Abstract
Objective
To improve the test standards for a version of the Romberg test and to determine if measuring kinematic variables improved its utility for screening.
Study design
Healthy controls and patients with benign paroxysmal positional vertigo, postoperative acoustic neuroma resection, and chronic peripheral unilateral weakness were compared.
Methods
Subjects wore Bluetooth-enabled inertial motion units while standing on the floor or medium density, compliant foam, with eyes open or closed, with head still or moving in pitch or yaw. Dependent measures were time to perform each test condition, number of head movements made, and kinematic variables.
Results
Patients and controls did not differ significantly with eyes open or with eyes closed while on the floor. With eyes closed, on foam, some significant differences were found between patients and controls, especially for subjects older than age 59. Head movement conditions were more challenging than head still. Significantly fewer patients than controls could make enough head movements to obtain kinematic measures. Kinematics indicated that lateral balance control is significantly reduced in these patients compared to controls. Receiver Operator Characteristics and sensitivity/specificity analyses showed moderately good differences with older subjects.
Conclusion
Tests on foam with eyes closed, with head still or moving, may be useful as part of a screening battery for vestibular impairments, especially for older people.
Keywords: balance testing, screening, Romberg, vestibular testing
INTRODUCTION
For many years variations of the Romberg test have been used for screening balance problems1, including vestibular disorders. Computerized versions show significant differences between normals and patients with vestibular disorders2, especially the Sensory Organization tests (SOT) of computerized dynamic posturography. That equipment, however, is not suitable for clinics with small equipment budgets or for situations requiring rapid screening.3, 4 The less precise Clinical Test of Sensory Interaction and Balance (CTSIB), described for use by therapists,5 is portable and inexpensive. Preliminary norms based on a small sample showed age-related changes. 6 Subsequent work verified that for older subjects standing on compliant foam with eyes closed is more challenging than with eyes open on foam or the floor and subjects display greater postural sway. 7,8 Recent work suggests that although CTSIB resembles SOT in some ways it is more challenging. 9
During some years the National Health and Nutrition Examination Survey collected data on CTSIB. 10 Normative values were reported for subjects aged 40 to 80+ and showed some age-related changes but the sample size per age decade and the upper end of the age range were not reported.11 We collected a smaller sample of controls but with a broader age range, plus kinematic data on postural sway. Also we compared controls to patients with vestibular disorders to obtain cut-points that, for the first time, were determined statistically rather than arbitrarily.
MATERIALS AND METHODS
Subjects
Subjects included asymptomatic healthy controls, and three groups of patients with known vestibular impairments: unilateral benign paroxysmal positional vertigo of the posterior semicircular canal (BPPV) prior to treatment with repositioning maneuvers; unilateral peripheral vestibular weakness (UW) excluding Meniere’s disease and migraine; and post-operative acoustic neuroma patients (AN) at least three months post-surgery. Controls were recruited from staff and visitors to the lab and were screened with a health history, head impulse tests, observation of gait, and Dix-Hallpike maneuvers. All subjects were independently ambulatory, had no joint replacements or history of neurologic disease, and functional vision with their corrective lenses.
All patients were diagnosed by board-certified physicians, mostly otolaryngologists and neurologists on the faculty of Baylor College of Medicine. BPPV patients were diagnosed based on a positive response to the Dix-Hallpike maneuver and any other clinical and laboratory tests used by the physician. UW patients all had at least a 20% weakness on bi-thermal caloric testing. Some patients also had decreased vestibulo-ocular reflex responses to low frequency sinusoidal rotations in darkness and/or impaired vestibular-evoked myogenic potentials.
This study was approved by the Institutional Review Board for Human Subjects Research for Baylor College of Medicine and Affiliated Hospitals. Subjects gave informed consent prior to participation.
