.2007.03436.x. PubMed: 17614975Available: . PubMed: 17614975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tuon FF, Gomes-Silva A, Da-Cruz AM, Duarte MI, Neto VA et al. (2008) Local immunological factors associated with recurrence of mucosal leishmaniasis. Clin Immunol 128: 442-446. doi: 10.1016/j.clim.2008.05.007. PubMed: 18585959Available: . PubMed: 18585959. [DOI] [PubMed] [Google Scholar]
- 18. Ji J, Masterson J, Sun J, Soong L (2005) CD4+CD25+ regulatory T cells restrain pathogenic responses during Leishmania amazonensis infection. J Immunol 174: 7147-7153. PubMed: 15905558Available: . PubMed: 15905558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Da-Cruz AM, Oliveira MP, De Luca PM, Mendonça SC, Coutinho SG (1996) Tumor necrosis factor-alpha in human American tegumentary leishmaniasis. Mem Inst Oswaldo Cruz. 91: 225-229. doi: 10.1590/S0074-02761996000200019. PubMed: 8736095Available: . PubMed: 8736095. [DOI] [PubMed] [Google Scholar]
- 20. Ribeiro-de-Jesus A, Almeida RP, Lessa H, Bacellar O, Carvalho EM (1998) Cytokine profile and pathology in human leishmaniasis. Braz J Med Biol Res 31: 143-148. doi: 10.1590/S0100-879X1998000100020. PubMed: 9686192Available: . PubMed: 9686192. [DOI] [PubMed] [Google Scholar]
- 21. Toledo VP, Mayrink W, Gollob KJ, Oliveira MA, Costa CA et al. (2001) Immunochemotherapy in American cutaneous leishmaniasis: immunological aspects before and after treatment. Mem Inst Oswaldo Cruz. 96: 89-98. doi: 10.1590/S0074-02762001000900013. PubMed: 11285479Available: . PubMed: 11285479. [DOI] [PubMed] [Google Scholar]
- 22. Mendes-Aguiar CO, Gomes-Silva A, Nunes E Jr, Pereira-Carvalho R, Nogueira RS et al. (2009) The skin homing receptor cutaneous leucocyte-associated antigen (CLA) is up-regulated by Leishmania antigens in T lymphocytes during active cutaneous leishmaniasis. Clin Exp Immunol 157: 377-384. doi: 10.1111/j.1365-2249.2009.03970.x. PubMed: 19664146Available: . PubMed: 19664146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de-Campos SN, Souza-Lemos C, Teva A, Porrozzi R, Grimaldi G Jr (2010) Systemic and compartmentalised immune responses in a Leishmania braziliensis-macaque model of self-healing cutaneous leishmaniasis. Vet Immunol Immunopathol 137: 149-154. doi: 10.1016/j.vetimm.2010.04.009. PubMed: 20546932Available: . PubMed: 20546932. [DOI] [PubMed] [Google Scholar]
- 24. Zaph C, Uzonna J, Beverley SM, Scott P (2004) Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat Med 10: 1104-1110. doi: 10.1038/nm1108. PubMed: 15448686Available: . PubMed: 15448686. [DOI] [PubMed] [Google Scholar]
- 25. Gollob KJ, Antonelli LR, Dutra WO (2005) Insights into CD4+ memory T cells following Leishmania infection. Trends Parasitol 21: 347-350. doi: 10.1016/j.pt.2005.06.007. PubMed: 15967724Available: . PubMed: 15967724. [DOI] [PubMed] [Google Scholar]
- 26. Okwor I, Uzonna J (2008) Persistent parasites and immunologic memory in cutaneous leishmaniasis: implications for vaccine designs and vaccination strategies. Immunol Res 41: 123-136. doi: 10.1007/s12026-008-8016-2. PubMed: 18389179Available: . PubMed: 18389179. [DOI] [PubMed] [Google Scholar]
- 27. Sallusto F, Lanzavecchia A, Araki K, Ahmed R ( 2010) From vaccines to memory and back. Immunity 33: 451-463. doi: 10.1016/j.immuni.2010.10.008. PubMed: 21029957Available: . PubMed: 21029957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Uzonna JE, Wei G, Yurkowski D, Bretscher P (2001) Immune elimination of Leishmania major in mice: implications for immune memory, vaccination, and reactivation disease. J Immunol 167: 6967–6974. PubMed: 11739516Available: . PubMed: 11739516. [DOI] [PubMed] [Google Scholar]
