Abstract
Anhedonia, or the inability to experience positive feelings is a hallmark of depression. However, few animal models have relied on decreased positive affect as an index of susceptibility to depression. Rats emit frequency modulated ultrasonic vocalizations (USVs), designated as “positive” calls in the 50kHz range. USVs have been associated with pharmacological activation of motivational reward circuits. Here we utilized selectively-bred rats differing in “emotionality” to ask whether there are associated differences in USVs. Rats bred based on locomotor response to novelty and classified as bred high-responders (bHRs) or bred low-responder (bLRs) exhibit inborn differences in response to environmental cues, stress responsiveness, and depression-like behavior. These animals also exhibit differences in anxiety-like behavior, which are reversed by exposure to environmental complexity (EC). Finally, these animals exhibit unique profiles of responsiveness to rewarding stimuli accompanied with distinct patterns of dopamine regulation. We investigated whether acute and chronic environmental manipulations impacted USVs in bHRs and bLRs. We found that, relative to bLRs, bHRs emitted significantly more 50 kHz USVs. However, if a bLR is accompanied by another bLR, there is a significant increase in 50kHZ USVs emitted by this phenotype. bHRs emitted increases in 50kHZ UVSs upon first exposure to EC, whereas bLRs showed a similar increase only after repeated exposure. bLRs’ increase in positive affect after chronic EC was coupled with significant positive correlations between corticosterone levels and c-fos mRNA in the accumbens. Conversely, a decline in the rate of positive calls in bHRs after chronic EC was associated with a negative correlation between corticosterone and accumbens c-fos mRNA. These studies demonstrate that inborn differences in emotionality interact with the environment to influence positive affect and underscore the potential interaction between glucocorticoids and the mesolimbic reward circuitry in modulating 50 kHz calls.
Keywords: Ultrasonic vocalization, Affect, Emotionality, Individual Differences
Major Depressive Disorder (MDD) is a clinical state characterized by unrelenting negative mood and the inability to experience positive thoughts and emotions. Consequently, efforts examining the neurobiology of depression have focused on animal models of negative affect, with a remaining major gap concerning the neurobiology of anhedonia as a core symptom of major depression. Advances have been made towards studying positive affect, however, our understanding of the neural circuitry of positive behaviors stems primarily from studies of reward mechanism—e.g. in response to food or drugs of abuse. These studies do not typically consider the spontaneous propensity to react positively to environmental stimuli, nor the role of physical and social features of the environment in triggering positive affective responses, or lack thereof, as seen in depressed individuals. Yet, basal differences in affective responsiveness likely lie at the core of vulnerability to mood and anxiety disorders. Addressing the question of propensity for positive affect vs. anhedonia requires a means of detecting positive affect as well as a validated animal model of differential vulnerability to anxiety- and depression-like behavior.
For more than 10 years, the measurement of 50 kHz Ultra-Sonic Vocalizations (USV) has offered a valuable index of positive affect in rodents (Burgdorf and Panksepp, 2006). Thus, frequency modulated 50-kHz calls have been associated with positive valence, and are reliably elicited during, and in anticipation of, various rewarding stimuli including food, sex and brain stimulation (Burgdorf et al., 2000) For example amphetamine administered either intraventricularly or directly into the shell of the nucleus accumbens (NAcc) increases 50 kHz USVs (Burgdorf et al., 2001). Thus, the mesocorticolimbic reward circuitry has been proposed to underlie 50-kHz USV calling and its associated positive state (Burgdorf et al., 2007). Here we will focus on USVs as a measure of positive affect and investigate its neural correlates in an animal model of differential reactivity to environmental stimuli.
Animals selectively bred based on their response to a novel environment, bred High Responders (bHR) and Low Responders (bLR), are characterized by significant differences in emotional reactivity. Thus bLRs, which are characterized by low exploration when exposed to a novel environment, are more prone to exhibit negative affect including high anxiety and increased depression-like behavior (Garcia-Fuster et al., 2012; Stedenfeld et al., 2011; Perez et al., 2009; Stead et al., 2006). Interestingly, repeated exposure to environmental complexity (EC) selectively benefits bLRs by reducing their anxiety-like behavior (Perez et al., 2009). However, it has yet to be determined whether the benefits of EC to bLRs’ emotionality are also manifested as increases in positive affect.
Beyond reducing anxiety, EC is known to have antidepressant effects (Llorens-Martin et al., 2011, Hendriksen et al., 2012) and confer stress resilience in several animal models of depression (Lehmann and Herkenham, 2011). Moreover, several brain regions including the infralimbic cortex, prelimbic cortex, and nucleus accumbens have been implicated in resilience mechanisms. EC has also been shown to positively influence antidepressant outcome response in mice exposed to chronic stress (Branchi et al., 2013). Still, while the mechanisms of EC have mostly been attributed to mitigating stress reactivity (Schloesser et al., 2010), it is not known whether EC has any impact on modulating positive affect.
The bHR and bLR lines also differ in reward signaling pathways. In particular, bHRs exhibit a higher frequency of spontaneous dopamine ‘release events’, enhanced DA response to reward-associated cues, and elevated sensitivity to dopamine agonists (Flagel et al., 2011; Flagel et al., 2010). Moreover, bHRs show greater psychomotor sensitization to repeated cocaine treatment (Garcia-Fuster et al., 2010) and increased motivation to take cocaine (Cummings et al., 2011).
In the current study we ask whether bHR and bLR rats exhibit differences in positive affect and whether the reward circuit may be implicated in affective responses to the environment in these animals. We first examined whether bHRs and bLRs exhibited individual differences in 50kHz USVs and determined the extent to which this measure could be altered by changes in the environment. Environmental manipulations included altering social group experience and increasing environmental complexity under acute and chronic conditions. Neural correlates of positive affect were studied by using c-fos in situ hybridization in the shell and core of the Nucleus Accumbens and in other regions implicated in reward including the infralimbic and prelimbic medial prefrontal cortex (mPFC) (Cardinal et al., 2002) and the dorsal and ventral periaqueductal gray matter (dPAG and vPAG) (Olmstead and Franklin, 1997). We also analyzed corticosterone (CORT) levels after the acute and chronic experimental manipulations. As CORT levels increase in conjunction with behavioral responses to rewarding stimuli such as cocaine and opiates (Marinelli and Piazza, 2002), we hypothesized that increased USVs would be associated with increases in CORT levels.
Experimental Procedures
Animals
Adult Male Sprague-Dawley rats were obtained from our in-house breeding colony at the Molecular and Behavioral Neuroscience Institute (MBNI) where we have maintained the bHR-bLR lines for over 35 generations. bHR-bLR lines are selectively bred based on differences in exploratory response to novelty, a trait initially used to predict individual differences in drug-taking behavior (Piazza, 1989). A detailed description of the breeding strategy and behavioral characterization of the bHR-bLR differences in anxiety- and depression-like behavior has been previously published (Stead et al., 2006; Garcia-Fuster et al.,2012). Rats were housed in pairs, with cagemates from the same phenotype group (i.e. one cage contains 2 bLRs or 2 bHRs) and kept on a 12 h light/dark cycle (lights on at 6:00 am) with food and water available ad libitum. Rats were allowed to acclimate to the housing conditions for at least 7 days prior to any experiments. All animals were treated in accordance with the National Institutes of Health guidelines on laboratory animal use and care, in accordance with the guidelines set by the University Committee on Use and Care of Animals (UCUCA) at the University of Michigan.