Materials and equipment
To obtain kinematic data during testing each subject wore a lightweight vest with an inertial motion sensor (IMU; Xsens North America Inc., Los Angeles, CA), 5.25 × 3.75 × 2 cm, weight 28.3 g, centered on the back at the mid-thoracic level and a plastic headband with another IMU. To cue head motions during head movement trials subjects heard a 0.33 Hz frequency-modulated auditory signal that oscillated between 170 and 450 Hz at a comfortable intensity level, via an iPod (Apple, Inc.) attached to external amplifiers placed on a desktop or clipped to the safety vest and heard via an ear bud earphone. 9 They were instructed to move their heads in time with the tone, and the staff member demonstrated making upward pitch when the tone was highest and downward pitch when the tone was lowest, simultaneously saying, “ Move your head in time with the tone like this: up, down, up, down.” For tests on foam subjects stood on Sunmate, medium density foam, 96 × 62 × 10 cm (Dynamic Systems Inc, Leicester, NC).
Procedure
Subjects arrived wearing a variety of shoes, from stiletto heels to flip-flops. To standardize footwear and maintain good hygiene subjects wore socks without shoes. 9 We loaned athletic socks to subjects who arrived without socks. They were tested in a quiet room with industrial carpeting. Subjects were guarded during all trials. For every trial they were instructed to stand quietly with feet together and arms crossed, for up to 30 seconds. 6,9 A trial ended if the subject took a step, moved one or both arms or, for tests with eyes closed, if the subject opened his eyes. To avoid a possible learning effect each subject had only one trial per condition. The time to complete the trial, in seconds, was recorded.
The 12 subtests were given to all subjects in the same order, in increasing order of difficulty: on the floor before on the foam; with eyes open before with eyes closed. Tests were given without augmented head motions (head still) before being given with yaw head rotations (yaw) and then with pitch head rotations (pitch). Initially, the patient groups and 10 subjects per decade from ages 21 to 79 were tested on all conditions. Later, to increase the sample size and improve statistical power on the more challenging conditions which showed greater variability, 24 more normals aged 24.9 to 78.8, and 24 UW patients aged 27.1 to 75.5, were added to the Eyes closed/foam (ECF)/head still (ECF still) and eyes closed/foam/head pitch (ECF pitch) conditions. To avoid fatiguing our subjects who were over the age of 80, after ascertaining that they could perform the condition on the floor with eyes open they were tested only on the conditions on foam with eyes closed, head still and head pitch.
Kinematic data from the torso-mounted IMU were analyzed if data from the head-mounted IMU indicated that subjects made five or more head movement cycles. For head still conditions the vector indices for each anticipated cycle of head motion were computed based on an assumed periodicity of 3 sec (f=0.33 Hz). Parameters were calculated to quantify sway in the mediolateral and anteroposterior directions for the trunk. The root mean squares of five balance parameters were calculated: anteroposterior acceleration (AX), mediolateral acceleration (AY), roll angular velocity (R), pitch angular velocity (P), yaw angular velocity (Y).
Statistical analyses
Patients and controls were compared on changes in the dependent measures of interest as a function of various conditions by multilevel statistical techniques.12 PROC GLIMMIX in SAS was used to fit generalized linear mixed models and to estimate the parameters by maximum likelihood. A separate model was fitted to each dependent variable (time to perform the task, number of head movements, and kinematic variables). For each model, within (condition) and between subjects (groups) effects were tested. Interaction effects were included in each model and tested. AIC, BIC and -2 log likelihoods were used to assess model fit. Adjustments were made for multiple comparisons. To determine if any test is useful in identifying people with vestibular disorders and to determine the optimal cut point on each test, we performed logistic regression and Receiver Operating Characteristic (ROC) analyses by age groups (21–59 and 60–79) and provided corresponding sensitivity and specificity values for various cut offs. P <0.05 was considered as statistically significant. All kinematics analyses were adjusted for age. All analyses were performed using SAS Statistical software, version 9.3 (SAS, Carry, NC).
RESULTS
The final sample included 156 controls, 18 AN subjects, 21 BPPV subjects, and 51 UW subjects; 27 UW subjects performed all conditions, 24 UW additional subjects performed tests on the three most challenging conditions. Controls included 24 subjects each in groups aged 21 to 29 and 30 to 39 years, 23 subjects 40 to 49, 22 subjects 50 to 59, 25 aged 60 to 69, 27 aged 70 to 79, and 11 aged 80 to 89 (elderly). Male and female controls did not differ on any head movement conditions. See Table 1.
Table 1.
