Abstract
Sorafenib, the first agent developed to target BRAF mutant melanoma, is a multi-kinase inhibitor that was approved by the FDA for therapy of kidney and subsequently liver cancer, and is currently in clinical trials for thyroid, lung and brain cancer. Colorectal cancer with V600E BRAF mutation has shown relative resistance to standard chemotherapy regimens, as well as lack of efficacy to vemurafenib in clinical trials. New treatments are needed for BRAF-mutant colorectal cancer. We report a case of a patient with BRAF-mutant metastatic colon cancer whose disease had progressed on FOLFOX plus bevacizumab and subsequent FOLFIRI plus cetuximab. Based on preclinical data published in Nature in 2012 suggesting that successful therapeutic targeting of BRAF in colorectal cancer may require concomitant targeting of the EGFR, we offered this patient without other attractive options the combination of sorafenib plus cetuximab, in off-label use with informed consent. Sorafenib and cetuximab therapy led to a mixed radiographic response with some areas showing dramatic improvement and other areas showing stable disease over a 7-month period which is a notably long period of progression-free survival for V600E BRAF mutated colon cancer. The cetuximab plus sorafenib therapy was very well-tolerated by the patient who remained on it long enough until another therapy option, regorafenib, was approved in September 2012. The patient was offered single agent regorafenib at the time of progression. At the time of progression on single agent regorafenib, panitumumab was combined with regorafenib and this was also well-tolerated and appeared to slow disease progression. Further study of these approaches in the clinic as personalized treatment of BRAF-mutant advanced colorectal cancer is warranted.
Keywords: sorafenib, BAY43-9006, cetuximab, sorafenib and cetuximab, combined therapy, colorectal cancer therapy, RAF inhibitor
Introduction
Sorafenib (BAY 43-9006), a multi-kinase inhibitor, has been shown to inhibit tumor growth and tumor angiogenesis by targeting Raf kinase, vascular endothelial growth factor receptor, c-kit and platelet-derived growth factor receptor.1-3 In previous studies, sorafenib demonstrated single-agent activity in patients with advanced solid tumors like renal cell carcinoma and hepatocellular carcinoma and has been approved by the FDA for the treatment of these tumors. In addition, sorafenib was studied in combination with oxaliplatin and mTOR (mammalian target of rapamycin) inhibitors in multiple preclinical studies. In phase I and phase II trials in colorectal cancer, continuous oral sorafenib 400 mg twice daily was safely combined with oxaliplatin, mTOR inhibitors, and irinotecan without detectable drug interactions and showed preliminary anti-tumor activity. A subset of patients with colorectal cancers carries V600E BRAF mutation which renders their tumors more aggressive and more rapidly refractory to available therapies. This suggested the possibility of adding a BRAF tyrosine kinase inhibitor as a possible therapeutic strategy to control those aggressive tumors.4 Based on a report in Nature in 2012, our patient was treated with sorafenib plus cetuximab as salvage off-label therapy for metastatic BRAF-mutant colorectal cancer to prevent EGFR activation feedback when BRAF is suppressed.8 The therapy was very well-tolerated by the patient who remained on it long enough until regorafenib was approved by the FDA, and the patient was offered single agent regorafenib at the time of disease progression. At the time of progression on single agent regorafenib, panitumumab was combined with regorafenib and this was also well-tolerated and appeared to slow disease progression. Those approaches will need further investigation as personalized therapy options for patients with BRAF-mutant metastatic colorectal cancer.