- 29. Scott P, Artis D, Uzonna J, Zaph C (2004) The development of effector and memory T cells in cutaneous leishmaniasis: the implications for vaccine development. Immunol Rev 201: 318-338. doi: 10.1111/j.0105-2896.2004.00198.x. PubMed: 15361250Available: . PubMed: 15361250. [DOI] [PubMed] [Google Scholar]
- 30. Bogdan C (2008) Mechanisms and consequences of persistence of intracellular pathogens: leishmaniasis as an example. Cell Microbiol 10: 1221-1234. doi: 10.1111/j.1462-5822.2008.01146.x. PubMed: 18363880Available: . PubMed: 18363880. [DOI] [PubMed] [Google Scholar]
- 31. Khamesipour A, Nateghi Rostami M, Tasbihi M, Miramin Mohammadi A, Shahrestani T et al. (2012) Phenotyping of circulating CD8+ T cell subsets in human cutaneous leishmaniasis. Microbes Infect 14: 702-711. doi: 10.1016/j.micinf.2012.02.006. PubMed: 22421108Available: . PubMed: 22421108. [DOI] [PubMed] [Google Scholar]
- 32. World Health Organization WHO (22-26 March 2010) Control of the leishmaniasis: report of a meeting of the WHO Expert Committee on the Control of Leishmaniasis, Geneva. World Health Organ Tech Rep Ser; no. 949: 1–xiii, 21485694. [Google Scholar]
- 33. Gaze ST, Dutra WO, Lessa M, Lessa H, Guimarães LH et al. (2006) Mucosal leishmaniasis patients display an activated inflammatory T-cell phenotype associated with a nonbalanced monocyte population. Scand J Immunol 63: 70-78. doi: 10.1111/j.1365-3083.2005.01707.x. PubMed: 16398703Available: . PubMed: 16398703. [DOI] [PubMed] [Google Scholar]
- 34. Conceição-Silva F, Dórea RC, Pirmez C, Schubach A, Coutinho SG (1990) Quantitative study of Leishmania braziliensis braziliensis reactive T cells in peripheral blood and in the lesions of patients with American mucocutaneous leishmaniasis. Clin Exp Immunol 79: 221-226. doi: 10.1111/j.1365-2249.1990.tb05182.x. PubMed: 2311299Available: . PubMed: 2311299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Da-Cruz AM, Oliveira-Neto MP, Bertho AL, Mendes-Aguiar CO, Coutinho SG (2010) T cells specific to Leishmania and other nonrelated microbial antigens can migrate to human leishmaniasis skin lesions. J Invest Dermatol 130: 1329-1336. doi: 10.1038/jid.2009.428. PubMed: 20107484Available: . PubMed: 20107484. [DOI] [PubMed] [Google Scholar]
- 36. Unutmaz D, Pileri P, Abrignani S (1994) Antigen-independent activation of naive and memory resting T cells by a cytokine combination. J Exp Med 180: 1159–1164. doi: 10.1084/jem.180.3.1159. PubMed: 8064232Available: . PubMed: 8064232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramanathan S, Gagnon J, Dubois S, Forand-Boulerice M, Richter MV et al. (2009) Cytokine synergy in antigen-independent activation and priming of naive CD8+ T lymphocytes. Crit Rev Immunol 29: 219-239. doi: 10.1615/CritRevImmunol.v29.i3.30. PubMed: 19538136Available: . PubMed: 19538136. [DOI] [PubMed] [Google Scholar]
- 38. Douek DC, Roederer M, Koup RA (2009) Emerging concepts in the immunopathogenesis of AIDS. Annu Rev Med 60: 471-484. doi: 10.1146/annurev.med.60.041807.123549. PubMed: 18947296Available: . PubMed: 18947296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Campanelli AP, Roselino AM, Cavassani KA, Pereira MS, Mortara RA et al. (2006) CD4+CD25+ T cells in skin lesions of patients with cutaneous leishmaniasis exhibit phenotypic and functional characteristics of natural regulatory T cells. J Infect Dis 193: 1313-1322. doi: 10.1086/502980. PubMed: 16586370Available: . PubMed: 16586370. [DOI] [PubMed] [Google Scholar]