Locomotion Testing
At postnatal day 55–60, rats were screened for locomotor response to a novel environment to confirm the bHR-bLR phenotype. Rats were placed in a standard size (43×21.5×24.5 cm) clear acrylic cage in a room adjacent to their colony room. Locomotor activity was monitored every 5 minutes for 1 hour by two panels of photocells connected to a computer. The first panel of three photocells was placed at the bottom of the cage to record horizontal locomotion, with the second panel of five photocells located near the top of the cage to determine rearing behavior. The locomotion testing rig and recording software were created in-house at the University of Michigan. Locomotor activity was tested between 9:00 and 11:30 am. Final locomotion scores were determined by summing horizontal and rearing activities. After screening for locomotor response to novelty, rats were obtained for the current studies around 70 days of age.
Experimental Arena
A 91.4 × 91.4 × 91.4 cm stainless steel cage was used for every session serving as the context for recording frequency modulated 50 kHZ USVs. The cage was always located in the same room adjacent to the colony room throughout both studies. The same cage was used as either the control environment (control, no toys) or that of environmental complexity (EC) for all experimental groups in Study 1 and Study 2. Complexity of the environment was achieved by the addition of the same 23 plastic toys differing in size and shape. Toys filled the EC cage to about 25 cm above the floor. Although all the EC sessions consisted of the same toys, the environmental setup may have been somewhat different based on the random reorganization of the toys at every new session by the experimenter. Animals could also climb over the toys, and explore the spaces above and beneath toys, which also could have made some toys move around. This also, at times, limited visibility of animals during their exploration.
USV Data Analysis
50 kHz USV calls were recorded with the UltraSoundGate condenser microphone CM16 (Avisoft, Bioacoustics, Germany), which was positioned at 40cm above from toys. The microphone was sensitive to frequencies of 20kHz–150kHz and was connected via the ultra 16 USB Audio Device (Avisoft Bioacoustics) to the computer containing the Avisoft Recorder software (version 2.95, Avisoft Bioacoustics), which recorded the calls in real time. Acoustic data was analyzed using SASLab Pro version 5.0 (Avisoft, Bioacoustics). Audio files were converted into a spectrogram and USV calls were quantified manually by an observer blind to experimental conditions. To be included in the analysis, quantified calls needed to be frequency modulated (FM) 50 kHz USV vocalizations showing a broad bandwidth within a frequency range of 35–85 kHz, and have a short duration (30–50 ms) (Knutson et al., 1998). USV data was sampled in 300 sec time bins for Study 1 and 200 sec time bins for Study 2.
Study 1. Recording of 50 kHz USV responses in bHR and bLR rats
To establish a baseline characterization of positive affective responses, rats (single or paired) were placed in a simple or complex environment (control vs. EC) either alone or in same-phenotype pairs for a single 30-min exposure, and 50kHz USV calls were recorded. The average number of FM 50kHz USV calls was obtained from six 5-min bins per session. No animal was repeated for this study as we were testing basal differences in USV responses in a complex or simple environment. Regardless of the number of animals exposed in each session, paired or single, the average number of calls was standardized to obtain the mean number of calls per individual animal in each session.
Study 2. Recording of 50 kHz USV responses during repeated exposure to environmental complexity in bHR groups vs. bLR groups
bHR and bLR rats experienced either acute (one exposure) or repeated exposure (3-times per week, every other day for 3 weeks = 9 exposures total) to a simple (control) or complex environment (EC). Animals were exposed in groups of six consisting of the same phenotype, with the same six animals being exposed as a group during each of the repeated EC or control sessions. This protocol design was chosen based on our preliminary data showing that such protocol duration was sufficient for reducing anxiety in bLR animals, and increasing USVs in outbred Sprague-Dawleys rats. In addition to USVs, behavior was video-recorded, corticosterone levels were measured, and brains were obtained (see below). For Study 2, 200 sec bins were sampled in the data analysis to maximize the number of data points available (9) for each session. The number of calls from each group of 6 animals for each experimental group session was averaged to obtain the mean number of calls per individual animal. The effects of exposure (control vs. EC) and duration (acute vs. chronic) were analyzed using a two-way ANOVA for each phenotype separately.
Behavioral Analysis
Behavior was analyzed by a blind observer using Noldus Observer (Leesburg, VA, USA) software to characterize locomotion, aggressive and social behaviors. All videos were collected on day 9 (capturing the first day of exposure for acute groups and the last day for chronic groups). Data presented illustrate the total number of bouts of a particular behavior, for any given animal in a specified quadrant of the screen. Locomotion was defined as walking, running, etc. without interacting with another rat. Social behavior was defined by sniffing, exploring, or playing with other rats without exhibiting aggression. Aggression was defined as clear exhibition of aggressive interactions as previously described by (Mackintosh and Grant, 1966) and (Miczek, 1974) (e.g. aggressive posture, both animals in mutual upright posture). The behavioral analysis was focused on a defined quadrant of the screen, and sampled based on what was readily visible behavior within that quadrant. It should be noted that some behaviors may have been missed when animals where exploring or hiding beneath the toys of the EC.
Corticosterone (CORT) Analysis
CORT levels were determined using an ImmuChem™ double antibody radioimmunoassay (MP Biomedicals, Orangeburg, NY). Plasma levels for corticosterone were obtained from trunk blood 20 minutes after the end of the first or last exposure to the EC or control environment (Day 9). In addition to the 8 treatment groups, we analyzed basal bHR and bLR control groups.
In situ Hybridization
At the conclusion of Study 2, rats were sacrificed by rapid decapitation 20 minutes after the end of their experimental manipulation (50 minutes after being placed in the environment, see Figure 1) and their brains removed, snap frozen in isopentane, and stored at −80°C. Brains were cryostat sectioned at −20°C at 10 μm in series throughout the brain, beginning around Bregma level 5.20 mm and ending around Bregma level −9.30 mm, mounted on Super Frost Plus slides (Fisher Scientific) and stored at −80°C until processed. In situ hybridization methodology has been described in detail elsewhere (Kabbaj et al., 2000). The c-fos probe was a 783-base-pair fragment directed against the rat c-fos mRNA. The probe was labeled in a reaction mixture consisting of 1 μg of linearized plasmid, 1X transcription buffer (Epicenter Technologies, Madison, WI), 125 μCi of 35S-labeled-UTP, 125 μCi of 35S-CTP, 150 μM ATP and GTP, 12.5 mM dithiothreitol, 1 μl of RNase inhibitor, and 1.5 μl of T7 RNA polymerase.
Figure 1.