Case Report
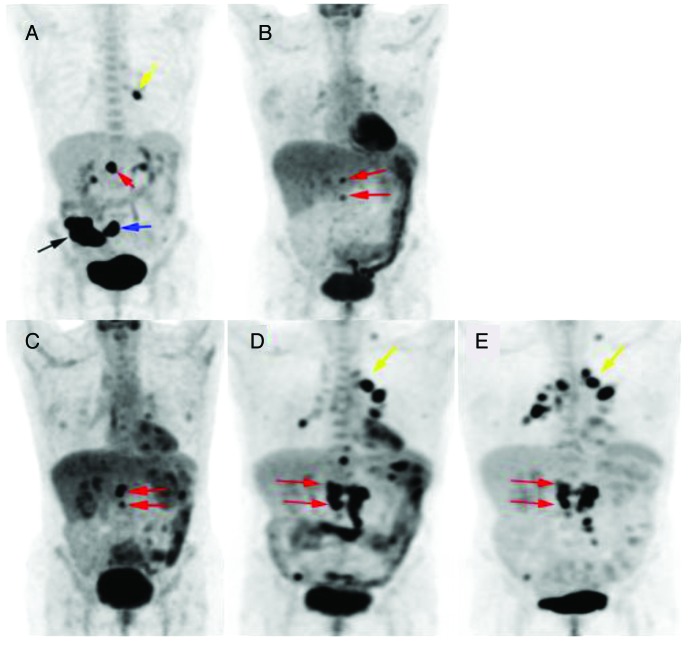
The patient is a 60-years-old Caucasian woman who presented to her primary care physician with blood in her stool on a routine office visit. This led to a colonoscopy and biopsy, which revealed a poorly differentiated adenocarcinoma of her ascending colon. A PET/CT demonstrated a large ascending colon mass, as well as left hilar, mesenteric, and aortocaval lymphadenopathy that were strongly FDG-avid. In addition, there were mildly FDG-avid masses in the left lower and upper lobes of the lung (Figs. 1, 2, 3, 4). A needle biopsy of the left lower lobe lung mass confirmed a diagnosis of metastatic colon cancer. She underwent an exploratory laparotomy with right hemicolectomy and right salpingo-oophorectomy. Surgical pathology revealed invasive adenocarcinoma with mucinous features and 7 out of 20 regional lymph nodes were positive for tumor (Figs. 5 and 6). Her primary tumor showed a variety of different morphologies including mucinous, well-differentiated glandular and poorly differentiated solid areas, and the metastatic tumor was mainly mucinous carcinoma. Heterogeneity is a common feature of colon cancer with MSI-H (High frequency of microsatellite instability). In addition, her pathology revealed an aberrant marker expression with CK7 positivity. Her CEA levels dropped from 402.1 to 184.3 ng/mL after surgery. She recovered well and in view of her stage IV metastatic disease, she was started on chemotherapy post-operatively with FOLFOX plus bevacizumab (oxaliplatin 100 mg/m2, leucovorin 400 mg/m2, 5FU 400 mg/m2, bevacizumab 5 mg/kg on days 1 and 15 with 46 h infusional 5FU at 2400 mg/m2 that was adjusted by pharmacokinetically-guided drug levels to 2633 mg/m2).
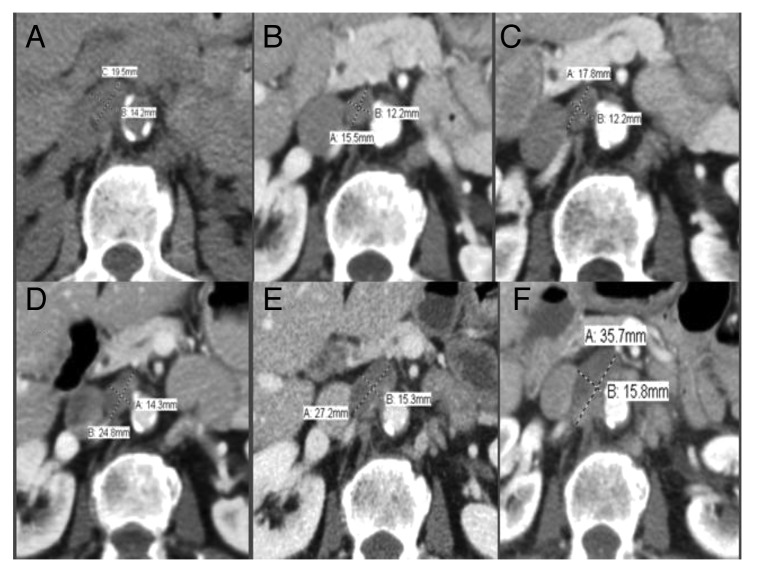
Figure 1. Slight disease progression in an upper aortocaval lymph node in response to sorafenib as revealed by PET-CT. (A) 6/1/11: 2.0 × 1.4 cm. (B) 11/7/11: 1.2 × 1.6 cm. (C) 1/17/12: 1.8 × 1.2 cm. (D) 4/13/12: 2.5 × 1.4 cm. (E) 6/26/12: 2.7 × 1.5 cm. (F) 11/02/12: 3.6 × 1.6 cm.
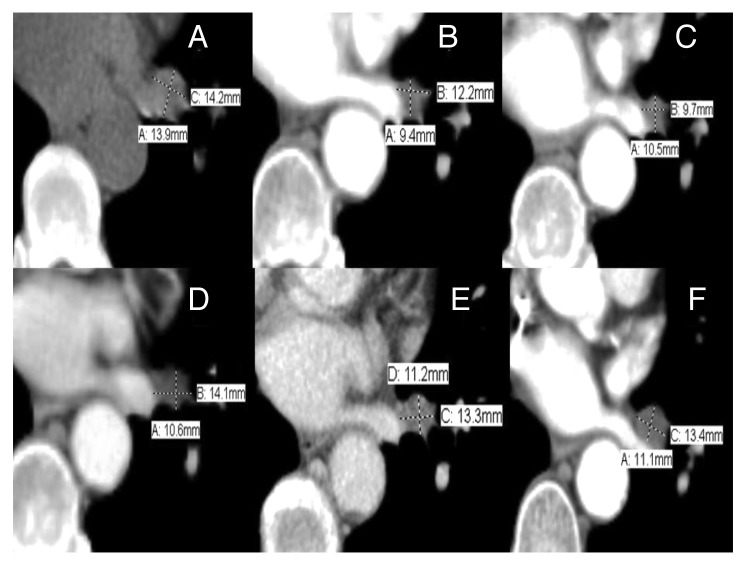
Figure 2. Objective response of left hilum lymph node by PET-CT revealing stable disease in response to Sorafenib. (A) 6/1/11: 1.4 × 1.4 cm. (B) 11/7/11: 1.2 × 0.9 cm. (C) 1/17/12: 1.0 × 1.1 cm. (D) 4/13/12: 1.4 × 1.1 cm. (E) 6/26/12: 1.3 × 1.1 cm. (F) 11/2/12: 1.3 × 1.1 cm.
