Abstract
Colorectal cancer patients with BRAF-mutant tumors have a more aggressive, rapidly progressing disease that is in critical need of novel therapeutic approaches. Indeed, whereas the median overall survival (OS) of colorectal cancer (CRC) patients receiving standard-of-care therapy is approximately two years or more if their tumors express wild-type BRAF and wild-type KRAS, median OS is less than twelve months with tumors expressing V600E-mutant BRAF and wild-type KRAS. Pro-apoptotic receptor agonists are a class of biologic agents under development to induce tumor-specific apoptosis and are being combined with classical chemotherapy or targeted agents in clinical trials. Herein, we present the case of a patient with bulky V600E-mutant BRAF hepatic flexure colon carcinoma, treated initially with FOLFOX plus bevacizumab neoadjuvant therapy and surgery. The patient had a rapid tumor relapse with metastatic disease to the liver and lung, and was enrolled in a phase 1b open-label clinical study, where he received the FOLFIRI regimen in combination with the pro-apoptotic receptor agonist dulanermin (rhApo2L/TRAIL). The patient maintained stable disease through 25 doses administered every two weeks before his disease progressed. After coming off study, the patient underwent surgical debulking and received intraperitoneal hyperthermic chemotherapy. He subsequently relapsed and was treated with FOLFIRI plus cetuximab. At the time of this report, the patient remains on active treatment. It is unclear what effect dulanermin may have had on the course of his disease, but it is noteworthy that the patient remained on FOLFIRI plus dulanermin therapy for a period that exceeded the median OS for patients with advanced, aggressive BRAF-mutant CRC. It is also noteworthy that at the time of this report the patient's overall survival since diagnosis has exceeded 30 months, which is beyond what is generally observed even for patients with CRC harboring wild-type BRAF and wild-type KRAS.
Keywords: Apo2L, BRAF, TRAIL, cancer therapy, clinical experience, colon cancer, dulanermin, patient
Case Presentation
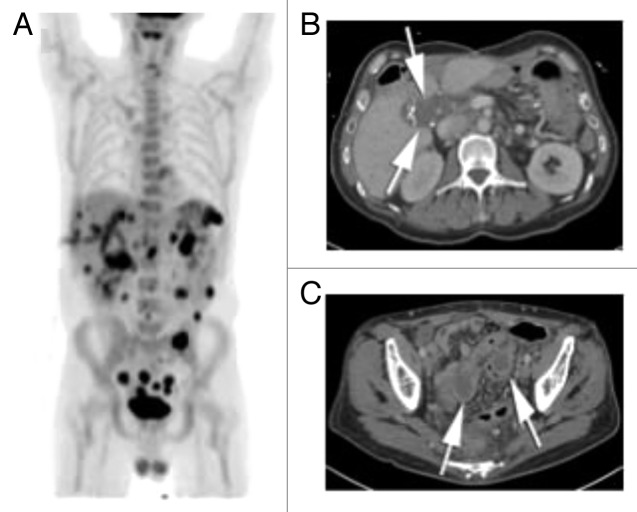
This patient is a 45-y old man with no significant previous past medical history, who presented to an outside hospital in May 2010 with a 3-mo history of weight loss and anemia. Workup revealed a large 7.9 × 7.9 × 9.8 cm mass involving the hepatic flexure of the colon and the right and left lobes of the liver (Fig. 1A–C). A fine-needle aspiration biopsy of the mass confirmed the presence of adenocarcinoma of the colon. Systemic staging revealed a pulmonary nodule of indeterminate significance, but no lymphadenopathy. His baseline CEA was not elevated above the normal range, but CA 19-9 was elevated at 360 unit/mL. The patient was started on modified FOLFOX6 and bevacizumab as neoadjuvant therapy. Tumor genetic testing showed the presence of the V600E BRAF mutation, low EGFR expression and low thymidine synthase expression in cancer tissue. After 3 mo of systemic chemotherapy, the patient was admitted to the hospital with a fistula between the duodenum and hepatic flexure of the colon. His disease burden measured by CT scan was similar to his initial presentation. A gastro-jejunal feeding tube was inserted for enteral nutrition.