Demographic details of the study sample. Mean age (standard deviation, ranges); number of males (m) and females (f); mean time (years) that UW and BPPV patients reported having illness or length of time post-operatively for AN patients (standard deviation, ranges). N, number of subjects.
| Age (yrs) | Sex | Length of illness | |
|---|---|---|---|
| Controls (N=156) | 52.8 (18.0, 23.3–89.5) | 80 m, 76 f | |
| AN (N=18) | 55.2 (10.8, 35.2–72.9) | 6 m, 12 f | 5.5 (5.8, 0.27 to 27) |
| BPPV (N=21) | 58.8 (11.7, 34.7–78.8) | 10 m, 11 f | 0.28 (0.45, 0.03 to 1.63) |
| UW (N=51) | 55.1 (15.6, 21.4 – 75.5) | 19 m, 32 f | 4.0 (9.0, 0.07 to 40) |
Time, less challenging conditions
For tests performed on the floor with eyes open no significant differences were found between controls and patient groups, among patient groups, or among age groups. For tests on foam with eyes open/head still no significant differences were found between controls and the combined patient group; for head pitch controls and patients differed significantly, p=0.015. Patients stood for significantly longer with head still than head pitch, p=0.01, or head yaw, p=0.01. See Table 2.
Table 2.
Time to perform the test, eyes open and eyes closed, on the floor and on foam. Head movement conditions are head still, head pitch and head yaw. Means, (standard deviation, ranges). Controls are aged 21–79. Data for elderly controls, aged 80+, are shown separately for conditions on foam with eyes closed.
| On the floor | On foam | |||||
|---|---|---|---|---|---|---|
| Still | Pitch | Yaw | Still | Pitch | Yaw | |
| Eyes open | ||||||
| Controls | 30 (0, 30) | 30 (0, 29.9–30) | 30 (0, 30) | 30 (0, 29.9–30) | 30 (0, 30) | 29.6 (2.9, 7.5–30) |
| AN | 30 (0, 30) | 30 (0, 30) | 30 (0, 30) | 30 (0, 30) | 28.1 (6.2, 4.5–30) | 26.4 (8.4, 4.1–30) |
| BPPV | 30 (0, 30) | 30 (0, 30) | 30 (0, 30) | 30 (0, 30) | 30 (0.1, 29–6–30) | 28.1 (5.8, 9.1–30) |
| UW | 30 (0, 30) | 30 (0, 30) | 30 (0, 30) | 30 (0, 30) | 27.0 (7.4, 7.7–30) | 29.0 (3.7, 13.0–30) |
| Eyes closed | ||||||
| Controls | 30 (0.1, 29.5–30) | 30 (0, 30) | 30 (0, 30) | 24.4 (10, 2.4–30) | 20.5 (10.4, 2.3–30) | 19.8 (10.4, 2.6–30) |
| AN | 30 (0, 30) | 29.2 (3.5, 15.3–30) | 29.1 (4.0, 13.2–30) | 8.4 (8.3, 2.5–30) | 5.9 (6.3, 2.5–30) | 6.7 (6.7, 2.7–30) |
| BPPV | 30 (0, 30) | 30 (0, 30) | 30 (0, 30) | 14.4 (11.3, 2.1–30) | 11.3 (9.9, 3.1–30) | 12.3 (9.5, 2.6–30) |
| UW | 28.4 (5.6, 7.2–30) | 28.4 (5.9, 7.6–30) | 27.4 (7.6, 2.7–30) | 14.9 (11.7, 1.6–30 7.7–30) | 9.7 (9.1, 1.8–30) | 10.3 (10.0, 2.1–30) |
For tests on the floor with eyes closed significant differences (p=0.01) were found between UW patients and controls at head still, pitch, and yaw. AN and BPPV subjects did not differ from controls. See Table 2. The differences between groups for conditions on the floor were not strong enough to consider for use as a screening test. Therefore the rest of the data analysis is presented only for the ECF conditions.