- 40. Salhi A, Rodrigues V Jr, Santoro F, Dessein H, Romano A et al. (2008) Immunological and genetic evidence for a crucial role of IL-10 in cutaneous lesions in humans infected with Leishmania braziliensis . J Immunol 180: 6139-6148. PubMed: 18424735Available: . PubMed: 18424735. [DOI] [PubMed] [Google Scholar]
- 41. Bacellar O, Lessa H, Schriefer A, Machado P, Ribeiro de Jesus A et al. (2002) Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun 70: 6734-6740. doi: 10.1128/IAI.70.12.6734-6740.2002. PubMed: 12438348Available: . PubMed: 12438348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Faria DR, Gollob KJ, Barbosa J Jr, Schriefer A, Machado PR et al. (2005) Decreased in situ expression of interleukyn-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect Immun 73: 7853-7859. doi: 10.1128/IAI.73.12.7853-7859.2005. PubMed: 16299275Available: . PubMed: 16299275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Macallan DC, Wallace D, Zhang Y, De Lara C, Worth AT et al. (2004) Rapid turnover of effector-memory CD4+ T cells in healthy humans. J Exp Med 200: 255-260. doi: 10.1084/jem.20040341. PubMed: 15249595Available: . PubMed: 15249595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Steel C, Nutman TB (2011) Altered T cell memory and effector cell development in chronic lymphatic filarial infection that is independent of persistent parasite antigen. PLOS ONE. 6: e19197. doi: 10.1371/journal.pone.0019197. PubMed: 21559422Available: . PubMed: 21559422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou X, McElhaney JE (2011) Age-related changes in memory and effector T cells responding to influenza A/H3N2 and pandemic A/H1N1 strains in humans. Vaccine. 29: 2169-2177. doi: 10.1016/j.vaccine.2010.12.029. PubMed: 21353149Available: . PubMed: 21353149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bomfim G, Andrade BB, Santos S, Clarêncio J, Barral-Netto M et al. (2007) Cellular analysis of cutaneous leishmaniasis lymphadenopathy: insights into the early phases of human disease. Am J Trop Med Hyg 77: 854-859. PubMed: 17984342Available: . PubMed: 17984342. [PubMed] [Google Scholar]
- 47. Brodskyn CI, Barral A, Boaventura V, Carvalho E, Barral-Netto M (1997) Parasite-driven in vitro human lymphocyte cytotoxicity against autologous infected macrophages from mucosal leishmaniasis. J Immunol 159: 4467-4473. PubMed: 9379046Available: . PubMed: 9379046. [PubMed] [Google Scholar]
- 48. Nylén S, Gautam S (2010) Immunological perspectives of leishmaniasis. J Glob Infect Dis 2: 135-146Available online at: PMID: 20606969] [DOI] [PMC free article] [PubMed]
- 49. Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM et al. (2002) Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med 8: 379-385. doi: 10.1038/nm0402-379. PubMed: 11927944Available: . PubMed: 11927944. [DOI] [PubMed] [Google Scholar]
- 50. Munitic I, Decaluwe H, Evaristo C, Lemos S, Wlodarczyk M et al. (2009) Epitope specificity and relative clonal abundance do not affect CD8 differentiation patterns during lymphocytic choriomeningitis virus infection. J Virol 83: 11795-11807. doi: 10.1128/JVI.01402-09. PubMed: 19726518Available: . PubMed: 19726518. [DOI] [PMC free article] [PubMed] [Google Scholar]