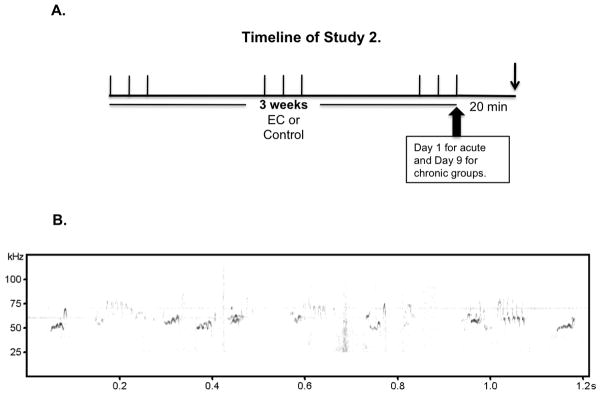
A) Chronic exposures where 30-minutes long every other day (labeled by each individual stand-up line in the diagram) throughout a three-week period. Acute exposures were performed on the last day of chronically exposed animals. On the last day blood was drawn for CORT analysis 20 minutes after exposure and brains were collected for in situ hybridizations studies (upside down arrow). (n=6 animals per group). B) Representative sonogram depicting frequency modulated 50-kHz USV calls.
Radioactive signals were quantified using computer-assisted optical densitometry software (Image J; Image processing and Analysis in Java). The regions analyzed were the prelimbic and infralimbic cortices (between Bregma 4.2 and 2.2 mm with values obtained from ~22 sections) the core and shell of the nucleus accumbens (between Bregma 2.2 and 0.7 mm with values obtained from ~10 sections), and the dorsal (dPAG) and ventral (vPAG) periaqueductal grey (between Bregma −6.30 and −8.50 mm with values obtained from ~12 sections) (see Figure. 7). These regions were targeted as they have previously been implicated in regulating affective responses to environmental stimuli (Quirk and Mueller, 2008) and in association with USVs (Burgdorf et al., 2007). The region of interest was outlined from both hemispheres and the signal threshold was defined as the mean gray value of background plus 3.5X its standard deviation. Only pixels with gray values exceeding the above-defined threshold were included in the analysis. Data from multiple sections per animal, obtained from one value per hemisphere, (i.e. 2 values per section) for each region of interest, were averaged resulting in a mean signal value for each animal and then averaged for each group.
Figure 7.
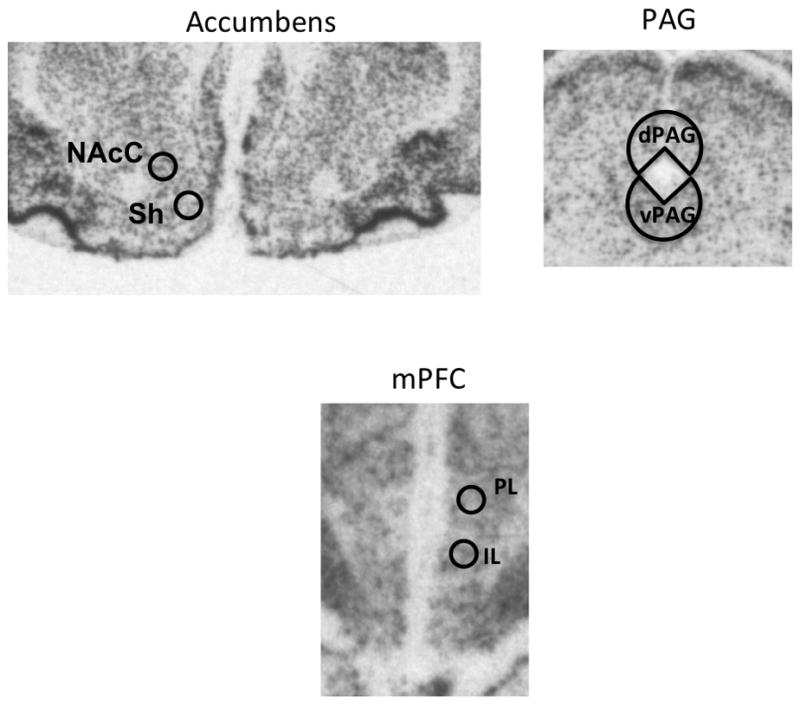
Representative images of c-fos expression for quantified regions; (Sh; Shell, NAcC; Core, IL; Infralimbic, PL; Prelimbic, dPAG; dorsal Periacueductal Gray.
Statistical Analyses
Data were analyzed using t-test or two-way ANOVA with Bonferroni corrected pairwise comparisons for post-hoc analysis. Two-way ANOVA was performed for both phenotypic groups separately in Study1, with exposure (control vs. EC) and group (Paired vs. Unpaired) as independent factors. In Study 2, two-way ANOVA, were performed with exposure (control vs. EC) and duration (acute vs. chronic) as independent factors. For Study 2, the USVs emitted during the last day of chronic exposure (Day 9) were compared with those emitted by the acute exposure group, as both occurred on the same day. For locomotion, social and aggressive behaviors, we performed a three-way ANOVA design with phenotype (bHR vs bLR), exposure (control vs. EC) and duration (acute vs. chronic) as independent factors. We also examined bivariate Pearson’s correlations for c-fos expression across the quantified structures and CORT. Data are presented as mean +/− standard error and statistical significance is assumed at p<0.05. Correlations were performed separately for every experimental group in Study 2 to determine how patterns of correlations differed between groups in terms of the relationship between c-fos expression and CORT. All data were analyzed using SPPS 20.
Results
Study 1
bHRs emit a higher number of frequency-modulated 50 kHz calls relative to bLRs
Given that bHRs and bLRs have been selectively bred based on individual differences in locomotor response to novelty, we first determined whether they exhibit differences in the number of FM 50kHz USV calls outside of their home cage. As presented in Figure 2A, bHRs show a significantly higher number of 50 kHz calls relative to bLRs under these conditions [t(10)= 2.7, p=0.02].
Figure 2.
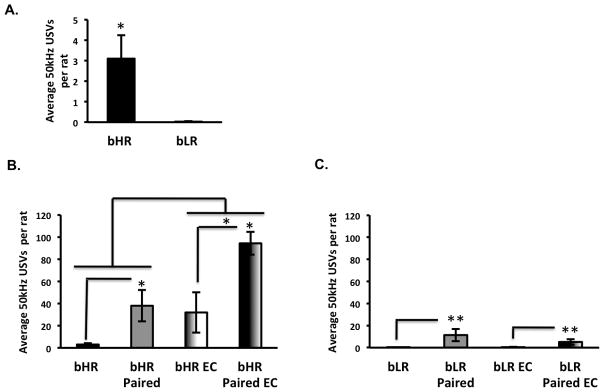
A) Individual bHR animals elicit a higher number of calls relative to single bLRs in the control cage. (n= 6 per group; *p<0.05) *** t-test pair-wise comparison from animals. B) bHRs show a significant increase in 50 kHz USVs when exposed to a complex environment. Presence of a cage mate also increased USVs in bHR. C) bLR animals show a significant increase in the number of 50 kHz USVs when accompanied by another bLR when acutely exposed to a control environment or EC. (*p<0.05) (**p<0.01) bonferroni post-hoc pair-wise comparisons.