Time, more challenging conditions with eyes closed on foam
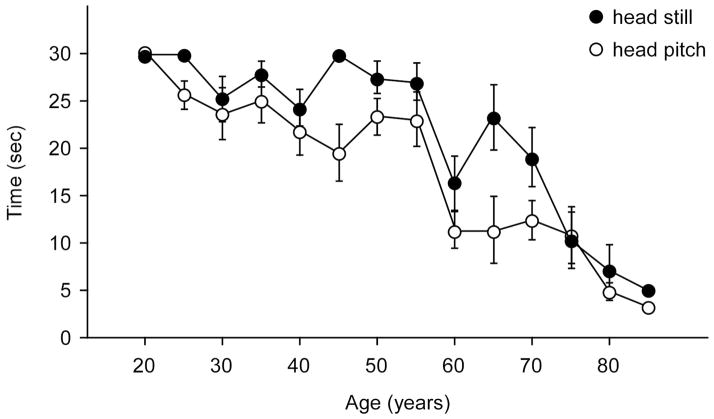
For head still and head pitch a significant effect of age was found for controls, p<0.001, such that Time was relatively stable until approximately age 59, decreased significantly to age 79, and then decreased significantly again for subjects in their 80’s. Consistent with this pattern significant differences were found among the three age groups at p<0.0001 for head still and at least p<0.01 for head pitch. See Figure 1. All elderly controls could stand on the floor with feet together for practice trials. Because clinicians see patients in this age range the reference values may be useful. Those data are shown in Table 3. Paired comparisons showed that the time scores for head still and head pitch did not differ significantly.
Figure 1.
Mean time scores of normal controls on head still and head pitch trials on foam with eyes closed across the age range in 5-year increments. Error bars are standard errors.
Table 3.
Summary table of time cut-off scores recommended for clinical testing. Recommended cut-points from ROC analyses are shown for younger subjects (aged 21 to 59) and older subjects (aged 60 to 79). Scores for elderly control subjects aged 80+ are shown as mean/median (standard deviation, ranges).
| Head still (sec) | Head pitch (sec) | |
|---|---|---|
| Younger | 29.8 | 29.9 |
| Older | 8.1 | 5.9 |
| Elderly | 7/4.1 (8.9, 2.0–30) | 4.7/3.4 (2.6, 2.1–9.8) |
Time, with eyes closed on foam and head still
For ECF/head still, controls performed all trials for significantly longer than the patient groups, combined, p<0.0001. AN patients approached performing the task for significantly less time than UW, p=0.056, or BPPV, p=0.09, groups. BPPV and UW groups did not differ significantly. For the combined patient compared to normals the significant odds ratio was: OR=0.93, 95% CI=0.90–0.95, p<0.0001. See Figure 2.
Figure 2.
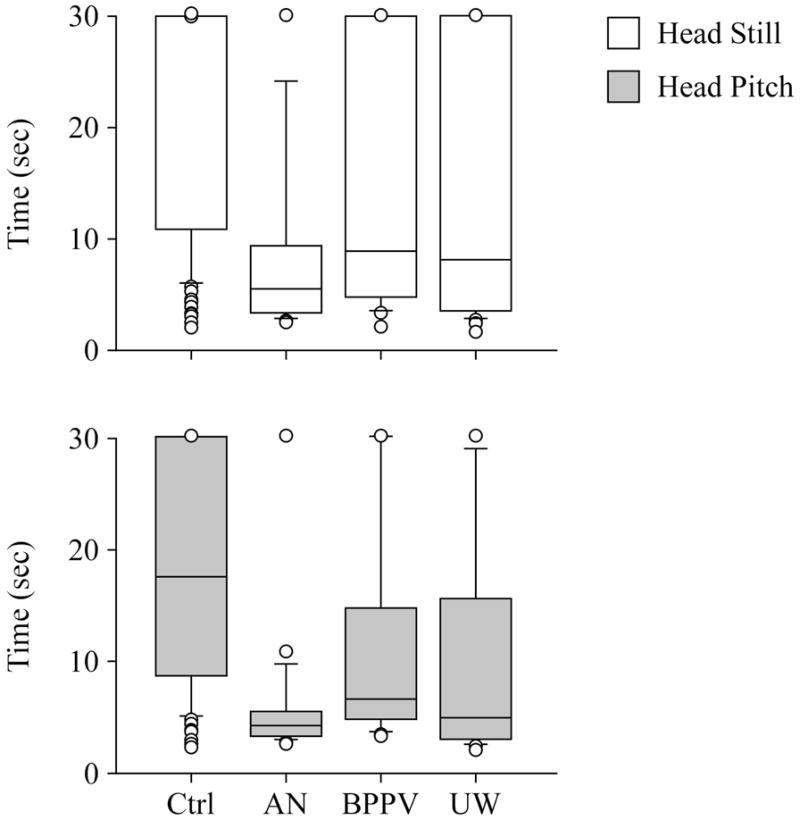
Time scores of controls and patient groups on foam with eyes closed. A. Head still condition. B. Head pitch condition. Center horizontal bars are medians; rectangle ends are interquartile ranges, error bars are 10th and 90th declines, and circles are outliers.
