Abstract
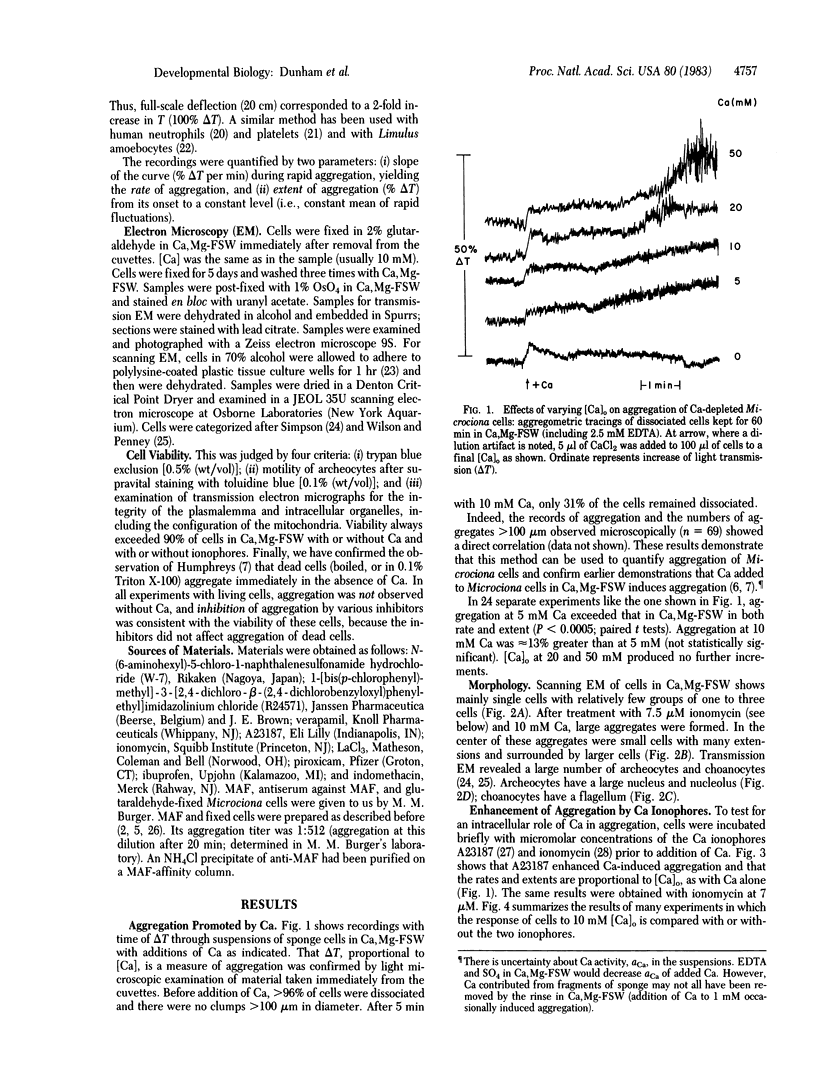
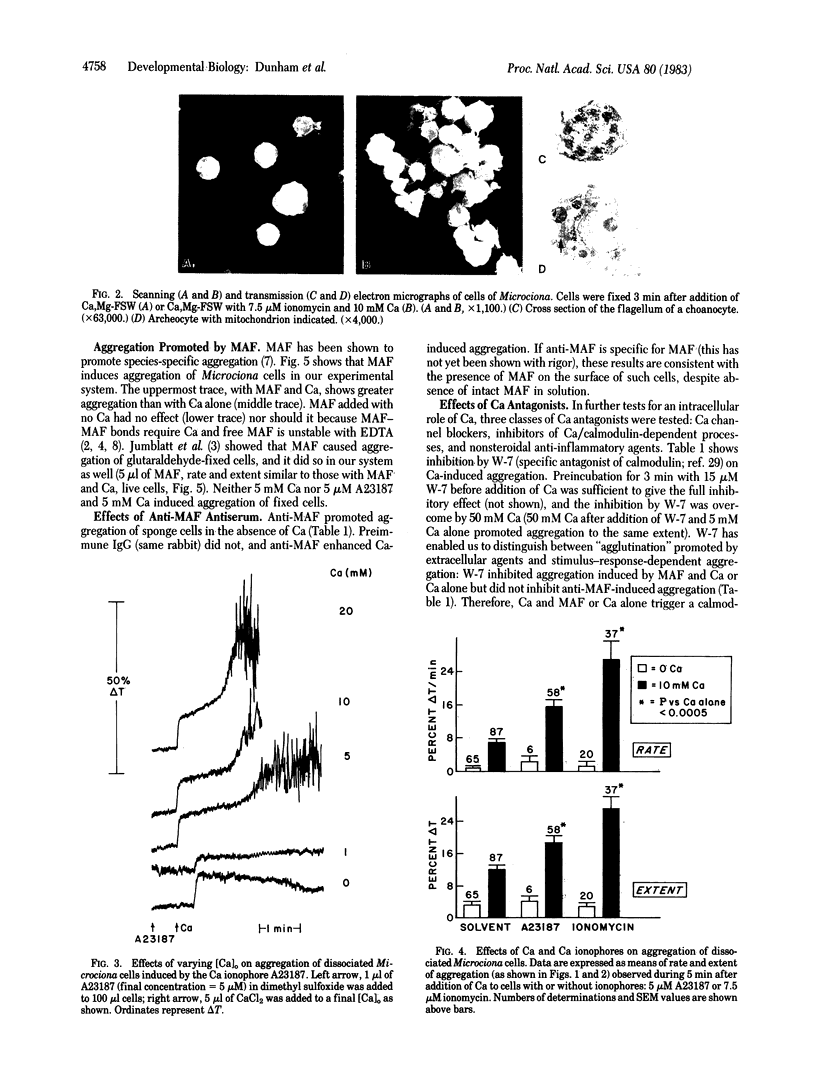
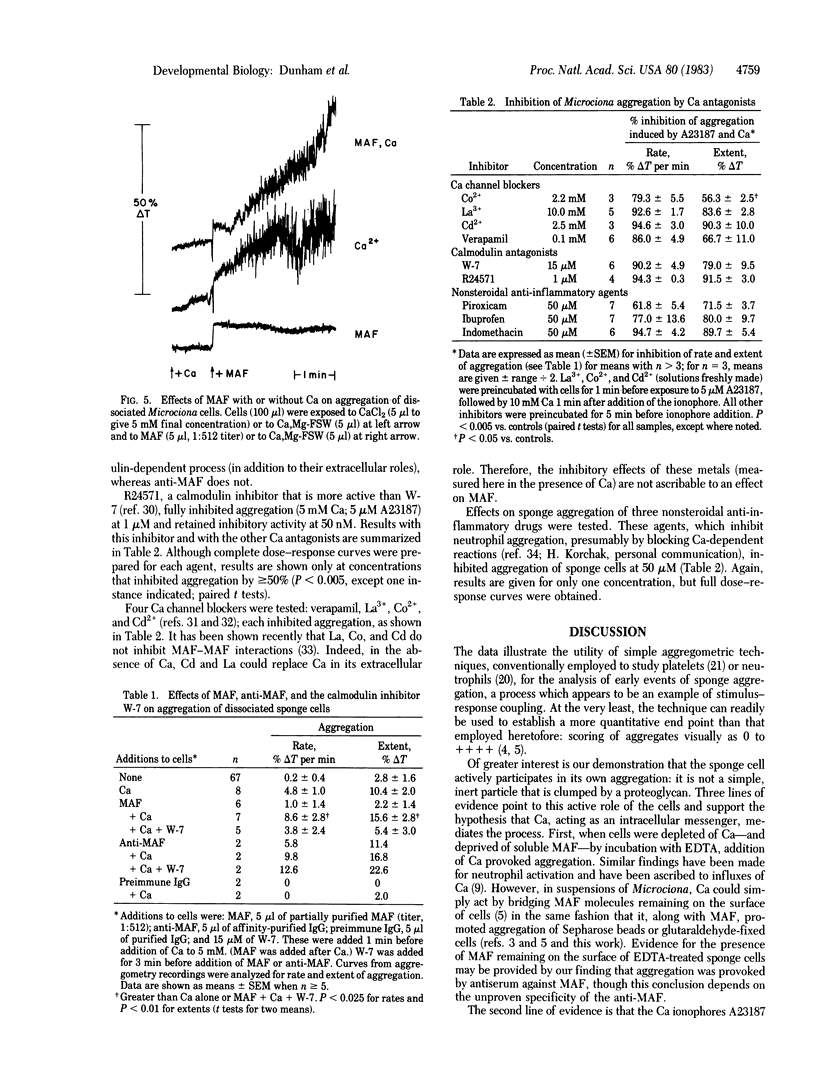
Aggregation of dissociated sponge cells has been proposed as a model for cell-cell recognition mediated by a specific proteoglycan aggregation factor (Microciona aggregation factor). To test whether sponge cells undergo stimulus-response coupling in which intracellular Ca is a messenger, aggregation of mechanically dissociated cells was studied. Changes in light transmission through cell suspensions paralleled aggregation as judged by microscopy. In the presence, but not absence, of Ca (>5 mM) partially purified Microciona aggregation factor aggregated both living and glutaraldehyde-fixed cells. Evidence for a messenger role of Ca was the following: (i) Addition of Ca to Ca-depleted cells induced aggregation that varied with [Ca]. (ii) Addition of Ca ionophores (A23187 and ionomycin) caused aggregation that varied with [Ca] and far exceeded that provoked by Ca alone. Glutaraldehyde-fixed cells did not respond to ionophores with or without Ca. (iii) Calcium antagonists inhibited aggregation. These included inhibitors of the Ca-calmodulin complex (N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide hydrochloride and 1-[bis(p-chlorophenyl)methyl]-3-[2,4-dichloro-β-(2,4-dichlorobenzyloxyl)phenylethyl]imidazolinium chloride), Ca channel blockers (La, Co, Cd, and verapamil), and three nonsteroidal anti-inflammatory agents (indomethacin, ibuprofen, and piroxicam). Results indicated not only that early events of sponge aggregation can be quantified by continuous recording but that aggregation is not simply due to passive agglutination of inert cells by an extracellular proteoglycan. Rather, sponge cells recognize surface ligands to which they respond by Ca-dependent stimulus-response coupling.
Keywords: Ca channel blockers, calmodulin antagonists, nonsteroidal anti-inflammatory agents, Microciona prolifera