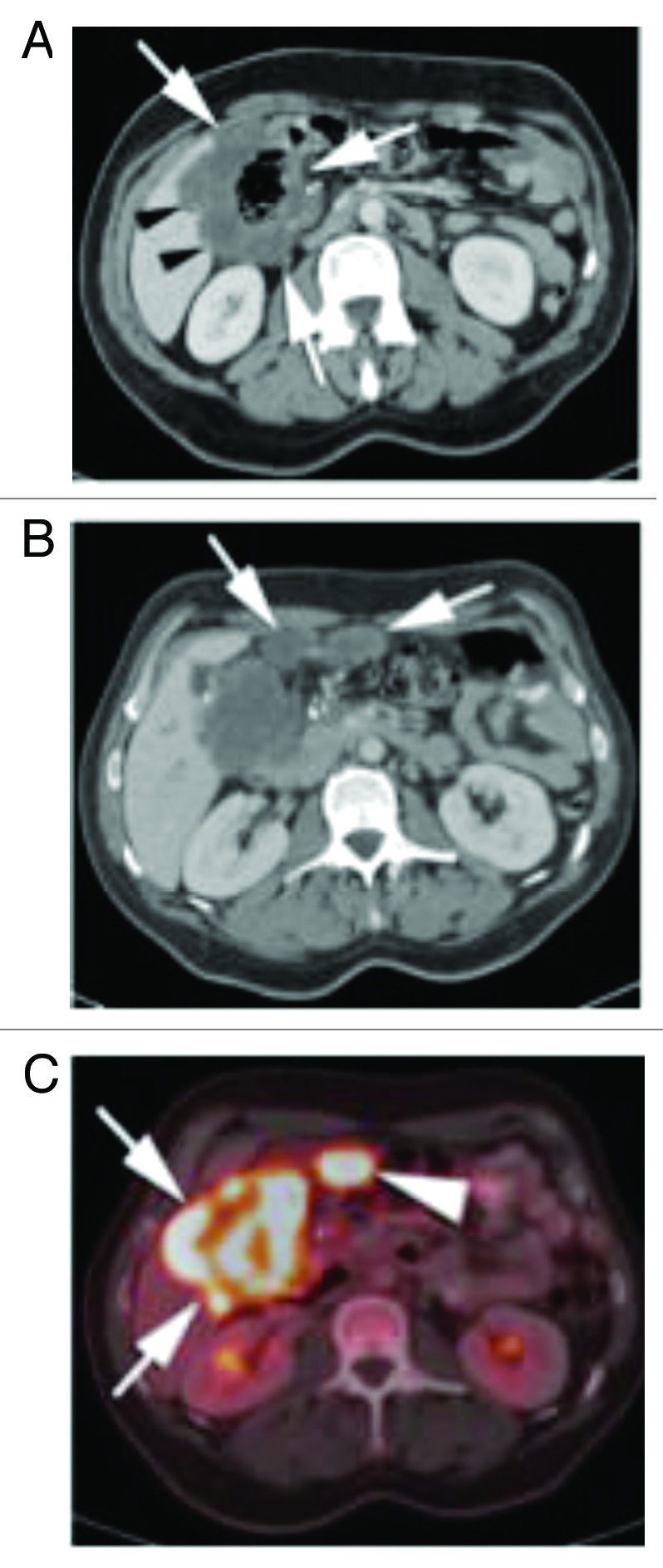
Figure 1. (A) Contrast enhanced CT of the abdomen shows a large, circumferential mass involving the hepatic flexure (white arrows). The mass infiltrates the adjacent right liver lobe (black arrows). (B) Contrast enhanced CT of the abdomen slightly more superiorly in the abdomen show two soft tissue nodules in the adjacent pericolonic fat (white arrows), consistent with peritoneal metastasis. (C) PET-CT fusion image at the level of the mass shown in (B) shows a markedly FDG avid hepatic flexure mass (white arrows), as well as marked FDG avidity of the pericolonic metastasis (arrow heads).
Two months after the feeding-tube placement, the patient was admitted to the hospital with sepsis. At that point, his serum CEA was not elevated but the CA 19-9 level was elevated at 252 unit/mL. After being treated with intravenous antibiotics and hemodynamic stabilization for several days, the decision was made to attempt surgical resection. He underwent an en bloc Whipple procedure, hemicolectomy, partial hepatectomy, and cholecystectomy. Final surgical pathology confirmed an invasive mucinous adenocarcinoma in the colonic mass (Fig. 2B). The pancreas, gallbladder, liver, duodenum, and omentum were also involved by adenocarcinoma. The stomach was negative for malignant involvement. Forty lymph nodes resected from around the colon, and 12 of them found to have metastatic disease. One out of 3 lymph nodes excised from the middle colic area was positive for malignancy. All margins were clear. Final pathological staging at the time of this surgery was T4b N2b. On immediate follow up after the surgery, the CEA level remained in the normal range at 0.8 ng/mL, and the CA 19-9 level normalized to 13.1 unit/mL. Because the patient developed a grade II neuropathy secondary to oxaliplatin during neoadjuvant therapy, systemic therapy was not introduced imminently post-surgery. Rather, he was closely monitored with imaging and analysis of serum tumor markers every two months.
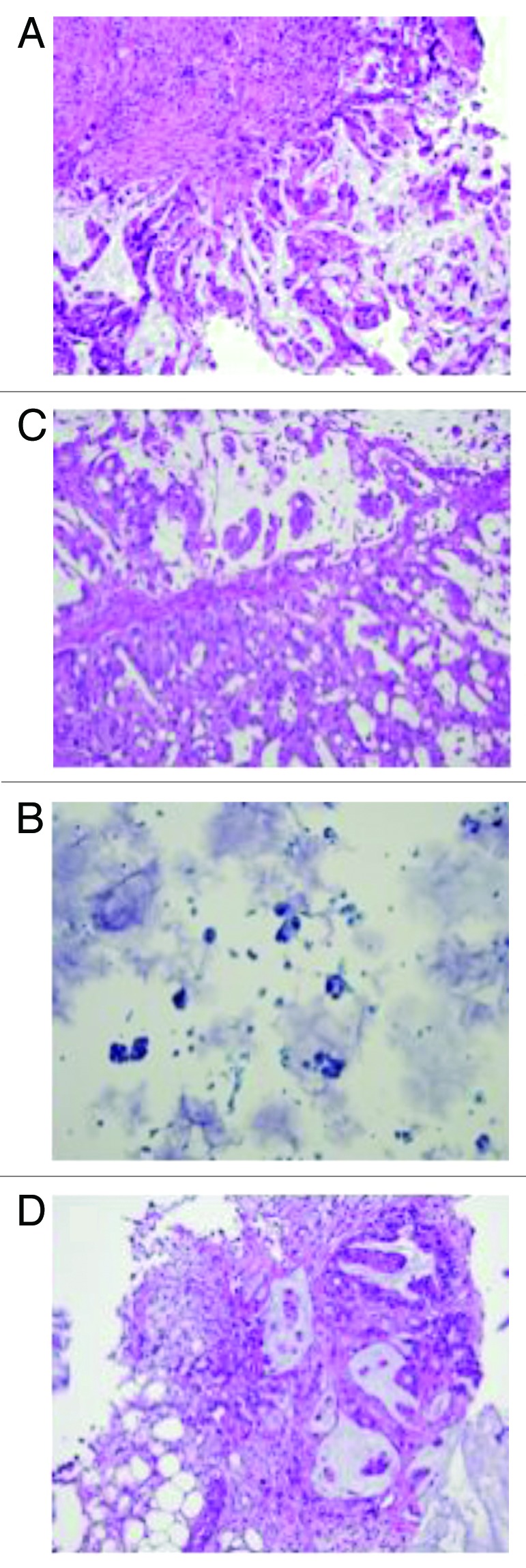
Figure 2. (A) Original liver fine needle aspiration biopsy performed in May 2010, slide with papanicolau staining (×200). Cancer cells are stained in blue with high nuclear cytoplasmic ratio. (B) Colon mucosa from first cytoreductive therapy, original H&E staining (×200): adenocarcinoma with mucinous feature is observed. (C) As shown in Figure 4C, LUQ mass grew despite stable other metastatic lesions. Biopsy was performed to confirm the malignancy of the mass. H&E staining (×200) confirmed colonic origin mucinous adenocarcinoma. (D) Biopsy of small bowel anastomosis site was re-biopsied to confirm the disease progression. H&E staining (×200) showed mucinous adenocarcinoma with similar morphology with original biopsy.