The ROC value was weak: ROC=0.73. Adjusting for age improved the odds ratio and ROC values very slightly: OR=0.92, 95% CI=0.88–0.95, p<0.0001; ROC=0.75. Without the BPPV subgroup the odds ratio and ROC values for the combined AN and UW group was slightly stronger: OR=0.90, 95% CI=0.865–0.937, p<0.0001; ROC=0.78. ROC’s for age groups of 21–59 and 60–79 were 0.72 and 0.79 respectively. Table 4 shows the best pairs of sensitivity and specificity values.
Table 4.
Sensitivity, specificity and related cut-points (time in sec) for eyes closed on foam, head still and head pitch, by age group. N, number of subjects, controls and patients combined. Three different combinations of sensitivity/specificity/cut-points are shown for each condition and age group so that the reader can see the range of values.
|
|
||||||
|---|---|---|---|---|---|---|
| Still | Pitch | |||||
|
| ||||||
| Sensitivity | Specificity | Cut-point | Sensitivity | Specificity | Cut-point | |
|
| ||||||
| Age | 0.50 | 0.99 | 9.8 | 0.81 | 0.58 | 24.8 |
| 21–59 | 0.55 | 0.83 | *29.8 | 0.82 | 0.54 | *29.9 |
| (N=123) | 1.0 | 0 | 30.0 | 1.0 | 0 | 30 |
|
| ||||||
| Age | 0.70 | 0.71 | 6.6 | 0.78 | 0.82 | 5.3 |
| 60–79 | 0.83 | 0.58 | *8.1 | 0.83 | 0.79 | *5.9 |
| (N=75) | 0.91 | 0.51 | 10.55 | 0.87 | 0.48 | 8.8 |
= best cut-point per condition based on best sensitivity/specificity combinations.
Time, with eyes closed on foam and head yaw
For ECF/head yaw controls performed the test for significantly longer than the combined patient group, p<0.0001. Time was significantly reduced compared to head still, p<0.0001, but was not significantly different from head pitch. Within the patient subgroups BPPV and AN groups approached significant differences, p<0.06, but no other paired comparisons showed any differences. See Table 2.
Time, with eyes closed on foam and head pitch
For ECF/head pitch using the combined patient groups compared to controls the significant odds ratio was: OR=0.91, 95% CI=0.88–0.94; and ROC= 0.77, p<0.0001. Adjusting for age improved the odds ratio and ROC values slightly: OR=0.895, 95% CI=0.857–0.93, p< 0.001, and ROC=0.78. Without the BPPV subgroup the odds ratio for the combined AN and UW group was stronger: OR=0.87, 95% CI=0.82–0.91, p<0.001, and ROC was 0.82. Table 3 shows the best sensitivity/specificity pairs by age. ROC’s for age groups of 21–59 and 60–79 were 0.80 and 0.84, respectively, i.e., better for older subjects. Table 4 shows that the best cut-point for the older group is lower than for the younger group; the exact cut point depends on the evaluator’s concern for sensitivity or specificity. Table 3 summarizes the recommended cut-off scores for normals aged 21 to 79 and lists the descriptive statistics of elderly subjects aged 80+. The clinician may use these values during clinical testing.
Analysis of head movement, with with eyes closed on foam
Due to a technical issue no kinematic data were recorded from one UW subject. Table 5 indicates the percentage of subjects per group who could perform enough head movements. Controls were significantly more likely than the total patient group to be able to perform at least 5 cycles of head motions, p ≤ 0.0003.
Table 5.