Acute exposure to a complex environment increases the number of frequency-modulated 50kHz calls in bHRs, but not bLRs.
Presence of a cagemate increases frequency-modulated 50kHz calls in both bHRs and bLRs.
As EC reduces anxiety (Perez et al., 2009), we examined whether EC would elicit an increase in the number of FM 50kHz USVs. We first asked whether such effects would occur during an acute exposure to a complex environment, and whether they would be altered by the presence or absence of a cagemate. There were main effects of exposure [F(1,17)= 8.7, p=0.009], and group [F(1,17)= 7.5, p=0.01] in bHRs, with EC and Paired animals showing an overall greater number of FM 50kHz USVs compared to controls and unpaired bHR rats. Thus, bHRs showed a significantly higher number of USVs in a complex environment regardless of whether they were alone or with a cagemate, while having a cagemate present also increases USVs in bHRs independent of whether or not the environment was complex (Figure 2B). In contrast EC does not increase USVs in bLRs. However, bLRs show a significantly higher number of USVs when they are accompanied by another bLR during exposure to a simple or complex environment as shown by a main effect of group [F(1,18)= 13.2, p=0.002] (Figure 2C).
Study 2
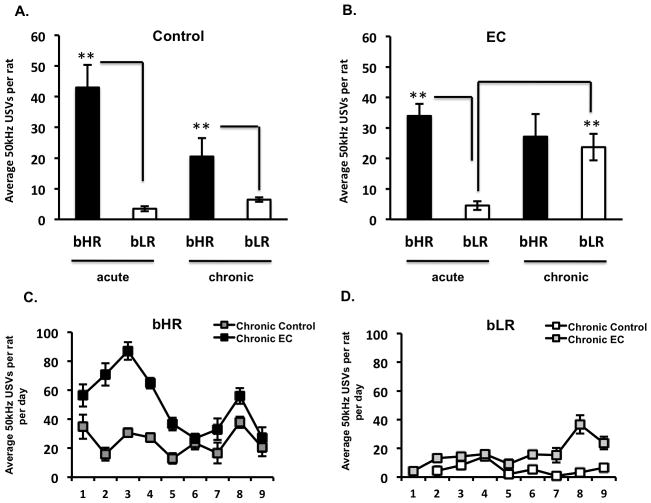
Repeated exposure to a complex environment increases 50-kHz USV calls only in bLRs
As described above, there are clear differences between bHRs and bLRs in the number of 50 kHz USVs emitted during acute exposure to a simple environment or to EC. However, given that these animals were bred for differences in response to novelty, we wanted to examine the impact of chronic exposure to EC on USVs in bHRs and bLRs. Our results showed that in bHR animals, there was a main effect of duration [F(1,32)= 7.97, p=0.008], with acute animals showing an overall higher number of FM 50kHz USVs relative to chronic animals (Figures 3A and 3B). On the other hand in bLRs, there were main effects of exposure [F(1,32)= 21.8, p=0.00005] and duration [F(1,32)= 16.4, p=0.0003] along with an interaction [F(1,32)= 17.7, p=0.0002], with significant increases in the average number of FM 50kHz USVs specifically observed after chronic EC (Figure 3B, 3D). Thus, upon repeated exposure to EC, bLRs’ rate of FM 50kHz USVs resembled that of bHRs’ (Figure 3B). Upon closer examination of the trajectory of the number 50kHz USV calls emitted by chronic groups we saw that in bHRs, chronic EC increased USVs on the 3rd day [t(16)= 2.99 p=0.009] relative to day 1. However, such increases in USVs in bHRs dissipated after repeated exposures (Figure 3C). Conversely, the increases in 50kHz USV calls emitted by bLRs during chronic EC were observed starting on the 6th day [t(16)= −3.5 p=0.003] relative to day 1 (Figure 3D).
Figure 3.
Chronic EC increases 50 kHZ USVs in bLRs and not bHRs. A) bHRs show no significant differences in 50 kHz USVs when exposed to EC under chronic conditions relative to controls. B) Conversely, bLR animals chronically exposed to EC showed a significant increase in the number of 50 kHz USVs. C) USVs responses to EC are weakened by repeated exposures in bHRs were an initial increase is lost upon repeated exposure. D) bLR showed an opposite trajectory in their USV response to chronic EC, where an increase emerged towards the end after repeated exposures (*p<0.05) (**p<0.01) bonferroni post-hoc pair-wise comparisons.
bHR and bLR differences in locomotion, social interaction, and aggression are reversed after repeated exposure to a complex environment
As expected bHRs showed significantly more locomotion [F(1,40)=59.7, p=0.0000001] than bLRs as seen by a main effect of phenotype (Figure 4A). There was also a main effect of EC on reducing locomotion [F(1,40)=58.9, p=0.0000001]. Interestingly, upon exposure to EC, the overall innate differences between the phenotypic groups disappeared due to the specific decreases in activity in bHRs as seen by a phenotype x exposure interaction [F(1,40)=14.8, p=0.0004]. bHRs also showed more social behavior than bLRs [F(1,40)=29.3, p=0.000003] as seen by main effects of phenotype (Figure 4B). Moreover, there was a main effect of EC exposure on social behavior [F(1,40)=18.3, p=0.0001] with EC decreasing overall social contacts. However, there was also a phenotype x exposure interaction [F(1,40)=9.5, p=0.003] on social behavior where EC reduced social contacts specifically in bHRs (Figure 4B). Finally, as expected (Kerman et al., 2011), bHRs show more aggressive behavior than bLRs as seen by a main effect of phenotype [F(1,40)=16.7, p=0.0002] (Figure 4C). There was also an interaction effect of exposure and duration [F(1,40)=6.0, p=0.019] on aggressive behavior along with a phenotype x exposure x duration interaction, [F(1,40)=7.7, p=0.008] showing that bHRs appear to increase their aggressive behavior after they have been chronically exposed to the control cage for 9 days relative to bHRs exposed to the control cage for 1 day.
Figure 4.