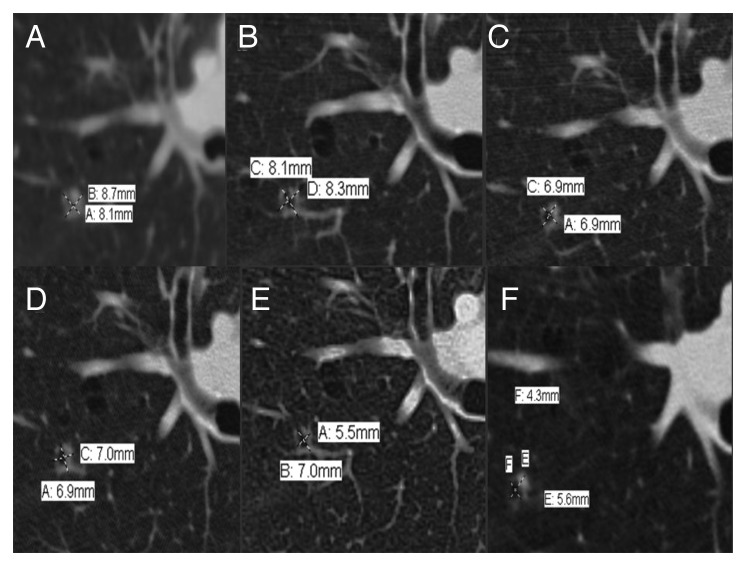
Figure 3. Objective response of right upper lobe lung nodule by PET-CTs showing stable disease: (A) 6/11/11: 0.9 × 0.8 cm with nodular component. (B) 11/7/11: 0.8 × 0.8 cm. (C) 1/17/12: 0.7 × 0.7 cm. (D) 4/13/12: 0.7 × 0.7 cm. (E) 6/26/12: 0.6 × 0.7 cm. (F) 11/2/12: 0.4 × 0.6 cm
Figure 4. Whole body PET images with overview of disease activity and response to treatment. (A) 6/1/11, (B) 1/17/12, (C) 4/13/12 (just prior to starting sorafenib), (D) 11/2/12 (slight disease progression with sorafenib use) and (E) 1/24/13. Black arrow, cecal mass; blue arrow, mesenteric lymph nodes; red arrows, aortocaval lymph nodes; yellow arrow, left hilum lymph node.
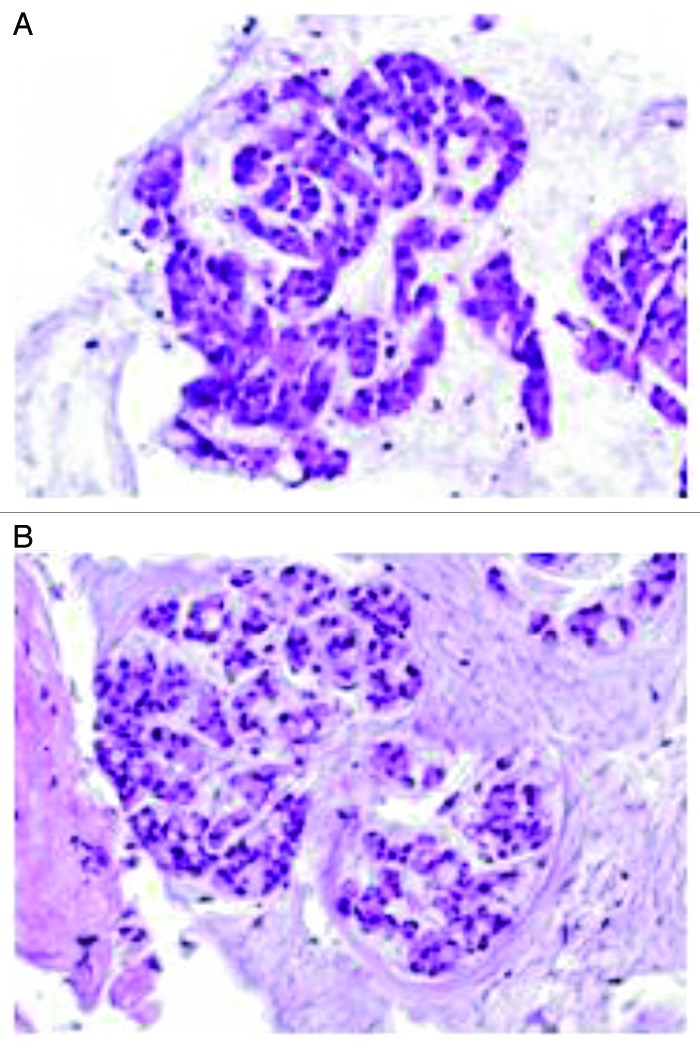
Figure 5. Colon resection specimen shows various morphologies in the primary tumor. (A) Area of well-differentiated adenocarcinoma; (B) area of mucinous adenocarcinoma; and (C) area of poorly differentiated adenocarcinoma. (Hematoxylin and eosin stain, original magnification × 400).