Percentages of subjects per group who could perform at least 5 cycles of head movements on foam with eyes closed: head still, head pitch, head yaw.
| Still | Pitch | Yaw | |
|---|---|---|---|
| Normals | 85 | 57 | 39 |
| BPPV | 73 | 19 | 19 |
| AN | 32 | 5 | 16 |
| UW | 67 | 24 | 6 |
Kinematic analyses of trunk movement, with eyes closed on foam
For AX controls and patients did not differ significantly with the head still or moving. Within the controls and within the patients, scores with head still differed from scores with head pitched, p<0.04 to p<0.03. For AY, controls and patients differed significantly regardless of head movement condition. For AY, within groups, both the control group and the patient group differed between head still and pitch conditions, p=0.001 and 0.01, respectively. Data from normals were not significantly correlated with age. See Table 6.
Table 6.
Kinematic data for eyes closed on foam, head still and head moving in pitch; controls and patient groups. Means (SD).
| Head condition | Controls | AN | BPPV | UW | |
|---|---|---|---|---|---|
| AX (m/sec2) | Still | 0.15 (0.12) | 0.22 (0.16) | 0.18 (0.10) | 0.12 (0.30) |
| Pitch | 0.18 (0.07) | *0.50 | 0.30 (0.13) | 0.21 (0.13) | |
| AY (m/sec2) | Still | 0.18 (0.13) | 0.27 (0.17) | 0.24 (0.12) | 0.26 (0.25) |
| Pitch | 0.20 (0.10) | *0.51 | 0.34 (0.13) | 0.24 (0.19) | |
| R (rad/sec) | Still | 0.04 (0.04) | 0.06 (0.03 | 0.06 (0.05) | 0.08 (0.12) |
| Pitch | 0.05 (0.03) | *0.15 | 0.09 (0.05) | 0.06 (0.40) | |
| P (rad/sec) | Still | 0.04 (0.04) | 0.07 (0.04) | 0.05 (0.03) | 0.09 (0.18) |
| Pitch | 0.07 (0.03) | *0.27 | 0.13 (0.09) | 0.07 (0.03) | |
| Y (rad/sec) | Still | 0.05 (0.04) | 0.06 (0.03) | 0.06 (0.02) | 0.08 (0.08) |
| Pitch | 0.06 (0.03) | *0.12 | 0.08 (0.03) | 0.07 (0.05) |
Represents only 1 subject. AX, root mean square (RMS) anteroposterior acceleration; AY, RMS mediolateral acceleration; R, RMS roll angular velocity; P, RMS pitch angular velocity; Y, RMS yaw angular velocity.
For R with head still controls did not differ significantly from BPPV patients but did differ significantly from the combined UW/AN group, p=0.02. Controls did not differ significantly from the combined patient group during head pitch. When the BPPV group was combined with the UW/AN group with head still controls differed significantly from patients, p=0.02, but the groups did not differ with head pitch. Controls differed between the head still and head pitched conditions, p=0.03 and the BPPV group differed between head still and head pitch, p=0.05, but the combined UW/AN group did not differ significantly between head still and head pitched. Data from normals were not significantly correlated with age.
For P controls differed from the combined patient group, p<0.0001; controls differed within themselves between head still and pitch, p=0.0025. No other differences were found. For Y controls differed significantly between head still and pitch, p=0.01. No other significant differences were found. Thus, across the five kinematic variables the parameters of sway in the mediolateral plane showed significant differences between controls and patients as well as among patient groups, indicating that lateral balance control is significantly reduced in these patient groups compared to normals.
ROC values for kinematic measures were not strong, < 0.80. When the best ROC values in kinematic measures were combined with the ROC values for time to perform the task the ROC values were all < 0.77. Therefore sensitivity and specificity values were not computed.
DISCUSSION
The goal of this study was to improve the usefulness of the CTSIB for screening standing balance in people suspected of having vestibular impairments, in general. The analyses of the diagnostic subgroups were secondary and exploratory, and should be considered with caution.
Time
The finding of age-related changes on the time performing the test confirms and extends the earlier work. 6,13,14 Unlike previous studies that arbitrarily divided the age range into equal age bins we allowed the data, shown in Figure 1, to dictate the cuts in the age range. This process provided three groups of different sizes -- ages 21 to 59, 60 to 79, and 80+ -- but the members within the groups were statistically similar. A larger sample might have allowed for more finer-grained analyses and might have found more subgroups. Thus, adding to the normal database may be a focus of future work.