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORN G. V., CROSS M. J. THE AGGREGATION OF BLOOD PLATELETS. J Physiol. 1963 Aug;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauldwell C. B., Henkart P., Humphreys T. Physical properties of sponge aggregation factor. A unique proteoglycan complex. Biochemistry. 1973 Jul 31;12(16):3051–3055. doi: 10.1021/bi00740a017. [DOI] [PubMed] [Google Scholar]
- Chandler D. E., Williams J. A. Intracellular divalent cation release in pancreatic acinar cells during stimulus-secretion coupling. II. Subcellular localization of the fluorescent probe chlorotetracycline. J Cell Biol. 1978 Feb;76(2):386–399. doi: 10.1083/jcb.76.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson H. S., Kaplan H. B., Korchak H. M., Smolen J. E., Weissmann G. Dissociation by piroxicam of degranulation and superoxide anion generation from decrements in chlortetracycline fluorescence of activated human neutrophils. Biochem Biophys Res Commun. 1982 Jan 15;104(1):247–253. doi: 10.1016/0006-291x(82)91966-0. [DOI] [PubMed] [Google Scholar]
- Feinstein M. B. Release of intracellular membrane-bound calcium precedes the onset of stimulus-induced exocytosis in platelets. Biochem Biophys Res Commun. 1980 Mar 28;93(2):593–600. doi: 10.1016/0006-291x(80)91119-5. [DOI] [PubMed] [Google Scholar]
- Gietzen K., Wüthrich A., Bader H. R 24571: a new powerful inhibitor of red blood cell Ca++-transport ATPase and of calmodulin-regulated functions. Biochem Biophys Res Commun. 1981 Jul 30;101(2):418–425. doi: 10.1016/0006-291x(81)91276-6. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Horn J. K., Kaplan H. B., Weissmann G. Calcium-induced lysozyme secretion from human polymorphonuclear leukocytes. Biochem Biophys Res Commun. 1974 Sep 23;60(2):807–812. doi: 10.1016/0006-291x(74)90312-x. [DOI] [PubMed] [Google Scholar]
- HUMPHREYS T. CHEMICAL DISSOLUTION AND IN VITRO RECONSTRUCTION OF SPONGE CELL ADHESIONS. I. ISOLATION AND FUNCTIONAL DEMONSTRATION OF THE COMPONENTS INVOLVED. Dev Biol. 1963 Aug;8:27–47. doi: 10.1016/0012-1606(63)90024-1. [DOI] [PubMed] [Google Scholar]