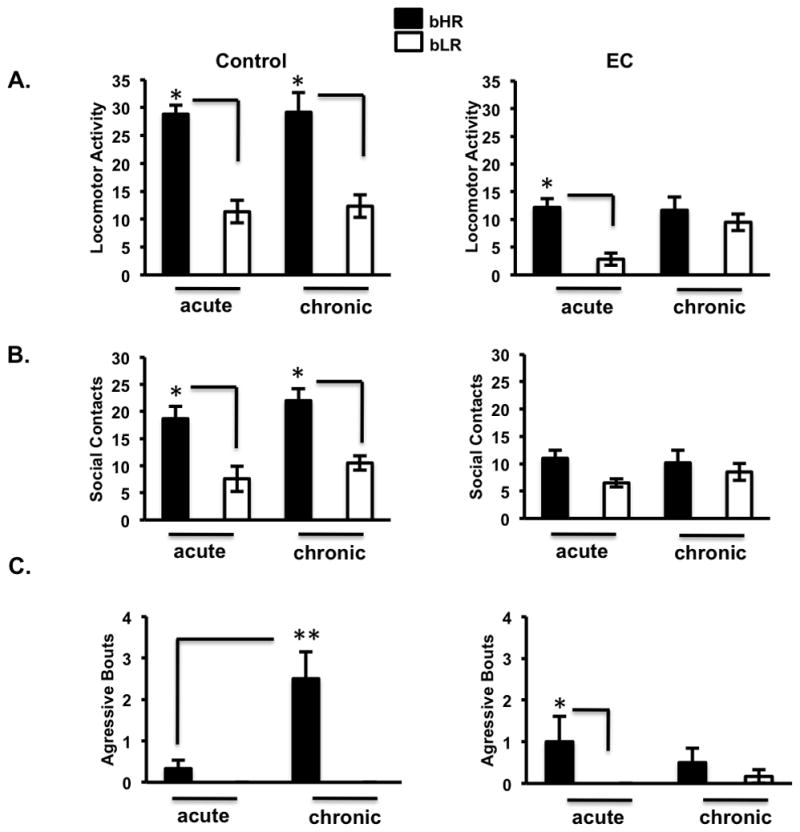
Exposure to a complex environment reverses bHR and bLR differences in locomotion, social interaction, and aggression. A) bHRs exposed to chronic EC showed significantly lower locomotion relative to chronic bHR controls. Thus, chronic EC reverses innate differences between the phenotypic groups where bHRs displayed the same levels of locomotion relative to bLRs. B) Exposure to EC decreases social behavior in bHRs, which reverses phenotype differences in acute and chronic EC exposed animals (n= 6 animals per group; *p<0.01). C) Exposure to EC reduces the significantly high levels of aggression seen in bHR under chronic exposure to a control cage. Such reversal disrupts phenotype differences between bHR and bLR under chronic EC conditions (*p<0.05; **p<0.01) bonferroni post-hoc pair-wise comparisons.
Exposure to a complex environment results in a differential increase in CORT in bHRs
Upon termination of chronic exposure to a complex environment we saw that bHRs show significantly higher levels of CORT relative to bLRs [F(1,50)=6.42 p=0.01] (Figure 5). There was also a main effect of EC [F(1,50)=4.21, p=0.04] as shown by increased CORT levels in animals exposed to EC (acute and chronic) relative to baseline. Moreover, chronic EC differentially increased CORT in bHRs relative to bLRs, as indicated by a significant phenotype x condition x duration interaction [F(1,50)= 4.1, p=0.04]. In sum, significant differences in CORT between the phenotypes were only apparent after chronic exposure to EC.
Figure 5.
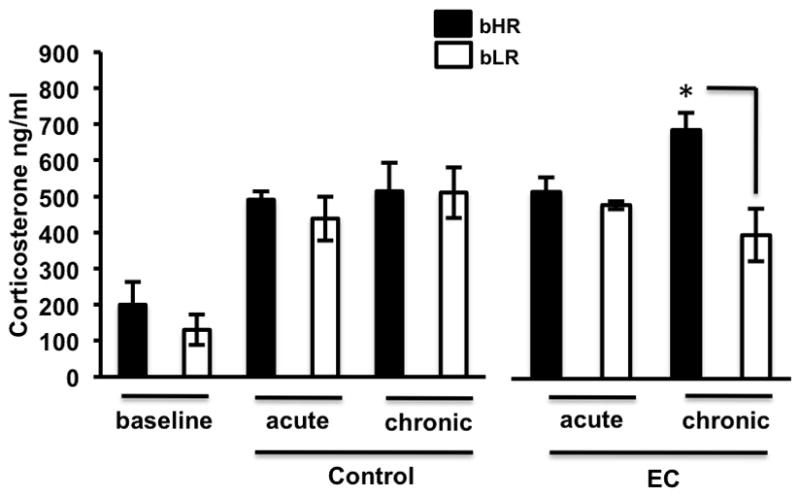
bHR animals exhibited a differential significant increase in CORT upon chronic exposure to EC relative to all groups. An interaction effect showed that chronic EC differentially increased CORT in bHRs relative to bLRs, thus after chronic EC bHRs showed significantly higher levels of CORT relative to chronic EC bLRs (*p=0.001).
Repeated exposure to a complex environment results in significant and distinct correlations between c-fos expression in the nucleus accumbens and CORT in bLRs vs bHRs
We found that c-fos mRNA was increased to a similar degree relative to controls for both phenotypic groups in response to all conditions and across brain regions. Thus, there was no condition that differentially activated bHRs vs. bLRs in a consistent manner, at least based on c-fos expression. This led us to focus our efforts on evaluating “connectivity patterns” and correlations, which revealed some interesting phenotype-dependent relationships in c-fos activation among several brain regions and between c-fos activation patterns and CORT response. As illustrated in Figure 6, we found a significant positive correlation between CORT and c-fos mRNA in the core and shell of the accumbens of bLR animals exposed to chronic EC (Core: r=0.89 and Shell: r=0.73; p=0.009 and p=0.05 respectively). Interestingly, an opposing pattern was observed in bHR animals exposed to chronic EC, where CORT correlates negatively with the accumbens c-fos expression (Core: r=−0.79 and Shell: r=−0.83; p=0.03 and p=0.02). Correlations of c-fos expression between structures for the acute bLR control groups also presented a distinct pattern of correlations for the dorsal PAG (dPAG). Specifically, the dPAG correlated negatively with the prelimbic cortex; r=−0.876, infralimbic cortex; r=−0.75, shell; r=−0.88, and core; r=−0.70. However, these negative correlations did not reach statistical significance.
Figure 6.
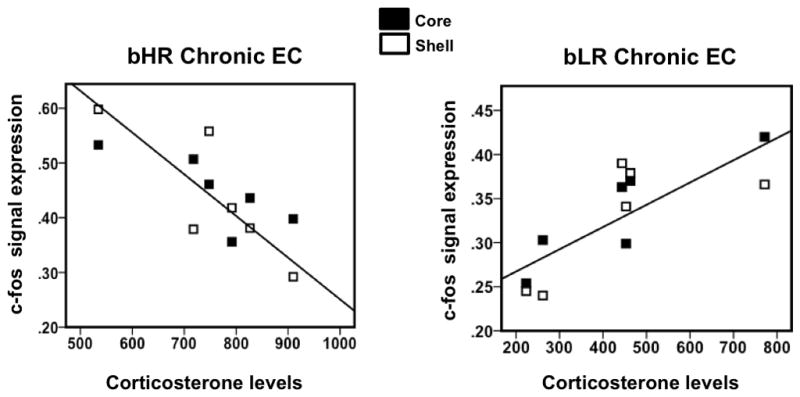
Bivariate correlations of CORT and accumbens c-fos expression for bHR and bLR animals exposed to chronic EC. bLRs displayed a distinct pattern of correlation of CORT with c-fos in the accumbens (Core: r=0.89 and Shell: r=0.73; p=0.009 and p=0.05 respectively), whereas bHR exposed to chronic EC displayed an opposing pattern were CORT correlates negatively with the accumbens c-fos expression (Core: r=−0.79 and Shell: r=−0.83; p=0.03 and p=0.02 respectively).