The finding that the conditions with eyes closed on foam are the most sensitive to patients replicates previous work. We have extended this work by examining more challenging conditions with head moving in yaw and pitch. As Table 4 shows, using Time as the dependent measure, ECF/head pitch is the condition most likely to differentiate patients from controls, for subjects aged 60 to 79 because that condition has the best combination of sensitivity/specificity. The conditions on the floor and with eyes open are too easy for many patients, especially younger people. ECF/head still is challenging but not as challenging as ECF/head pitch. Also, controls aged 60 to 79 had poorer specificity with head still than with head pitch. These findings are supported by previous work in the literature: Jacobson et al reported poor sensitivity and specificity for eyes open and eyes closed conditions with head still.15
Analysis of Head movements
The finding that patients could perform a reduced number of head movements is relatively new, supports our previous study, 9 and provides another measure that clinicians can use during testing. Similar to the finding about Time, as shown in Table 5 the number of head movements is a more challenging measure, especially in head pitch with eyes closed on foam. This measure is easily observed in the clinic during head pitch and can augment testing. For example, a strong 65 year old male might perform the condition for 9 seconds but might make only 3 head motions. Thus he would be considered abnormal.
The finding that the patient groups had difficulty performing head motions is consistent with the underlying pathophysiology. Even the head still conditions were challenging for some subjects and many of these subjects could not perform enough head movements. The differences among the subgroups on time and number of head movements are interesting but the subgroup sizes were too small to draw any conclusions. Future research my address this problem.
Kinematics of trunk movements
As expected we found kinematic differences between the patients and controls with increased scores on kinematic variables in the patient groups. Previous work showed increased trunk sway and other parameters in patients compared to controls 7,16,17,18 but we had different percentages of subjects who provided kinematic data. Notably, our patient groups differed from controls in maintaining mediolateral stability. This finding has implications for performance of functional motor skills. Thus, if the necessary hardware and software are available kinematics provide a useful third level of analysis. These data should be interpreted with caution, however, due to the decreased sample size.
CONCLUSION
Time is a good primary measure, especially for older subjects. The ability to make at least 5 cycles of head movements during each ECF condition is a good secondary measure. If the equipment is available, kinematics are useful tertiary measures. The data from controls indicate a few false postives might still be detected. Balance screening with CTSIB is useful to idicate vestibular function and perhaps to indicate functional skill. It is very inexpensive and useful where computerized dynamic posturogrpahy is not available. It should not, however, be the only determinant of whether someone has normal vestibular function. Other screening measures should also be used.
Acknowledgments
i. Supported by NIH/NIDCD grant 1R01DC009031 to HSC and by a grant from the National Space Biomedical Research Institute through NASA NCC 9-58 (SA02001) to APM.
We thank the staff of the Center for Balance Disorders, and Ross Tonini, AuD, Baylor College of Medicine; and Christopher Miller, Wyle Science, Technology and Engineering Group; for their invaluable assistance.
Footnotes
Location of work:
Data were collected at the Bobby R Alford Department of Otolaryngology – Head and Neck Surgery, Baylor College of Medicine.
Financial Disclosure:
ii/iii. No financial interests in outside companies, etc.
Conflict of interest: None
Presented:
Preliminary analyses were presented as posters at the Association for Research in Otolaryngology Mid-Winter Meeting, Baltimore, February 20, 2011, and the Society for Neuroscience Annual Meeting, Washington, DC, November 15, 2011.
