Abstract
Objectives
To determine symptoms and findings in patients with dysphagia related to epiglottic dysfunction. To analyze outcomes in patients who underwent partial epiglottidectomy due to dysphagia related to epiglottic dysfunction
Study Design
Review and analysis of clinical data obtained as part of the diagnosis and treatment of patients with dysphagia related to epiglottic dysfunction.
Methods
A retrospective review was performed of all post-treatment head and neck cancer patients who underwent epiglottidectomy at a single tertiary care referral center. Objective pre- and post-procedure swallow findings, endoscopic evaluation, and subjective improvement based on patient self-report were reviewed.
Results
Seven patients were identified based on endoscopic evaluation and modified barium swallow study (MBSS) as having epiglottic pathology leading to dysphagia. Specific anatomic and functional findings included thickening of the epiglottis, absence of epiglottic deflection, vallecular bolus retention during and after the swallow, and bolus backflow from the pharynx to the oral or nasal cavity. Partial epiglottidectomy was performed in these patients. Post-operative MBSS was analyzed for changes in swallow efficiency and safety. Nearly all patients demonstrated improved pharyngeal bolus passage with little to no added swallowing morbidity.
Conclusions
Preliminary findings suggest a role for partial epiglottidectomy in post-treatment head and neck cancer patients with swallowing disorders. Ideal candidates have intact tongue base contraction and poor retroflexion of the epiglottis, which result in bolus obstruction at the level of the valleculae. Partial epiglottic resection enables improved bolus passage in the pharyngeal phase. Minimal post-operative morbidity occurs in the appropriately selected patient.
Keywords: dysphagia, epiglottidectomy, epiglottectomy, head and neck cancer, radiation, chemotherapy, quality of life
Introduction
Dysphagia is a common and important complaint in head and neck cancer patients who are treated with radiation therapy, with or without addition of chemotherapy1. Although estimates vary, the addition of chemotherapy to radiation therapy may increase the incidence of dysphagia by nearly a factor of three, from 9% to 23%2. In fact, as many as 51% of patients may be gastrostomy tube-dependent two years after completion of hyperfractionated radiotherapy with concurrent chemotherapy3. Not surprisingly, dysphagia has a markedly detrimental impact upon these patients' quality of life4,5.
A normal, functional swallow is a complex process that incorporates oral, pharyngeal, and esophageal phases; the tongue, larynx, pharynx, and esophagus, therefore, all play important roles. They are interconnected by intricate neuromuscular coordination, both voluntary and involuntary. Chemoradiotherapy can affect the swallowing process at one or more of these steps6. For example, reduced tongue base contraction, hyolaryngeal range of motion, and upper esophageal sphincter opening can result in increased pharyngeal residue, which may lead to increased aspiration7. Chemoradiation therapy is also associated with pharyngeal and esophageal strictures in up to 46% of patients, requiring multiple endoscopies with dilation for treatment of the stenoses8-10.
Although the typical anatomical changes in the appearance of the epiglottis following radiotherapy are familiar to the head and neck endoscopist, only sporadic mention of this is found in the literature11. Nonetheless, a dysfunctional epiglottis is not an uncommon finding in irradiated head and neck cancer patients, and the co-existence of a dysfunctional epiglottis in patients with dysphagia has been well described, often in association with aspiration11-13. Garon et al, in particular, described the presence of vallecular (and to a lesser extent, pyriform sinus) stasis of food as a strong indicator of abnormal epiglottic movement patterns and dysfunction. Nasal backflow was also described as an indicator of epiglottic dysfunction. However, the authors simply pointed out that the epiglottic dysfunction itself contributes to dysphagia, but they stopped short of suggesting possible treatment options11.
In reviewing our own post-treatment head and neck cancer population, we have noticed a common finding of a stiff and thickened epiglottis on endoscopic evaluation, which also typically correlated with a lack of normal epiglottic deflection on modified bariums swallow studies (MBSS) (Figure 1). We noted that this dysfunction results in an inefficient pharyngeal phase of swallow, with excessive vallecular residue that is difficult to clear, even with behavioral maneuvers. We became interested in treating the obstructive nature of the dysfunctional epiglottis and began performing partial epiglottidectomies in an effort to improve swallow efficiency in this otherwise recalcitrant dysphagia condition. Given the long, controversial history in the literature regarding the role of the epiglottis in a normal, functional swallow, we performed a retrospective review of an initial series of patients who underwent partial epiglottidectomy as part of their dysphagia management14. The purpose of this report is to describe our preliminary experience and results in a small group of patients. Additionally, we identify patient factors that increase the likelihood of surgical success. We provide novel information on a therapeutic modality that could be included in the armamentarium of interventions available to the dysphagia specialist in the management epiglottic dysfunction after chemoradiation therapy for head and neck cancer.