- Henkart P., Humphreys S., Humphreys T. Characterization of sponge aggregation factor. A unique proteoglycan complex. Biochemistry. 1973 Jul 31;12(16):3045–3050. doi: 10.1021/bi00740a016. [DOI] [PubMed] [Google Scholar]
- Hoffstein S. T., Friedman R. S., Weissmann G. Degranulation, membrane addition, and shape change during chemotactic factor-induced aggregation of human neutrophils. J Cell Biol. 1982 Oct;95(1):234–241. doi: 10.1083/jcb.95.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstein S., Goldstein I. M., Weissmann G. Role of microtubule assembly in lysosomal enzyme secretion from human polymorphonuclear leukocytes. A reevaluation. J Cell Biol. 1977 Apr;73(1):242–256. doi: 10.1083/jcb.73.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys S., Humphreys T., Sano J. Organization and polysaccharides of sponge aggregation factor. J Supramol Struct. 1977;7(3-4):339–351. doi: 10.1002/jss.400070307. [DOI] [PubMed] [Google Scholar]
- Jumblatt J. E., Schlup V., Burger M. M. Cell-cell recognition: specific binding of Microciona sponge aggregation factor to homotypic cells and the role of calcium ions. Biochemistry. 1980 Mar 4;19(5):1038–1042. doi: 10.1021/bi00546a032. [DOI] [PubMed] [Google Scholar]
- Kaplan H. B., Edelson H. S., Friedman R., Weissmann G. The roles of degranulation and superoxide anion generation in neutrophil aggregation. Biochim Biophys Acta. 1982 Sep 13;721(1):55–63. doi: 10.1016/0167-4889(82)90023-4. [DOI] [PubMed] [Google Scholar]
- Kenney D. M., Belamarich F. A., Shepro D. Aggregation of horseshoe crab (Limulus polyphemus) amebocytes and reversible inhibition of aggregation by EDTA. Biol Bull. 1972 Dec;143(3):548–567. doi: 10.2307/1540183. [DOI] [PubMed] [Google Scholar]
- Kobayashi R., Tawata M., Hidaka H. Ca2+ regulated modulator protein interacting agents: inhibition of Ca2+-Mg2+-ATPase of human erythrocyte ghost. Biochem Biophys Res Commun. 1979 Jun 13;88(3):1037–1045. doi: 10.1016/0006-291x(79)91513-4. [DOI] [PubMed] [Google Scholar]