Discussion
The current studies were aimed at uncovering whether bHRs and bLRs rats, which show innate differences in negative affect-related behaviors (Garcia-Fuster et al., 2012; Stead et al., 2006) and differences in accumbens response to the rewards and environmental stimuli (Flagel et al., 2011), displayed differences in positive affect. Given that the nucleus accumbens has been proposed as a mediator of USVs (Burgdorf et al., 2007) we explored whether accumbens c-fos activation was associated with increased USVs. Moreover, since we have previously shown that differences in anxiety-like behaviors in bHR vs. bLRs could be modulated by environmental factors (Perez et al., 2009), we specifically examined the impact of environmental modulators (i.e. complex environment, social interactions) on 50 kHz USVs. Our main findings show that; 1) bHRs emit more 50 kHz USVs relative to bLRs in a simple context different from their home cage. 2) The presence of a familiar animal (i.e. cagemate) increases 50 kHz USVs in both bHRs and bLRs in this same context. 3) Acute EC increases USVs, only in bHRs, whereas chronic exposure decreases USVs in bHRs. 4) bLRs require repeated exposure to EC to show a significant increase in USVs. 5) bLRs exhibited a highly significant positive correlation between CORT levels and c-fos mRNA in the core and shell following repeated exposure to EC. Conversely, a significant increase in CORT levels after chronic EC, correlated negatively with c-fos expression in the shell and core of the nucleus accumbens of bHRs.
bHRs emit higher levels of 50kHZ USVs
Our initial study showed that bHRs emit higher levels of USVs compared to bLR rats in a context different from their home cage. This extends our previously reported differences in anxiety and depression like-behavior in these bred lines (Stead et al., 2006; Garcia-Fuster et al., 2012). These differences in USV between bHRs and bLRs are based on a comparison of USV calls in a different context. Thus, we acknowledge that without having a baseline level of USVs we can’t know for certain if there is a true increase in bHR and a decrease in bLRs upon exposure to a new context. However, our data is consistent with previous reports showing that heritable factors influencing emotionality are related to individual differences in USV responses (Burgdorf et al., 2005; Panksepp et al., 2000). For example, animals that were bred for high rates of 50 kHz USVs display decreased anxiety-like behavior (Burgdorf et al., 2009), resembling our bHR rats (Stead et al., 2006). Thus, differences in 50 kHz USV in our bHR-bLR lines may in part be driven by heritable factors contributing to variation in positive affect.
Effects of social interactions on USVs
Environmental factors may also modulate individual differences in positive affect. The influence of the environment on USVs was initially assessed in the presence or absence of a cagemate during exposure to an unfamiliar context. Our results showed that while bLRs are characterized by low positive affect in an unfamiliar context, exposing them with their cagemate significantly enhances their number of 50 kHz USVs. This suggests that bLRs, may not sense the environment the same way when they are accompanied by a cagemate vs. when they are alone, and that social interactions may promote positive affect. Indeed, social pairing in bLRs may be serving a social buffering phenomenon (Kiyokawa et al., 2007), reducing anxiety and aversion to unfamiliar environments, which in turn may facilitate positive affect. bHR rats exposed to either the control or enriched environment with a cagemate also emitted more USV calls relative to those exposed alone. These data are consistent with recent reports showing that social interaction increases USVs (Willey and Spear, 2012) and suggest that social interactions are invoking a reward response in both phenotypes (Fritz et al., 2011 Panksepp et al., 2007, Panksepp and Lahvis, 2007). In the current study, we did not assess whether the increase in USVs is dependent upon whether the “paired animal” is familiar or not. bLRs are by definition a bit more averse to novelty relative to bHRs, and it could very well be that familiarity is an important factor for bLRs; whereas in bHRs, USVs might equally be enhanced by the presence of an unfamiliar animal. This is an interesting issue that will be addressed in future studies.
Differential effects of EC on bHR and bLR USVs
It is quite clear that chronic exposure reduces USVs in bHRs both under control and EC conditions. However, USV call reductions under chronic control and chronic EC reflect different dynamic profiles, suggesting that different factors might be driving the reduction of USV calls.
A complex environment (EC) increases USVs in bHRs, at least acutely, relative to the simple control context. Following this initial rise, 50 kHz USVs decline upon repeated exposure to EC. In contrast, the decline in USVs under chronic control conditions seems more stable. These differential trends in reduction of USVs are likely indicative of overall differences in habituation processes. For example, after chronic exposure to control conditions, there is an increase in aggressive encounters that accompanies unchanging high social interactions. On the other hand, after chronic EC, both of these behaviors are reduced. It is possible that in controls a reduction in USVs might be due, in part, to the increases in aggressive encounters (Brunelli and Hofer, 2007). Conversely, chronic EC may be creating a dynamic shift in the overall behavioral profile of bHRs, with effects of reduced social contacts extending to the observed reduction of USVs. Whether reduced social interactions in chronic bHRs are due to the familiar and non-novel nature during the final exposure is unknown and will be a focus of future studies.
In contrast, for bLRs an increase in USVs occurred only after repeated exposure to EC. That is, with chronic exposure, bLRs eventually elicited high levels of positive affect comparable to that of bHRs. This could be reflective of the fact that it takes time for the bLRs to become comfortable (i.e. less anxious) in such an environment (Perez et al., 2009). In this context, bLRs’ initial cautious response to acute EC may be compounded by the presence of unfamiliar social interactions with other bLR animals. After a certain number of encounters from repeated exposures to EC there may be an opportunity for these social interactions to eventually become familiar and rewarding for bLRs (Fritz et al., 2011 Panksepp et al., 2007, Panksepp and Lahvis, 2007). Thus, progressive “social familiarization” could enable them to experience positive affect after repeated EC, thereby supporting habituation to the complex environment and reversing the initial anxiogenic response during acute EC.