Approximately 2 mo after surgery, a repeat CT scan showed enlargement of several lymph nodes within the mesenteric root. The scan also detected persistent stable small nodules in the right lower pulmonary lobe. CEA and CA 19-9 levels remained normal. Systemic therapy was discussed with the patient, but not started at that point, since he was still healing from surgery. At four months after surgery, his CEA rose to 4.3 ng/mL. PET/CT scan showed multifocal hepatic metastatic disease throughout the subcapsular and parenchymal area. There was also metastatic lymphadenopathy along the celiac axis mesentery, as well as the right psoas muscle. A bone scan showed a lesion involving the left anterior fourth rib and possible metastatic spread to the left anterior eighth rib.
Since the patient’s disease was progressing rapidly, with new bony involvement and underlying poor prognostic factors, the decision was made to enroll the patient in a phase 1b open-label dose escalation clinical trial evaluating the safety and pharmacokinetics of multiple doses of dulanermin (recombinant human Apo2L/TRAIL), administered intravenously in combination with the FOLFIRI regimen, in patients with previously treated metastatic colorectal cancer. He was started on the clinical trial with FOLFIRI + dulanermin on March 29, 2011. The regimen involved a 14-d cycle of administration of FOLFIRI (leucovorin 400 mg/m2, 5-FU 400 mg/m2 bolus followed by 2400 mg/m2 over 46 h, irinotecan 180 mg/m2) plus dulanermin (9 mg/kg/day on day 1–3 of each cycle).
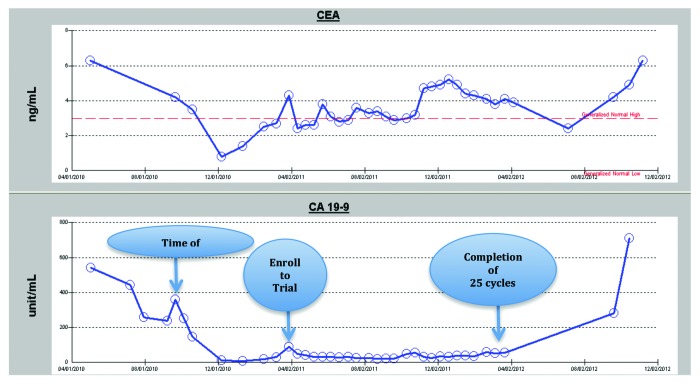
After the first cycle of treatment, his CEA came down to 2.4 ng/mL, while CA 19-9 dropped from 89.8 to 50.1 unit/mL. The CA 19-9 level remained low throughout the active treatment period on study, but the CEA level was fluctuating slightly regardless of clinical disease status. Hematologic, liver, and renal functions remained stable. The serum tumor marker changes are summarized in Figure 3.
Figure 3. Serum marker changes over the course of treatment Each arrow indicates the time of surgery, the patient’s enrollment into the clinical trial, and the date of the 25th treatment cycle following protocol. His disease progressed as documented after the 25th dose and he came off active treatment on the clinical trial.
After completing a total of 25 cycles of the FOLFIRI plus dulanermin, a CT scan revealed enlargement of a soft-tissue left upper quadrant (LUQ) abdominal nodule that was suspicious for progression, but 5 other lesions as well as the CA19.9 level remained stable. A CT-guided biopsy of the LUQ nodule confirmed colon mucinous adenocarcinoma (Figs. 4 and 5C). The patient came off study after the pathological confirmation of progressive disease.