- Liu W. C., Slusarchyk D. S., Astle G., Trejo W. H., Brown W. E., Meyers E. Ionomycin, a new polyether antibiotic. J Antibiot (Tokyo) 1978 Sep;31(9):815–819. doi: 10.7164/antibiotics.31.815. [DOI] [PubMed] [Google Scholar]
- Misevic G. N., Jumblatt J. E., Burger M. M. Cell binding fragments from a sponge proteoglycan-like aggregation factor. J Biol Chem. 1982 Jun 25;257(12):6931–6936. [PubMed] [Google Scholar]
- Naccache P. H., Molski T. F., Alobaidi T., Becker E. L., Showell H. J., Sha'afi R. I. Calmodulin inhibitors block neutrophil degranulation at a step distal from the mobilization of calcium. Biochem Biophys Res Commun. 1980 Nov 17;97(1):62–68. doi: 10.1016/s0006-291x(80)80134-3. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Involvement of membrane calcium in the response of rabbit neutrophils to chemotactic factors as evidenced by the fluorescence of chlorotetracycline. J Cell Biol. 1979 Oct;83(1):179–186. doi: 10.1083/jcb.83.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Rice D. J., Humphreys T. Two Ca2+ functions are demonstrated by the substitution of specific divalent and lanthanide cations for the Ca2+ required by the aggregation factor complex from the marine sponge, Microciona prolifera. J Biol Chem. 1983 May 25;258(10):6394–6399. [PubMed] [Google Scholar]
- Smolen J. E., Korchak H. M., Weissmann G. The roles of extracellular and intracellular calcium in lysosomal enzyme release and superoxide anion generation by human neutrophils. Biochim Biophys Acta. 1981 Nov 5;677(3-4):512–520. doi: 10.1016/0304-4165(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Weissmann G. The effect of various stimuli and calcium antagonists on the fluorescence response of chlorotetracycline-loaded human neutrophils. Biochim Biophys Acta. 1982 Apr 29;720(2):172–180. doi: 10.1016/0167-4889(82)90009-x. [DOI] [PubMed] [Google Scholar]