Figure 6. Metastatic adenocarcinoma shows predominant mucinous component. (A) Lung biopsy in 2011; (B) lymph node biopsy 2012. (Hematoxylin and eosin stain, original magnification × 400).
Repeat imaging after 3 cycles showed multiple enlarged lymph nodes in the chest and abdomen which were less FDG-avid and there was a consistent downward trend in her CEA levels (her CEA dropped to 17 ng/mL after cycle 3). The patient required a dose reduction of oxaliplatin to 80 mg/m2 due to paresthesia, but continued on the above therapy with persistent benefit. However, her CEA started to rise after the fourth cycle of therapy. Her next PET/CT revealed increased FDG avidity in the enlarged aortocaval nodes which represented disease progression. She was switched to mFOLFIRI plus cetuximab after 6 cycles (irinotecan 180mg/m2, leucovorin 400 mg/m2, 5FU 400 mg/m2, cetuximab 500 mg/m2 on days 1 and 15 with 46 h infusional 5FU again at 2633 mg/m2 based on prior drug levels). Her genetic work up had revealed a wild-type KRAS gene and a BRAF V600E mutation (seen in 8–10% of colorectal carcinoma),6 which predicts a worse prognosis and less response to panitumumab or cetuximab as compared with BRAF wild-type tumors in metastatic colorectal cancer.5-7
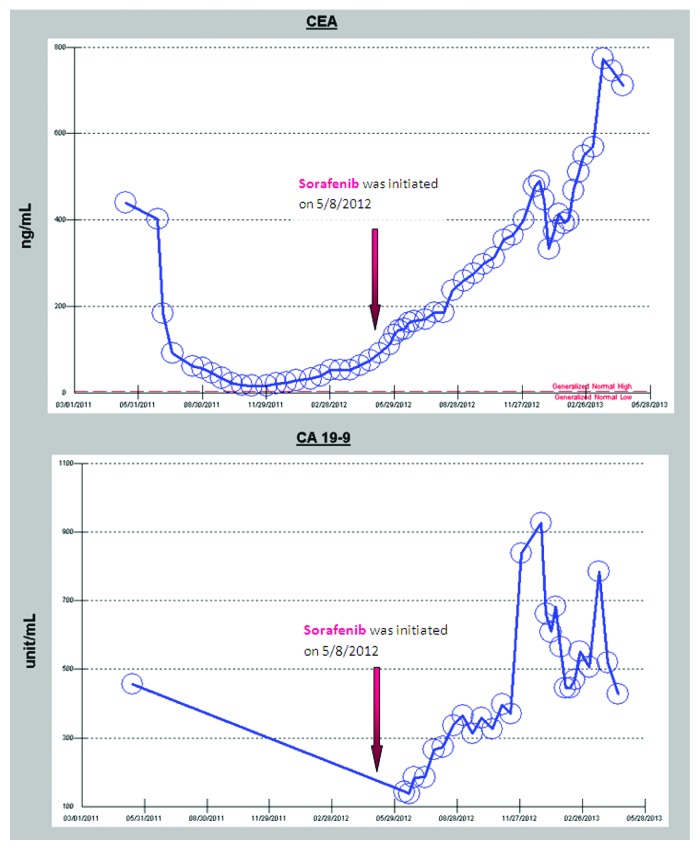
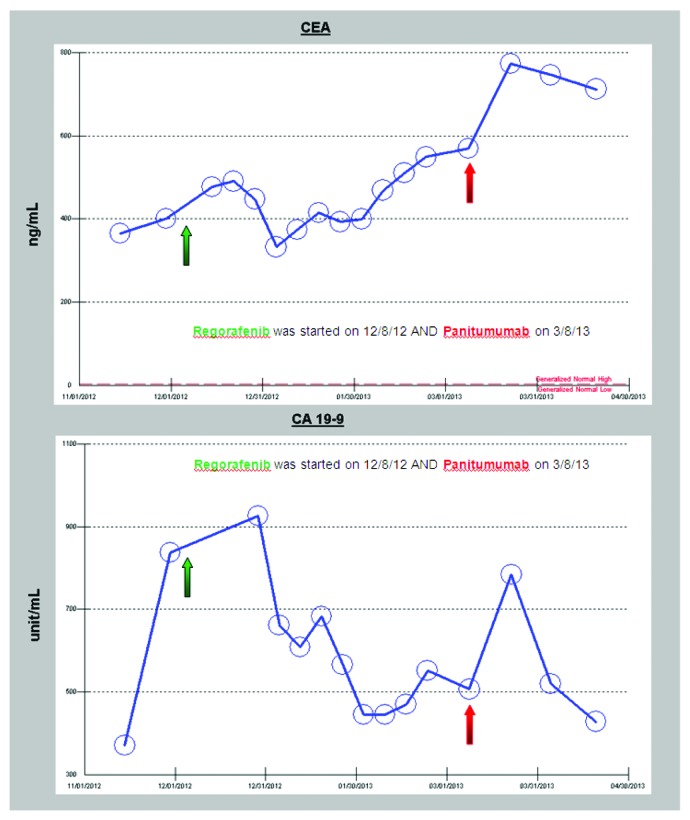
Despite the treatment with FOLFIRI plus cetuximab, her CEA levels progressively rose and a repeat PET/CT after 3 cycles revealed increased size of the aortocaval nodes. After an extensive multidisciplinary discussion of treatment options, the patient opted to treatment with an off-label combination therapy of twice daily sorafenib (400 mg twice daily), a BRAF/multi-kinase inhibitor and weekly cetuximab (500 mg/m2). This regimen, although unproven in any clinical trials, was based on recent discoveries that BRAF inhibitors such as vemurafenib show limited effect in V600E BRAF mutant colon cancer due to rapid feedback activation of EGFR activation which compensates for BRAF inhibited cell proliferation.8 She started sorafenib and weekly cetuximab three weeks post her last PET/CT scan. Her follow-up evaluations revealed continuous slow rise of her CEA and CA19-9 levels while on this regimen (Fig. 7); however, her 2 mo follow-up PET/CT scan showed an apparent mixed response with increase in size of the aortocaval nodes but decrease/near resolution of the lung nodules (Figs. 1–4). She, therefore, was