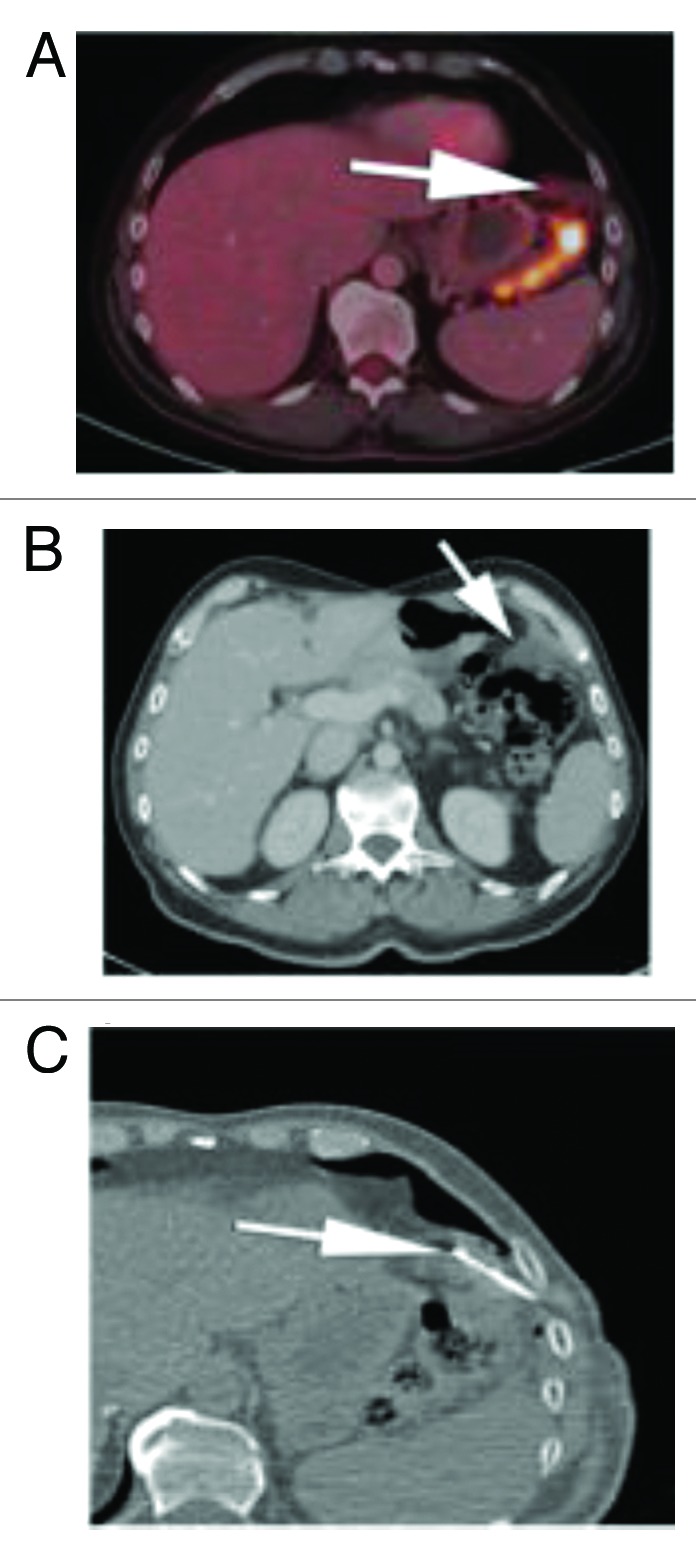
Figure 4. (A) Subsequent PET CT fusion image after 25 cycles showed no FDG avidity of the left upper quadrant soft tissue mass, favoring a good response to therapy (arrow). (B) Contrast enhanced CT performed soon after the PET image shown in Figure 6A showed interval increase in size of the left subphrenic metastasis. As there was disparity between an interval growth of the mass and lack of FDG activity, tissue sampling was recommended. (C) Intra-procedural CT scan shows the tip of a core biopsy needle (arrow) sampling the mass in the left subphrenic space. Pathology result was positive for viable disease.
Figure 5. (A) PET maximum intensity projection (MIP) image on latest follow-up exam shows diffuse FDG activity scattered throughout the abdomen and pelvis, consistent with peritoneal metastatic disease. (B) Latest contrast enhanced CT scan show post-surgical changes in the right abdomen following colectomy, with soft tissue mass in the porta hepatis (arrows). There are biliary drainage catheters in place. The left subphrenic masses have resolved. (C) Contrast enhanced CT through the pelvis show 2 peripherally enhancing masses along the sigmoid colon, which were FDG avid (not shown) and consistent with metastatic disease.
The patient requested surgical consultation at this point and received debulking surgery with hyperthermic intraperitoneal chemotherapy (HIPEC). Approximately 3 weeks after completing that treatment, he was admitted to the hospital with fever and was found to have a sub-hepatic abscess. He underwent drainage of the abscess and completed a course of antibiotics. He was readmitted to the hospital approximately 3 mo later for cholangitis and was found at that time to have recurrent metastatic mucinous adenocarcinoma confirmed from biopsies taken from duodenum and small bowel anastomosis site (Fig. 5D). The patient continued to have elevated liver function tests (LFTs) and was referred to interventional radiology for placement of a percutaneous biliary drainage catheter (PBDC) on September 15, 2012. The PBDC was placed without complications, and after stabilization of his LFTs, he was started on FOLFIRI plus cetuximab. This regimen was stopped by end of March due to progression of disease.
Discussion
BRAF mutation and colon cancer
The activating V600E BRAF mutation, which occurs in about 10% of patients with colorectal cancer, is associated with a more aggressive and rapidly progressing disease. Although patients with BRAF mutated tumors benefit from the standard-of-care irinotecan or oxaliplatin-containing chemotherapies,1 the outcome of these patients remains poor. The median OS for colorectal cancer patients with BRAF-mutant and KRAS wild-type tumors is less than one year, as compared with two years or greater for patients with wild-type BRAF and wild-type KRAS.2,3 Because of the poor prognosis associated with BRAF-mutant colorectal cancer despite available therapies, new treatment approaches are urgently needed.