It is not known what drives increases in positive affect in bHRs after acute EC. Most of the factors increasing 50 kHz USVs are reward-related stimuli such as food, mating and psychostimulants (Knutson et al., 1998; Burgdorf et al., 2000; Burgdorf et al., 2001; Mu et al., 2009; Ma et al., 2010), which suggests that these calls are at least in part regulated by dopamine (DA) responses in the reward circuitry. This is supported by reports showing that DA antagonists reduce 50 kHZ USV calls (Burgdorf et al., 2007; Ciucci et al., 2009). In this context, bHR animals show a strong drive for novelty exploration, have a higher sensitivity to DA agonists and an elevated dopaminergic response to reward-related cues (Flagel et al., 2010; Flagel et al., 2011). These innate characteristics could potentially make bHRs more reactive to a complex environment given the increased environmental stimulation. Thus, bHRs may be emitting more calls when acutely exposed to a complex environment by virtue of the potentially rewarding effects of novelty exploration for these animals. However, after repeated EC, such stimulation appears to have blunted the positive affective responses in bHRs.
bHR and bLR animals are known to present differential responses to chronic EC, where EC preferentially reduces anxiety in bLRs after 3 weeks (Perez et al., 2009). Interestingly, reliable changes in positive affect in bLRs after EC seem to require a similar duration. Thus, such effects may rely on changes in experience dependent plasticity from exposure to EC. It is plausible that neural circuits associated with positive affect such as the accumbens DA system might undergo neuroplasticity changes, which may lend a positive affective response in bLRs. Indeed, previous studies have highlighted changes in the reward circuitry in response to EC. For example, exposure to EC has been shown to alter dopamine transporter (DAT) and NMDA expression in the nucleus accumbens (Zakharova et al., 2009; Wood et al., 2005). This suggests that EC does in fact impact the mesolimbic DA circuit. Thus, while acute effects of EC are most likely related to bHRs’ innate DA-related responses to environmental stimuli (Flagel et al., 2011), chronic effects seen in bLRs may be related to changes in the expression of DA-related molecules such as DAT. Such changes might reflect increases in DA related activity in the accumbens during EC, which might contribute to more positive affect in bLRs.
C-fos expression in the nucleus acumbens differentially correlates with CORT in bHRs vs. bLRs after chronic EC
The fact that bLRs displayed an increase, whereas bHRs displayed a decrease in USVs following chronic EC suggests that positive affect stemming from EC exposure is subject to dynamic fluctuations and individual variation. It also suggests that an optimal “neural equilibrium” in emotionality may be required for sustained positive affect. Our c-fos expression and CORT correlations showing a parallel directionality in the dynamic change of USVs in the bHRs and bLRs supports such a view. There was a highly significant positive correlation between CORT levels and c-fos expression in the accumbens core and shell in bLRs. In bHRs the opposite pattern was observed, where CORT was negatively correlated with accumbens core and shell c-fos expression. Clearly, the meaning of activation in the accumbens is different for bHRs vs. bLRs after repeated exposure to EC. Yet, what underlies these differences and how they may be related to the respective changes in positive affect in these animals is not known.
Previous evidence has shown that CORT increases DA reward responses in the accumbens to a higher degree in outbred HRs (Piazza et al., 1996, Rouge-Pont et al., 1998) relative to LRs. Moreover, outbred HRs have been shown to self-administer CORT. That is, CORT serves as a positive reinforcer and is thought to promote novelty exploration in outbred HRs (Piazza et al., 1993; Piazza et al., 1996; Kabbaj et al., 2000). Thus, CORT may be rewarding and euphorogenic (Piazza, 1997) and this may occur for bLRs during chronic EC. Our correlation data suggests that following chronic EC bLRs may have “converted” to an HR-like phenotype by sharing the reward-like response to CORT (Piazza et al., 1996; Rouge-Pont et al., 1998). Moreover, our observations show that chronic EC elicited a profile in bLRs that was comparable to that of bHRs. This includes: analogous levels of locomotion, social interaction and aggression, comparable levels of 50 kHz USVs calls, and a significant positive correlation for CORT and accumbens activation (as in acute control conditions bHRs show CORT positively correlating with c-fos expression in the shell (data not shown)).
It should also be noted that increased levels of CORT are not always associated with increased reward. In fact increased levels of CORT after EC have also been shown to interfere with the rewarding and reinforcing effects of psychostimulants (Xu et al., 2009; Green et al., 2002; Zakharova et al., 2009). Thus, it is plausible that increased levels of CORT, as seen in bHRs after chronic EC, may have negatively influenced DA response in the accumbens core and shell, and in turn resulted in decreased positive affect. These effects might also explain why bHRs show decreased locomotor activity after EC, as this behavior is also influenced by fluctuations in the levels of glucocorticoids (Piazza and Le Moal, 1997). Thus, bHRs’ significant increase in CORT levels may have intensified the negative relationship between CORT and the nucleus accumbens, thus potentially decreasing bHRs’ positive affect to EC.
Finally, we have observed a consistent pattern of negative correlations for the dPAG with the infralimbic cortex, prelimbic cortex and accumbens core and shell in acute control bLRs. This pattern of negative correlations was not evident in any other experimental condition for bLRs or bHRs. Such findings support a potential involvement of the dPAG in regulating 50 kHz USVs for bLRs at least in an opposing manner, based on c-fos expression, to that seen in other reward-related circuit regions.
Although a specific role for the dPAG in 50 kHz USVs is not known, this structure has been implicated in coordinating brains systems involved in emotional vocalizations (Sadananda et al., 2008). Specifically, the dPAG has been implicated in the elicitation of 22 kHz USVs calls (Sadananda et al., 2008; Kroes et al., 2007), which are emitted during aversive experiences like social-defeat (Kroes et al., 2007). Interestingly, while the playback of 22 kHz USVs increases c-fos in the dPAG, c-fos expression in the accumbens and mPFC was reported upon playback of 50 kHz USVs (Sadananda et al., 2008). Such results suggest that the valence of the experience and associated neural activation may be differentially regulated. Indeed, our data implicate an opposing pattern of activation in relation to the mPFC and accumbens. Still, the involvement of the dPAG may be more complex and the extent to which these structures, in concert, play a role in bLRs’ USV response to novelty is difficult to clarify based on the current results.
In conclusion, our studies suggest that the environment differentially modulates inborn differences in positive affect between bHRs and bLRs, and highlight the relationship bewteen glucocorticoid levels and neuronal activity in the nucleus accumbens as a potential mediating mechanism.
Highlights.
bHRs emit more 50 kHz USVs relative to bLRs when exposed to a novel environment.
The presence of a cagemate increases 50 kHz USVs in both bHRs and bLRs.
Acute EC increases USVs, in bHRs, whereas chronic exposure decreases USVs in bHRs.
bLRs require repeated exposure to EC to show a significant increase in USVs.
Acknowledgments
This research was supported by NIMH 20030 (JAPS), NIDA P01 DA021633 (HA) and Office of Naval Research (ONR) N00014-09-1-0598 (HA) and Hope for Depression Research Foundation (HDRF) RGA 10-011 (HA). MJGF is a ‘Ramón y Cajal’ Researcher (MINECO-UIB).
We are grateful to James Stewart, Sarah Wong, Andrea Betrus and Krystin Harper for technical assistance.