continued on the same regimen for 7 mo with excellent quality of life, exceeding the expected survival for the population of patients with BRAF-mutant colorectal cancer. It is clear to the clinicians who cared for this patient that the trajectory of her disease progression was favorably impacted by the combined BRAF and EGFR inhibitor approach over a period of seven months, and the therapy was very well-tolerated with excellent quality of life/minimal toxicity. She has since been switched to regorafenib 160 mg daily for days 1 through 21 of a 28 d cycle, another recently approved multi-kinase inhibitor with structure very similar to sorafenib. After three and a half months of monotherapy on regorafenib the EGFR inhibitor panitumumab (6 mg/kg every 14 d) was added to avoid resistance that may have developed against cetuximab leading to prior progression. This combination led to improvement in CEA and CA19-9 levels after she started to progress on monotherapy, (Fig. 8). Both the single agent regorafenib and combination of regorafenib plus panitumumab were well-tolerated in this patient. Tumor marker trends since diagnosis are shown in Figures 7 and 8.
Figure 7. CEA and CA 19-9 responses since diagnosis and also in response to sorafenib plus cetuximab therapy.
Figure 8. CEA and CA 19-9 responses to regorafenib and combination of regorafenib plus panitumumab began on 3/8/13.
Discussion
The development of targeted therapies has provided new options for the personalized management of patients with advanced solid tumors.
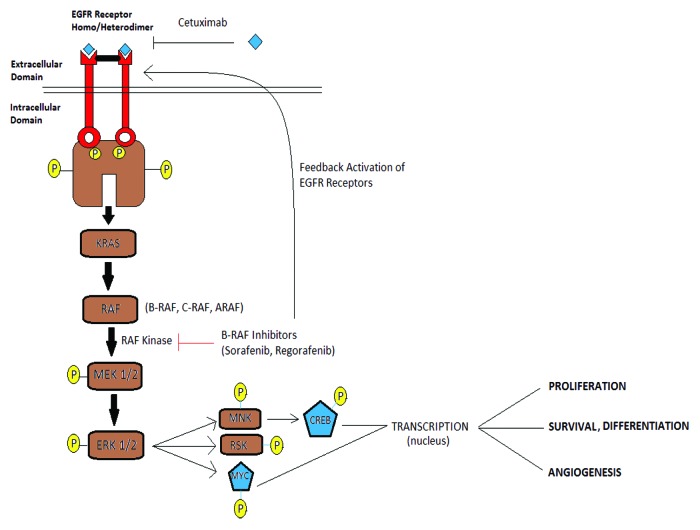
Sorafenib (BAY43-9006) is an oral multikinase inhibitor that can block the Ras/Raf/MEK/ERK signaling cascade that is important for the growth of solid tumors.1,3,9 The MEK-ERK signaling pathway is a downstream target of oncogenic Raf mutations9-11 that occur in ~10% of human colorectal carcinomas.11 Sorafenib also targets several other receptor tyrosine kinases, including vascular endothelial growth factor receptor 2 (VEGFR2), platelet-derived growth factor receptor (PDGFR), FLT3, Ret, and c-Kit.1,2 Sorafenib has shown preclinical activity against a variety of tumor types and is a standard treatment for hepatocellular and renal cell carcinomas12,13 (Fig. 9). Multiple targeted therapeutic agents have been studied based on their predicted pathway inhibition to see their role in colorectal cancer.14
Figure 9. EGFR and BRAF/ERK signaling pathway.