Dulanermin as a pro-apoptotic receptor agonist
Apoptosis is a major mechanism of cell elimination during normal physiology and functions as a component of an endogenous surveillance mechanism to suppress cancer development.4 Two major signaling pathways, known as the intrinsic and extrinsic pathways, control activation of apoptotic cell death: these pathways are activated in cancer cells by chemotherapy and radiotherapy as well as by anti-tumor immune surveillance mechanisms.5 The intrinsic pathway is also known as the mitochondrial cell death pathway and is regulated by the Bcl-2 family of pro-apoptotic and anti-apoptotic proteins, as well as IAP proteins and negative regulatory pro-survival kinases in the cell.6,7 Extrinsic cell death can be activated by pro-apoptotic members of the tumor necrosis factor (TNF) cytokine family, including TNFα, Fas/CD95 ligand, and Apo2 ligand/TNF-related apoptosis-inducing ligand (Apo2L/TRAIL)8,9 Complicated cross-talk between these pathways can attenuate pro-apoptotic signals, as well as the impact of chemotherapy to achieve an anti-tumor effect.9-11
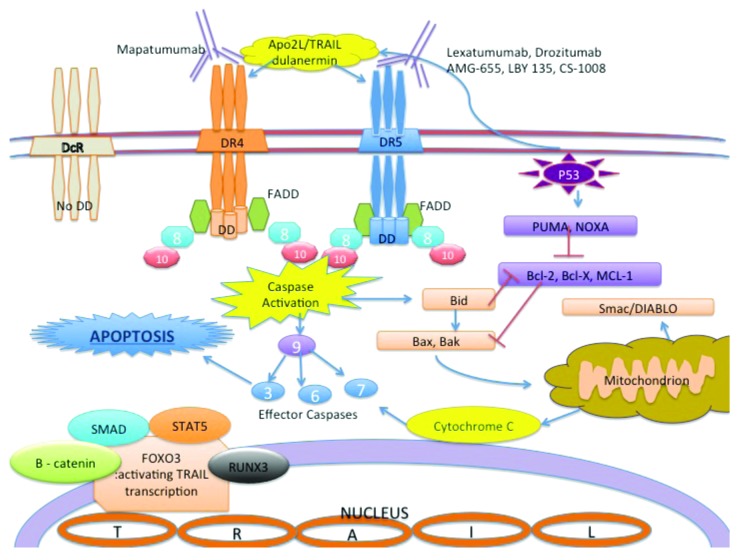
Apo2L/TRAIL and its receptors have been the subject of intense scientific efforts for over 15 y owing to the preferential killing by this ligand of cancerous and transformed cells vs. most normal cells. There are 5 different receptors that bind Apo2L/TRAIL, namely, DR4, DR5, DcR1, DcR2, and OPG (osteoprotegrin).9,12 The endogenous Apo2L/TRAIL protein is present in various tissues, predominantly in spleen, lung and prostate and expressed on the surface of cytotoxic T cells and NK cells.9,13 The human Apo2L/TRAIL gene is located on chromosome 3 at position 3q26 and encodes a type II transmembrane protein that has homology to other TNF family members. DR4 and DR5 are death domain-containing pro-apoptotic receptors that bind specifically to Apo2L/TRAIL and transduce apoptosis-inducing signals into the cell (Fig. 6) via a caspase-mediated mechanism.9,11 Interestingly, Apo2L/TRAIL-induced cell death is largely tumor cell-specific and has very low or no effect on normal cells.15-17 Decoy receptors 1 and 2 may contribute to the selective anti-tumor effect of Apo2L/TRAIL. These decoy receptors bind to Apo2L/TRAIL and are expressed on normal cells but absent from most tumor cells. Because of the lack a death domain, these decoys counteract apoptosis activation, thus provide protection for normal cells.
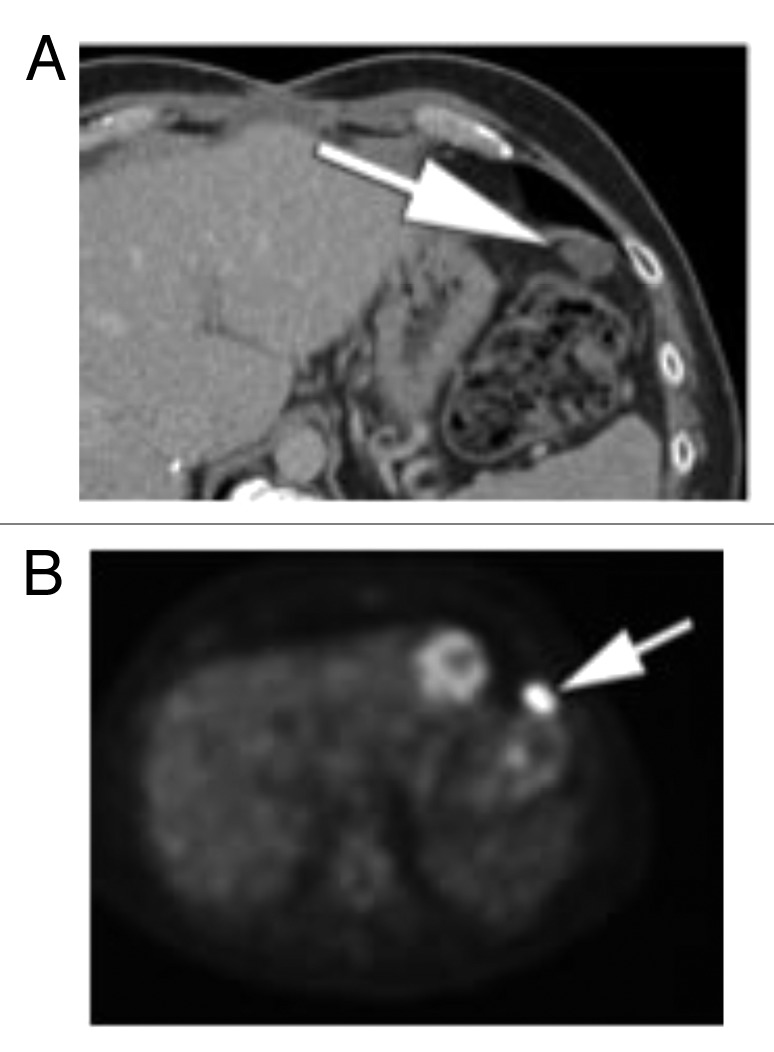
Figure 6. (A) Follow up after initial cytoreductive surgery. Contrast enhanced CT through the upper abdomen shows. progression of omental and peritoneal disease, with development of new oval soft tissue nodule in the left upper quadrant of the abdomen (arrow). (B) PET performed at the same time shows marked FDG avidity of the oval soft tissue nodules in the left upper quadrant of the abdomen (arrow), consistent with metastatic disease.