List of abbreviations
- bHR
bred High Responder
- bLR
bred Low Responder
- EC
Environmental Complexity
- CORT
Corticosterone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Branchi I, Santarelli S, Capoccia S, Poggini S, D’Andrea I, Cirulli F, Alleva E. Antidepressant treatment outcome depends on the quality of the living environment: a pre-clinical investigation in mice. PLoS One. 2013;8:e62226. doi: 10.1371/journal.pone.0062226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli SA, Hofer MA. Selective breeding for infant rat separation-induced ultrasonic vocalizations: developmental precursors of passive and active coping styles. Behav Brain Res. 2007;182:193–207. doi: 10.1016/j.bbr.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behav Neurosci. 2000;114:320–327. [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J, Ikemoto S. Nucleus accumbens amphetamine microinjections unconditionally elicit 50-kHz ultrasonic vocalizations in rats. Behav Neurosci. 2001;115:940–944. doi: 10.1037//0735-7044.115.4.940. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J. The neurobiology of positive emotions. Neurosci Biobehav Rev. 2006;30:173–187. doi: 10.1016/j.neubiorev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J, Brudzynski SM, Kroes R, Moskal JR. Breeding for 50-kHz positive affective vocalization in rats. Behav Genet. 2005;35:67–72. doi: 10.1007/s10519-004-0856-5. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J. Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping, lesion, and pharmacology studies. Behav Brain Res. 2007;182:274–283. doi: 10.1016/j.bbr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Ciucci MR, Ahrens AM, Ma ST, Kane JR, Windham EB, Woodlee MT, Schallert T. Reduction of dopamine synaptic activity: degradation of 50-kHz ultrasonic vocalization in rats. Behav Neurosci. 2009;123:328–336. doi: 10.1037/a0014593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H, Becker JB. Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biol Sex Differ. 2:3. doi: 10.1186/2042-6410-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PE, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz M, El Rawas R, Salti A, Klement S, Bardo MT, Kemmler G, Dechant G, Saria A, Zernig G. Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict Biol. 16:273–284. doi: 10.1111/j.1369-1600.2010.00285.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Fuster MJ, Parks GS, Clinton SM, Watson SJ, Akil H, Civelli O. The melanin-concentrating hormone (MCH) system in an animal model of depression-like behavior. Eur Neuropsychopharmacol. doi: 10.1016/j.euroneuro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fuster MJ, Perez JA, Clinton SM, Watson SJ, Akil H. Impact of cocaine on adult hippocampal neurogenesis in an animal model of differential propensity to drug abuse. Eur J Neurosci. 2010;31:79–89. doi: 10.1111/j.1460-9568.2009.07045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology (Berl) 2002;162:373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- Hendriksen H, Meulendijks D, Douma TN, Bink DI, Breuer ME, Westphal KG, Olivier B, Oosting RS. Environmental enrichment has antidepressant-like action without improving learning and memory deficits in olfactory bulbectomized rats. Neuropharmacology. 2012;62:270–277. doi: 10.1016/j.neuropharm.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa Y, Takeuchi Y, Mori Y. Two types of social buffering differentially mitigate conditioned fear responses. Eur J Neurosci. 2007;26:3606–3613. doi: 10.1111/j.1460-9568.2007.05969.x. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J Comp Psychol. 1998;112:65–73. doi: 10.1037/0735-7036.112.1.65. [DOI] [PubMed] [Google Scholar]
- Kroes RA, Burgdorf J, Otto NJ, Panksepp J, Moskal JR. Social defeat, a paradigm of depression in rats that elicits 22-kHz vocalizations, preferentially activates the cholinergic signaling pathway in the periaqueductal gray. Behav Brain Res. 2007;182:290–300. doi: 10.1016/j.bbr.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Herkenham M. Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J Neurosci. 2011;31:6159–6173. doi: 10.1523/JNEUROSCI.0577-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens-Martin M, Tejeda GS, Trejo JL. Antidepressant and proneurogenic influence of environmental enrichment in mice: protective effects vs recovery. Neuropsychopharmacology. 2011;36:2460–2468. doi: 10.1038/npp.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ST, Maier EY, Ahrens AM, Schallert T, Duvauchelle CL. Repeated intravenous cocaine experience: development and escalation of pre-drug anticipatory 50-kHz ultrasonic vocalizations in rats. Behav Brain Res. 212:109–114. doi: 10.1016/j.bbr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh JH, Grant EC. The effect of olfactory stimuli on the agonistic behaviour of laboratory mice. Zeitschrift fur Tierpsychologie. 1966;23:584–587. doi: 10.1111/j.1439-0310.1966.tb01614.x. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- Miczek KA. Intraspecies aggression in rats: effects of d-amphetamine and chlordiazepoxide. Psychopharmacologia. 1974;39:275–301. doi: 10.1007/BF00422968. [DOI] [PubMed] [Google Scholar]
- Mu P, Fuchs T, Saal DB, Sorg BA, Dong Y, Panksepp J. Repeated cocaine exposure induces sensitization of ultrasonic vocalization in rats. Neurosci Lett. 2009;453:31–35. doi: 10.1016/j.neulet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Franklin KB. The development of a conditioned place preference to morphine: effects of microinjections into various CNS sites. Behav Neurosci. 1997;111:1324–1334. doi: 10.1037//0735-7044.111.6.1324. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6:661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci. 2009;29:6379–6387. doi: 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc Natl Acad Sci U S A. 1993;90:11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Res Brain Res Rev. 1997;25:359–372. doi: 10.1016/s0165-0173(97)00025-8. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deroche V, Maccari S, Simon H, Le Moal M. Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc Natl Acad Sci U S A. 1996;93:8716–8720. doi: 10.1073/pnas.93.16.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouge-Pont F, Deroche V, Le Moal M, Piazza PV. Individual differences in stress-induced dopamine release in the nucleus accumbens are influenced by corticosterone. Eur J Neurosci. 1998;10:3903–3907. doi: 10.1046/j.1460-9568.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- Sadananda M, Wohr M, Schwarting RK. Playback of 22-kHz and 50-kHz ultrasonic vocalizations induces differential c-fos expression in rat brain. Neurosci Lett. 2008;435:17–23. doi: 10.1016/j.neulet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Molecular psychiatry. 2010;15:1152–1163. doi: 10.1038/mp.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, Vazquez DM, Watson SJ, Akil H. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- Stedenfeld KA, Clinton SM, Kerman IA, Akil H, Watson SJ, Sved AF. Novelty-seeking behavior predicts vulnerability in a rodent model of depression. Physiol Behav. 2011;103:210–216. doi: 10.1016/j.physbeh.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey AR, Spear LP. Development of anticipatory 50 kHz USV production to a social stimuli in adolescent and adult male Sprague-Dawley rats. Behav Brain Res. 226:613–618. doi: 10.1016/j.bbr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DA, Buse JE, Wellman CL, Rebec GV. Differential environmental exposure alters NMDA but not AMPA receptor subunit expression in nucleus accumbens core and shell. Brain Res. 2005;1042:176–183. doi: 10.1016/j.brainres.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Xu Z, Hou B, Zhang Y, Gao Y, Wu Y, Zhao S, Zhang C. Antidepressive behaviors induced byenriched environment might be modulated by glucocorticoid levels. Eur Neuropsychopharmacol. 2009;19:868–875. doi: 10.1016/j.euroneuro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Miller J, Unterwald E, Wade D, Izenwasser S. Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience. 2009;163:890–897. doi: 10.1016/j.neuroscience.2009.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]