Sorafenib has been shown to enhance TRAIL (TNF-related apoptosis-inducing ligand)-mediated apoptosis in human leukemia and colon cancer cell lines.20 It has also shown efficacy when combined with radiation; radiation treatment followed sequentially by sorafenib was found to be associated with the greatest delay of the xenograft tumor growth.15 Using sorafenib as a single agent or in a combination with other chemotherapy has shown efficacy and safety when combined with other cancer-related therapeutic agents.16-19
Sorafenib plus irinotecan shows acceptable toxicity and promising activity as a second-line treatment for patients with metastatic colorectal cancer (mCRC) with KRAS mutated tumors, according to a previous study presented at the European Society for Medical Oncology’s 13th World Congress on Gastrointestinal Cancer (ESMO-GI).
Sorafenib is generally well tolerated and has shown promise in a variety of malignancies24-26 including gastrointestinal malignancies,19 renal cell carcinoma,21 hepatocellular carcinoma,22 and lung cancer.
Combination of rapamycin with sorafenib synergistically inhibits proliferation of CRC cells via abrogating rapamycin-induced activation of PI3K/Akt and Ras-MAPK signaling pathways.23 CRCs harboring coexistent KRAS and PIK3CA mutations are partially sensitive to either rapamycin or sorafenib monotherapy, but highly sensitive to combination treatment with rapamycin and sorafenib. Combination with sorafenib enhances therapeutic efficacy of rapamycin on induction of apoptosis and inhibition of cell cycle progression, migration, and invasion of CRCs.23 Similarly, we hypothesize that the combination of sorafenib and cetuximab may demonstrate efficacy and safety at inhibiting growth of xenografts from CRC cells with existent V600E mutation in BRAF in the presence of wild-type KRAS gene, which predicts a worse prognosis as compared with BRAF wild-type tumors. This BRAF V600E gene mutation is seen in 8–10% of colorectal carcinoma.6
Sorafenib has proven efficacy as a single-agent in renal cell carcinoma and hepatocellular carcinoma, and there is a strong rationale for investigating its use in combination with other agents. In particular, targeting multiple Raf isoforms with sorafenib may help to overcome resistance to other agents, while the ability of sorafenib to induce apoptosis may increase the cytotoxicity of chemotherapeutic agents. Based on positive results in preclinical studies, further investigation in phase I and II studies has shown potential antitumor activity when sorafenib is combined with cytotoxic agents in different solid tumors, including hepatocellular carcinoma and melanoma. Promising results have been reported in phase I and II studies of sorafenib combined with paclitaxel and carboplatin, with oxaliplatin in gastric and colorectal cancer,17,18 with docetaxel in breast cancer, with gemcitabine in ovarian cancer and with capecitabine in different solid tumors. Phase II and III studies are currently investigating the use of sorafenib in combination with different agents in a variety of solid tumors. Our case supports the safety of the combined therapy of sorafenib and cetuximab in a patient with colorectal carcinoma who experienced an excellent quality of life with slowed disease progression and mixed radiographic response. A current NIH study, NCT 00326495 sponsored by National Cancer Institute (NCI), is recruiting participants with colorectal carcinoma to evaluate the efficacy and safety of combining sorafenib and cetuximab to assess this combination efficacy and safety.27
To assess sorafenib with cetuximab in treating metastatic colorectal cancer, 35 patients with metastatic colorectal cancer were enrolled in a previous study and were randomized to receive cetuximab with or without oral sorafenib. Patients received cetuximab i.v. weekly for 4 weeks and oral sorafenib twice daily on days 1–28, with recycling every 4 weeks. The primary end point was the response rate (partial and complete), while the secondary end points were the adverse effects, time to progression and overall survival. That study revealed that the partial response was higher in cetuximab–sorafenib, which constituted 33.3% compared with 17.6% in the cetuximab group but with a P value of 0.44. Progression-free survival had a statistically higher significant difference in wild-type KRAS as compared with mutant KRAS cases (P = 0.0001). Median overall survival was seven and five months in the sorafenib plus cetuximab and cetuximab groups respectively with P value of 0.49. The conclusion was that KRAS and BRAF was a predictor of response, so genotyping of the tumors was needed for defining the patient population that was likely to benefit from the targeted therapy. A combination of therapy that simultaneously targets KRAS and BRAF could be a useful approach to increase the number of patients who may benefit from anti-EGFR therapy.28
BRAF inhibitors might be improtant agents in manging advance colon cancer after failure of front-line treatments. A list of the BRAF inhibitors is shown in Table 1. Regorafenib was approved in 2012 for advanced metastatic colorectal cancer that has progressed on other therapies. We explored in this article the outcome of combined sorafenib and cetuximab for treatment of colorectal cancer. It is interesting that use of regorafenib after the patient’s tumor progressed on sorafenib plus cetuximab was associated with some response and subsequent combination of regorafenib plus panitumumab was well tolerated and was associated with slowing of progression. The results reveal the safety and show an activity of combined agents of sorafenib and cetuximab against colorectal cancer, revealing novel effects of sorafenib on anti-apoptotic signaling mediators and suggesting the combination of sorafenib plus cetuximab as possible combined therapy for advanced colorectal cancer.