Clinical trials targeting the Apo2L/TRAIL pathway
Activation of the extrinsic apoptotic pathway as an anti-cancer therapy has been investigated pre-clinically and clinically. In the 1980s to90s, TNF was studied as a potential therapy for cancer in combination with interferon or chemotherapy; while suitable for isolated limb perfusion, this approach showed too much toxicity for systemic administration.9,15,16 The discovery of Apo2L/TRAIL and the observation that it was substantially less toxic than TNF toward normal cells stimulated an interest in its development as a potential therapeutic agent to treat human cancer.
The main approaches to target this attractive “cancer-specific pathway” include dulanermin—a recombinant soluble form of human Apo2L/TRAIL, which activates both DR4 and DR5,18-20 and agonistic antibodies that specifically activate either DR4 or DR5.19 Various forms of recombinant Apo2L/TRAIL have been developed and studied in the pre-clinical setting in single-agent mode or as a part of combinatorial therapeutic regimens. Through in vitro and in vivo studies, Apo2L/TRAIL showed promising activity as a potential therapeutic agent in various human cancer models including colorectal cancer, glioma, breast, uterine, ovarian, liver, prostate, kidney, and thyroid cancers.9,17-23 Dulanermin (rhApo2L/TRAIL) is the only recombinant human Apo2L/TRAIL protein targeting both DR4 and DR5 that has been available for clinical testing.
Dulanermin and several pro-apoptotic receptor agonistic antibodies—mapatumumab for DR4, and lexatumumab, drozitumab, AMG-655 (conatumumab), LBY 135, CS-1008 (tigatuzumab) for DR5—have been developed by different companies. Clinical trials using these agents are summarized in Table 1.
Table 1. Apo2L/TRAIL signaling pathway and all the available agents in clinical development/trials.
| TRAIL agent | Clinical trial | Combined agents | Target cancers | Results and trial status |
|---|---|---|---|---|
| Dulanermin (rhApo2L/TRAIL) |
Phase I |
Single agent |
Advanced or metastatic solid tumors or non Hodgkin lymphoma |
Completed MTD not reached; maximum-administered dose 30 mg/kg x 5 d per 21-d cycle Two patients with chondrosarcoma achieved sustained partial response Development discontinued |
| Dulanermin (rhApo2L/TRAIL) |
NCT00400764 Phase Ib/II |
Rituximab |
CD20+ B cell Non Hodgkin lymphoma |
Completed Acceptable safety profile Overall response: 61.5% in rituximab only vs. 63.6% in combination Development discontinued |
| Dulanermin (rhApo2L/TRAIL) |
NCT00508625 Phase Ib/II |
Paclitaxel Carboplatin Bevacizumab |
Non-small cell lung cancer |
Completed No improvement in response rate or progression free survival Development discontinued |
| Dulanermin (rhApo2L/TRAIL) |
NCT00671372 Phase Ib |
Cetuximab/irinotecan Or FOLFIRI Bevacizumab |
Previously treated metastatic colorectal cancer |
Completed Acceptable safety profile Development discontinued |
| Dulanermin (rhApo2L/TRAIL) |
NCT00873756 Phase Ib |
FOLFOX Bevacizumab |
First line metastatic colorectal cancer |
Ongoing Stopped accrual Development discontinued |
| Drozitumab |
Phase I |
Single agent |
Advanced or metastatic solid tumors or NHL |
Completed MTD not reached; maximum-administered dose 20 mg/kg per 14-d cycle Development discontinued |
| Drozitumab |
NCT00543712 Phase II |
Single agent |
Chondrosarcoma |
Completed No objective responses observed Development discontinued |
| Drozitumab |
NCT00480831 Phase II |
Paclitaxel Carboplatin Bevacizumab |
Non-small cell lung cancer |
Completed Development discontinued |
| Drozitumab |
NCT00497497 Phase Ib |
Cetuximab/irinotecan Or FOLRIFI Bevacizumab |
Previously treated metastatic colorectal cancer |
Completed Acceptable safety profile Development discontinued |
| Drozitumab |
NCT00851136 Phase Ib |
FOLFOX Bevacizumab |
First line metastatic colorectal cancer |
Completed Acceptable safety profile Development discontinued |
| Drozitumab |
NCT00517049 Phase II |
Rituximab |
CD20+ B cell Non Hodgkin lymphoma |
Completed Generally well tolerated Development discontinued |
| Mapatumumabbreak/>(HGS-ETR1) |
NCT00092924 Phase II |
Single agent |
Non-small cell lung cancer |
No result reported to date |
| LBY 135 |
Phase I |
Capecitabine |
Advanced solid tumors |
Acceptable safety profile Shown clinical activity |
| Lexatumumab (HGS-ETR2) |
NCT00428272 Phase I |
Lexatumumab with or without interferon gamma |
Refractory pediatric solid tumor |
No result reported to date |
| Lexatumumab (HGS-ETR2) |
Phase Ib |
Gemcitabine Pemetrexed Doxorubicin FOLFIRI |
Various solid tumors |
Acceptable safety profile Continuing to phase II |
| Conatumumab (AMG-655)12 |
Phase II |
Paclitaxel Carboplatin |
First line advanced NSCLC |
Improved PFS in SCC group (HR 0.47 [0.23–0.94]) Trend toward improved OS (HR 0.72 [0.43–1.23]) |