References
- 1.Lanska DJ. The Romberg sign and early instruments for measuring postural sway. Semin Neurol. 2002;22:409–18. doi: 10.1055/s-2002-36763. [DOI] [PubMed] [Google Scholar]
- 2.Nashner LM. Computerized dynamic posturography: clinical applications. In: Jacobson GP, Newman CW, Kartush JM, editors. Handbook of Balance Function Testing. St. Louis, MO: Mosby-Year Book; 1993. pp. 308–34. [Google Scholar]
- 3.Analytic and Reporting Guidelines: The National Health and Nutrition Examination Survey (NHANES) National Center for Health Statistics Centers for Disease Control and Prevention. Hyattsville, MD: National Center for Health Statistics; 2006. [Google Scholar]
- 4.Cohen HS, Cox C, Springer G, Hoffman HJ, Young MA, Margolick JB, et al. Prevalence of abnormalities in vestibular function and balance among HIV-seropositive and HIV-seronegative women and men. PLoS One. 2012;7:e3841. doi: 10.1371/journal.pone.0038419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shumway-Cook A, Horak FB. Assessing the influence of sensory interaction on balance. Phys Ther. 1986;66:1548–50. doi: 10.1093/ptj/66.10.1548. [DOI] [PubMed] [Google Scholar]
- 6.Cohen H, Blatchly CA, Gombash LL. A study of the Clinical Test of Sensory Interaction and Balance. Phys Ther. 1993;73:346–51. doi: 10.1093/ptj/73.6.346. [DOI] [PubMed] [Google Scholar]
- 7.Gill J, Allum JHJ, Carpenter MG, Held-Ziolkowska M, Adkin AL, Honegger F, et al. Trunk sway measures of postural stability during clinical balance test: effects of age. J Gerontol A Biol Sci Med Sci. 2001;56A(7):M438–M47. doi: 10.1093/gerona/56.7.m438. [DOI] [PubMed] [Google Scholar]
- 8.Weirich G, Bemben DA, Bemben MG. Predictors of balance in young, middle-aged, and late middle-aged women. J Geriatr Phys Ther. 2010;33:110–7. [PubMed] [Google Scholar]
- 9.Mulavara AP, Cohen HS, Peters BT, Sangi-Haghpeykar H, Bloomberg JJ. New analyses of the Sensory Organization Test compared to the Clinical Test of Sensory Integration and Balance in patients with benign paroxysmal positional vertigo. Laryngoscope. 2013 doi: 10.1002/lary.24075. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Health and Nutrition Examination Survey Balance Procedures Manual. Centers for Disease Control and Prevention, National Center for Health Statstics. United States Department of Health and Human Services; 2003. [Google Scholar]
- 11.Agrawal Y, Carey JP, Hoffman HJ, Sklare DA, Schubert MC. The modified Romberg Balance Test: normative data in U.S. adults. Otol Neurotol. 2011;32:1309–11. doi: 10.1097/MAO.0b013e31822e5bee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snijders T, Bosker R. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. Thousand Oaks, CA: Sage Publications; 1999. [Google Scholar]
- 13.Ricci NA, de Faria Goncalves D, Coimbra AMV, Coimbra IB. Sensory interaction on static balance: a comparison concerning the history of falls of community-dwelling elderly. Geriatrics and Gerontology. 2009;9:165–71. doi: 10.1111/j.1447-0594.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- 14.Vereeck L, Wuyts F, Truijen S, Van de Heyning PH. Clinical assessment of balance: normative data, and gender and age effects. Int J Audiol. 2008;47:67–75. doi: 10.1080/14992020701689688. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson GP, McCaslin DL, Piker EG, Gruenwald J, Grantham S, Tegel L. Insensitivity of the “Romberg test of Standing Balance on Firm and Compliant Support Surfaces” to the results of caloric and VEMP tests. Ear Hear. 2011;32:e1–5. doi: 10.1097/AUD.0b013e31822802bb. [DOI] [PubMed] [Google Scholar]
- 16.Allum JHJ, Adkin AL, Carpenter MG, Held-Ziolkow M, Honegger F, Pierchala K. Trunk sway measures of postural stability during clinical balance tests: effects of a unilateral vestibular deficit. Gait Posture. 2001;14:227–37. doi: 10.1016/s0966-6362(01)00132-1. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto C, Murofushi T, Sugasawa K, Chihara Y, Ushio M, Yamasoba T, et al. Assessment of postural stability using foam posturography at the chronic stage after acute unilateral peripheral vestibular dysfunction. Otol Neurotol. 2012;33:432–6. doi: 10.1097/MAO.0b013e3182487f48. [DOI] [PubMed] [Google Scholar]
- 18.Balaguer García R, Pitarch Corresa S, Baydal Bertomeu JM, Morales Suárez-Varela MM. Static posturography with dynamic tests. Usefulness of biomechanical parameters in assessing vestibular patients. Acta Otorhinolaryngol Esp. 2012;63:332–8. doi: 10.1016/j.otorri.2012.03.006. [DOI] [PubMed] [Google Scholar]