Table 1. List of available BRAF inhibitors.
| Name of drug | Type | Targets | Stage of development |
|---|---|---|---|
| Sorafenib tosylate (Bay 43-9006 or Nexavar) |
Multi TKI |
Raf-1, B-Raf, VEGFR2/3, PDGFRβ, Flt 3, and c-KIT |
Approved for RCC and HCC |
| Vemurafenib (RG7204 or PLX4032 or Zelboraf) |
Multi TKI |
B-RafV600E, C-Raf, MAP4K5 (KHS1), ACK-1, FGR, and SRMS |
Approved for late-stage melanoma with BRAF mutation, phase I in CRC29 |
| Regorafenib (BAY 73-4506 or fluoro-sorafenib or Stivarga) |
Multi TKI |
Raf- 1, VEGFR1/2/3, PDGFRβ, Kit, and RET |
Approved in CRC, phase III for GIST |
| RAF265 (CHIR-265) |
Selective inhibitor of B-Raf and VEGFR2 |
B-Raf and VEGFR2 |
Phase II in metastatic melanoma, phase Ib in advanced solid tumors with BRAF V600E mutation or RAS mutation in combination with MEK162 |
| XL281 (BMS-908662) |
Selective RAF kinase inhibitor |
B-RAF, C-RAF, and the B-RAFV600E |
Phase I/II in CRC30,31 |
| SB-59088532 |
Selective BRAF kinase inhibitor |
B-RafV600E |
|
| GDC-0879 |
Selective B-Raf inhibitor for B-RafV600E |
B-RafV600E |
In vivo mice studies |
| PLX-4720 |
Selective inhibitor of B-RafV600E and c-Raf-1Y340D/Y341D |
B-RafV600E and c-Raf-1Y340D/Y341D |
In vivo mice studies |
| AZ628 | Multi TKI | Wild-type CRAF, BRAFV600E, and wild-type BRAF | In vitro studies |
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/25191
References
- 1.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 2.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–40. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adjei AA. Blocking oncogenic Ras signaling for cancer therapy. J Natl Cancer Inst. 2001;93:1062–74. doi: 10.1093/jnci/93.14.1062. [DOI] [PubMed] [Google Scholar]
- 4.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 5.Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–12. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 6.Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–74. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 7.Richman SD, Seymour MT, Chambers P, Elliott F, Daly CL, Meade AM, et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27:5931–7. doi: 10.1200/JCO.2009.22.4295. [DOI] [PubMed] [Google Scholar]
- 8.Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–3. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 9.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 10.Hilger RA, Scheulen ME, Strumberg D. The Ras-Raf-MEK-ERK pathway in the treatment of cancer. Onkologie. 2002;25:511–8. doi: 10.1159/000068621. [DOI] [PubMed] [Google Scholar]
- 11.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 12.Lang L. FDA approves sorafenib for patients with inoperable liver cancer. Gastroenterology. 2008;134:379. doi: 10.1053/j.gastro.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 13.Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006;12:7271–8. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- 14.Joudeh J, Allen JE, Das A, Prabhu V, Farbaniec M, Adler J, et al. Novel antineoplastics targeting genetic changes in colorectal cancer. Adv Exp Med Biol. 2013;779:1–34. doi: 10.1007/978-1-4614-6176-0_1. [DOI] [PubMed] [Google Scholar]
- 15.Plastaras JP, Kim SH, Liu YY, Dicker DT, Dorsey JF, McDonough J, et al. Cell cycle dependent and schedule-dependent antitumor effects of sorafenib combined with radiation. Cancer Res. 2007;67:9443–54. doi: 10.1158/0008-5472.CAN-07-1473. [DOI] [PubMed] [Google Scholar]
- 16.Moore M, Hirte HW, Siu L, Oza A, Hotte SJ, Petrenciuc O, et al. Phase I study to determine the safety and pharmacokinetics of the novel Raf kinase and VEGFR inhibitor BAY 43-9006, administered for 28 days on/7 days off in patients with advanced, refractory solid tumors. Ann Oncol. 2005;16:1688–94. doi: 10.1093/annonc/mdi310. [DOI] [PubMed] [Google Scholar]