| Conatumumab (AMG-655)13 |
Phase II |
Gemcitabine in all arms Open label ganitumab |
Metastatic pancreatic cancer |
6-mo survival 57% (95% CI 41–70) in the ganitumab arm 59% (42–73) in the conatumumab arm 50% (33–64) in the placebo arm |
| Conatumumab (AMG-655)14 |
Phase I/II |
Doxorubicin |
Unresectable soft tissue sarcoma |
No unexpected side effects PFS 5.6 mo conatumumab arm vs 6.4 mo placebo arm (stratified HR: 1.00; P = 0.973) |
| Conatumumab (AMG-655) |
Phase Ib/II |
Panitumumab |
Metastatic colorectal cancer—WT KRAS MT KRAS Two group |
Safely administered Median overall Survival: 7.3 mo in WT KRAS group, 4.4 mo in MT KRAS group No improved activity to date |
| Conatumumab (AMG-655) |
Phase II |
FOLFOX6 Ganitumab Bevacizumab |
Advanced colorectal, locally advanced lymphoma, metastatic NSCLC |
Stopped accrual No result to date |
| Tigatuzumab (CS-1008) |
NCT00945191 Phase II |
Paclitaxel Carboplatin |
Ovarian cancer |
Completed but no result reported to date |
| Tigatuzumab (CS-1008) |
NCT01033240 Phase II |
Sorafenib |
Advanced liver cancer |
Stopped accrual Safe completion of phase IB, result to be reported |
| Tigatuzumab (CS-1008) |
NCT00521404 Phase II |
Gemcitabine |
Untreated, unresectable pancreatic cancer |
Completed No result reported to date |
| Tigatuzumab (CS-1008) |
NCT00991796 Phase II |
Paclitaxel Carboplatin |
Metastatic or unserectable NSCLC | Significant trend toward to increased PFS No improvement in OS |
To date, a phase III trial has not been conducted for this category of therapeutic agents, but as seen in Table 1, currently several phase II trials across solid tumors and hematologic malignancies are ongoing. In the case reported herein, a patient with V600E BRAF-mutated and KRAS wild-type colon cancer, which is associated with a poor prognosis, was treated with a novel combination therapy of dulanermin plus FOLFIRI. His disease remained stable for a period of over one year while on this combination therapy.
Whether the effect of treatment by FOLFIRI plus dulanermin was actually of benefit to the patient described in this report, and whether this combined approach may be more generally beneficial for the subgroup of colorectal cancer patients with BRAF mutations is unknown. The patient’s survival extended beyond 30 mo at the time of this report, where he remains on active treatment. It is unclear if the dulanermin component of therapy specifically impacted on his relatively prolonged survival; however, it is noteworthy that the patient remained on FOLFIRI plus dulanermin therapy for a period that exceeds the known median OS for patients with advanced, aggressive BRAF-mutant malignancy that are treated with the FOLFIRI or FOLFIRI plus cetuximab. It is also noteworthy that the survival status of this patient (>30 mo) is well beyond what is generally observed for colorectal cancer patients with wild-type BRAF and wild-type KRAS who are treated with standard-of-care therapies. It is also interesting to note that the patient presented high levels of the O-glycosylation serum marker CA19-9 prior to therapy, suggesting higher O-glycosylation of some proteins in tumors. Although the O-glycosylation status of DR4 and DR5 of his tumor is unknown, sensitivity of colorectal cancer cell lines to dulanermin has been reported to correlate with O-linked glycosylation of DR4 and DR5.
In conclusion, targeting the Apo2L/TRAIL pro-apoptotic receptor pathway should be further investigated with available agents, to treat patients who otherwise have a poor prognosis, including colorectal cancer patients with mutant BRAF (Fig. 7).
Figure 7. Apo2L/TRAIL signaling pathway and available agents in clinical trials DD: death domain; FADD: Fas-associated death domain; 3,6,7,8,9,10 indicates each caspase 3 and caspase 6,7,8,9,10 accordingly. Other agent names are listed in the legend section. DR4 and DR5 have a cytoplasmic death domain that is lacking in the decoy receptors (DcR) that bind to Apo2L/TRAIL. Mapatumumab is an agonistic DR4-targeting monoclonal antibody and Lexatumumab, Drozitumab, AMG-655, LBY 135 and CS-1008 are agonistic DR5-targeting monoclonal antibodies. Dulanermin (recombinant human Apo2L/TRAIL) can stimulate pro-apoptotic death receptors on the surface of cancer cells as does native endogenous Apo2L/TRAIL. The extrinsic apoptosis pathway is mediated by the initiator caspase 8 and 10 and the effector caspase 3, 6 and 7. In many cancer cell types, pro-apoptotic receptor agonists induce both the extrinsic and intrinsic (mitochondrion mediated) apoptosis pathways. FOXO3, a direct regulator of the TRAIL gene, binds to the promoter site and regulates transcription. FOXO3 is regulated by other regulators shown in this figure, as well as other mechanisms via c-MET, JNK, IKK and NFkB.
Acknowledgments
WSE-D is an American Cancer Society Research Professor. WSE-D served as the Principal Investigator at Penn State University of the phase 1b open label dose escalation clinical trial evaluating the safety and pharmacokinetics of multiple doses of dulanermin (TRAIL) administered intravenously in combination with the FOLFIRI regimen in subjects with previously treated metastatic colorectal cancer.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/25310
References
- 1.Richman SD, Seymour MT, Chambers P, Elliott F, Daly CL, Meade AM, et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27:5931–7. doi: 10.1200/JCO.2009.22.4295. [DOI] [PubMed] [Google Scholar]
- 2.Samowitz WS, Sweeney C, Herrick J, Albertsen H, Levin TR, Murtaugh MA, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063–9. doi: 10.1158/0008-5472.CAN-05-0404. [DOI] [PubMed] [Google Scholar]
- 3.Laurent-Puig P, Cayre A, Manceau G, Buc E, Bachet JB, Lecomte T, et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27:5924–30. doi: 10.1200/JCO.2008.21.6796. [DOI] [PubMed] [Google Scholar]
- 4.Halliwell B. A super way to kill cancer cells? Nat Med. 2000;6:1105–6. doi: 10.1038/80436. [DOI] [PubMed] [Google Scholar]
- 5.von Roretz C, Lian XJ, Macri AM, Punjani N, Clair E, Drouin O, et al. Apoptotic-induced cleavage shifts HuR from being a promoter of survival to an activator of caspase-mediated apoptosis. Cell Death Differ. 2013;20:154–68. doi: 10.1038/cdd.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–11. doi: 10.1016/S1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 7.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNFfamily death receptors. Oncogene. 2003;22:8628–33. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- 9.Roy S, Nicholson DW. Cross-talk in cell death signaling. J Exp Med. 2000;192:21–6. doi: 10.1084/jem.192.8.F21. [DOI] [PubMed] [Google Scholar]
- 10.Kandasamy K, Srinivasula SM, Alnemri ES, Thompson CB, Korsmeyer SJ, Bryant JL, et al. Involvement of proapoptotic molecules Bax and Bak in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced mitochondrial disruption and apoptosis: differential regulation of cytochrome c and Smac/DIABLO release. Cancer Res. 2003;63:1712–21. [PubMed] [Google Scholar]
- 11.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–90. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 12.Paz-Ares L, Bálint B, de Boer RH, van Meerbeeck JP, Wierzbicki R, De Souza P, et al. A randomized phase 2 study of paclitaxel and carboplatin with or without conatumumab for first-line treatment of advanced non-small-cell lung cancer. J Thorac Oncol. 2013;8:329–37. doi: 10.1097/JTO.0b013e31827ce554. [DOI] [PubMed] [Google Scholar]
- 13.Kindler HL, Richards DA, Garbo LE, Garon EB, Stephenson JJ, Jr., Rocha-Lima CM, et al. A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Ann Oncol. 2012;23:2834–42. doi: 10.1093/annonc/mds142. [DOI] [PubMed] [Google Scholar]
- 14.Demetri GD, Le Cesne A, Chawla SP, Brodowicz T, Maki RG, Bach BA, et al. First-line treatment of metastatic or locally advanced unresectable soft tissue sarcomas with conatumumab in combination with doxorubicin or doxorubicin alone: a phase I/II open-label and double-blind study. Eur J Cancer. 2012;48:547–63. doi: 10.1016/j.ejca.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Lenk H, Tanneberger S, Müller U, Ebert J, Shiga T. Phase II clinical trial of high-dose recombinant human tumor necrosis factor. Cancer Chemother Pharmacol. 1989;24:391–2. doi: 10.1007/BF00257449. [DOI] [PubMed] [Google Scholar]
- 16.Smith TL, Lee JJ, Kantarjian HM, Legha SS, Raber MN. Design and results of phase I cancer clinical trials: three-year experience at M.D. Anderson Cancer Center. J Clin Oncol. 1996;14:287–95. doi: 10.1200/JCO.1996.14.1.287. [DOI] [PubMed] [Google Scholar]
- 17.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–62. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitsiades N, Poulaki V, Mitsiades CS, Koutras DA, Chrousos GP. Apoptosis induced by FasL and TRAIL/Apo2L in the pathogenesis of thyroid diseases. Trends Endocrinol Metab. 2001;12:384–90. doi: 10.1016/S1043-2760(01)00441-6. [DOI] [PubMed] [Google Scholar]
- 19.Zhan C, Wei X, Qian J, Feng L, Zhu J, Lu W. Co-delivery of TRAIL gene enhances the anti-glioblastoma effect of paclitaxel in vitro and in vivo. J Control Release. 2012;160:630–6. doi: 10.1016/j.jconrel.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 20.Kelly MM, Hoel BD, Voelkel-Johnson C. Doxorubicin pretreatment sensitizes prostate cancer cell lines to TRAIL induced apoptosis which correlates with the loss of c-FLIP expression. Cancer Biol Ther. 2002;1:520–7. doi: 10.4161/cbt.1.5.169. [DOI] [PubMed] [Google Scholar]
- 21.Ashley DM, Riffkin CD, Lovric MM, Mikeska T, Dobrovic A, Maxwell JA, et al. In vitro sensitivity testing of minimally passaged and uncultured gliomas with TRAIL and/or chemotherapy drugs. Br J Cancer. 2008;99:294–304. doi: 10.1038/sj.bjc.6604459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitsiades N, Poulaki V, Mitsiades C, Tsokos M. Ewing’s sarcoma family tumors are sensitive to tumor necrosis factor-related apoptosis-inducing ligand and express death receptor 4 and death receptor 5. Cancer Res. 2001;61:2704–12. [PubMed] [Google Scholar]
- 23.Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–21. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]