- 17.Kupsch P, Henning BF, Passarge K, Richly H, Wiesemann K, Hilger RA, et al. Results of a phase I trial of sorafenib (BAY 43-9006) in combination with oxaliplatin in patients with refractory solid tumors, including colorectal cancer. Clin Colorectal Cancer. 2005;5:188–96. doi: 10.3816/CCC.2005.n.030. [DOI] [PubMed] [Google Scholar]
- 18.Penland SK, Goldberg RM. Combining anti-VEGF approaches with oxaliplatin in advanced colorectal cancer. Clin Colorectal Cancer. 2004;4(Suppl 2):S74–80. doi: 10.3816/CCC.2004.s.012. [DOI] [PubMed] [Google Scholar]
- 19.Heim M, Scharifi M, Zisowsky J, Jaehde U, Voliotis D, Seeber S, et al. The Raf kinase inhibitor BAY 43-9006 reduces cellular uptake of platinum compounds and cytotoxicity in human colorectal carcinoma cell lines. Anticancer Drugs. 2005;16:129–36. doi: 10.1097/00001813-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Ricci MS, Kim SH, Ogi K, Plastaras JP, Ling J, Wang W, et al. Reduction of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or sorafenib sensitizes resistant human cancer cells to TRAIL-induced death. Cancer Cell. 2007;12:66–80. doi: 10.1016/j.ccr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Ratain MJ, Eisen T, Stadler WM, et al. Final findings from a Phase II, placebo controlled, randomized discontinuation trial (RDT) of sorafenib (BAY 43-9006) in patients with advanced renal cell carcinoma (RCC) Proc Am Assoc Cancer Res. 2005;23:388s. [Google Scholar]
- 22.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of BAY 43-9006 in patients with advanced hepatocellular carcinoma (HCC) Eur J Cancer. 2004;2:16. doi: 10.1016/S1359-6349(04)80050-8. [DOI] [Google Scholar]
- 23.Gulhati P, Zaytseva YY, Valentino JD, Stevens PD, Kim JT, Sasazuki T, et al. Sorafenib enhances the therapeutic efficacy of rapamycin in colorectal cancers harboring oncogenic KRAS and PIK3CA. Carcinogenesis. 2012;33:1782–90. doi: 10.1093/carcin/bgs203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–72. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 25.Awada A, Hendlisz A, Gil T, Bartholomeus S, Mano M, de Valeriola D, et al. Phase I safety and pharmacokinetics of BAY 43-9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer. 2005;92:1855–61. doi: 10.1038/sj.bjc.6602584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark JW, Eder JP, Ryan D, Lathia C, Lenz HJ. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005;11:5472–80. doi: 10.1158/1078-0432.CCR-04-2658. [DOI] [PubMed] [Google Scholar]
- 27.BAY 43-9006 Plus Cetuximab to Treat Colorectal Cancer. Identifier: NCT00326495. Available online at http://clinicaltrials.gov/ct2/show/NCT00326495? Accessed 5/1/13.
- 28.Galal KM, Khaled Z, Mourad AM. Role of cetuximab and sorafenib in treatment of metastatic colorectal cancer. Indian J Cancer. 2011;48:47–54. doi: 10.4103/0019-509X.75825. [DOI] [PubMed] [Google Scholar]
- 29.Safety Study of PLX4032 in patients with Solid Tumors. Identifier No. -NCT00405587. Available online at http://www.clinicaltrials.gov/ct2/show/NCT00405587 Accessed 5/1/13
- 30.Safety and Efficacy Study of BMS-908662 Alone or in Combination With Cetuximab in Subjects With K-RAS or B-RAF Mutation Positive Advanced or Metastatic Colorectal Cancer. Identifier No. - NCT01086267. Available online at http://clinicaltrials.gov/show/NCT01086267 Accessed 5/1/13.
- 31.Schwartz GK, Robertson S, Shen A, et al. A phase I study of XL281, a selective oral RAF kinase inhibitor, in patients (Pts) with advanced solid tumors. J Clin Oncol. 2009;27:15s. [Google Scholar]
- 32.King AJ, Patrick DR, Batorsky RS, Ho ML, Do HT, Zhang SY, et al. Demonstration of a genetic therapeutic index for tumors expressing oncogenic BRAF by the kinase inhibitor SB-590885. Cancer Res. 2006;66:11100–5. doi: 10.1158/0008-5472.CAN-06-2554. [DOI] [PubMed] [Google Scholar]