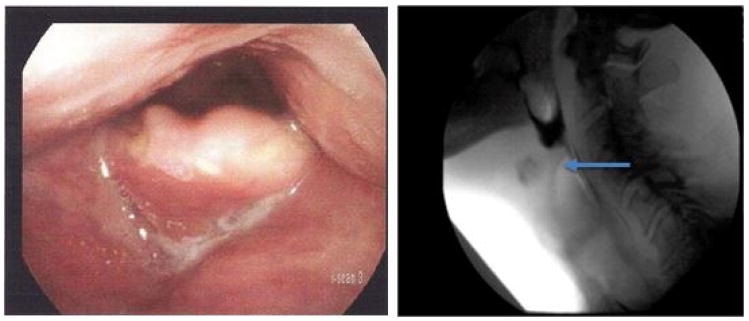
Figure 1.
Left – typical thickened appearance of epiglottis in a head and neck cancer patient following radiation. Right – MBSS image of thickened epiglottis (arrow) that is unable to deflect, causing pharyngeal phase obstruction of the bolus.
Materials and Methods
This retrospective study was approved by the Internal Review Board (IRB) of the University of California, Los Angeles. Medical records were reviewed to identify all consecutive patients with dysphagia who underwent epiglottidectomy during a 14-month period. Criteria for inclusion in the study were: history of treatment with radiation therapy for head and neck cancer and documentation of pre- and post-operative MBSS. Criteria for exclusion were: evidence of ongoing cancer based on pathology and lack of upper esophageal sphincter (UES) opening.
Pre-operative laryngeal endoscopies were reviewed to assess the epiglottic to pharyngeal diameter ratios because of the traditional observation of a thickened epiglottis in the irradiated patient, and due to the presumption that an epiglottis that occupies a greater pharyngeal area will cause increased obstruction to passage of the food bolus, especially when it does not deflect in a functional manner. To assess these diameters, a still image of laryngeal videoendoscopy, taken while the subject is breathing comfortably at rest, was used. Epiglottic diameter was measured as the distance from the posterior-most aspect of the tongue base to the posterior-most aspect of the epiglottis in the midline. Pharyngeal diameter was measured in the same anterior-to-posterior line, and measured as the distance from the posterior-most position of the tongue base to the posterior pharyngeal wall. The calculated ratio (epiglottic diameter over the pharyngeal diameter) was used to determine the relative pharyngeal area occupied by the epiglottis at the oropharyngeal level. As comparable standardized measurements are not available in the literature, the same calculation was performed for age-matched controls (one control for each subject) without a history of cancer or irradiation who were seen in our voice clinic for benign laryngopharyngeal pathology (vocal fold lesions, pharyngitis, etc.) (Figure 2). These calculations were similarly performed using still images acquired during office endoscopy.
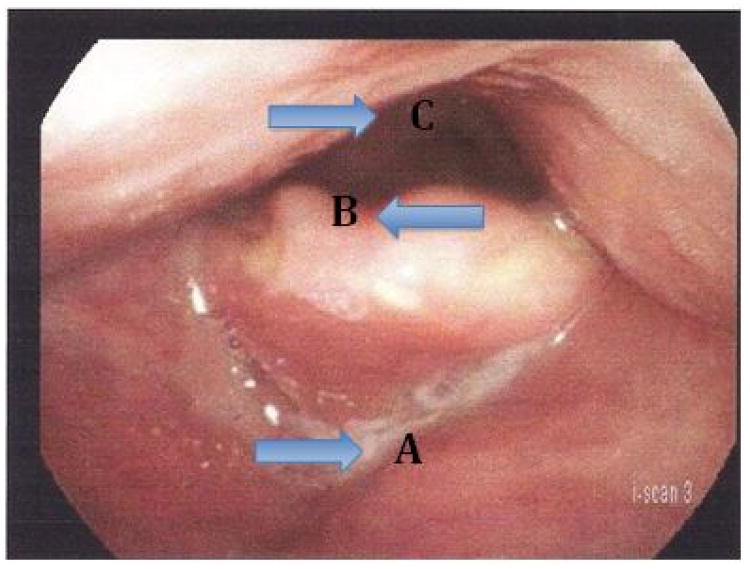
Figure 2.
Calculation of epiglottic to pharyngeal (EP) ratio. Diameters are measured in an anterior to posterior direction in the midline. Epiglottic diameter is measured from A to B. Pharyngeal diameter is measured from A to C. The epiglottic diameter is divided by the pharyngeal diameter and converted to percentage form to represent the EP ratio.
Pre-operative and post-operative MBSS's were compared to assess for the following: (1) pharyngeal phase time, defined as the time taken from initiation of the pharyngeal phase (bolus entering the pharynx) until cessation of volitional bolus clearance; (2) presence or absence of tongue base contact with the posterior pharyngeal wall as an indicator of driving force, evidenced by lack of an air or contrast column between these two structures at the height of the swallow; (3) amount of vallecular residue after the first swallow, as an indicator of the amount of obstruction provided by the epiglottis; (4) amount of residue in the pyriform sinuses (Table 1); and (5) degree of penetration-aspiration as defined by Rosenbeck15 (Table 2). Additionally, the UES was assessed to determine if opening was sufficiently narrow to obstruct bolus flow, since that would provide another reason for pharyngeal residue. When more than one pre- or post-operative MBSS were available, the final MBSS before surgery and the initial MBSS after surgery were used. Patients served as their own controls (pre- versus post-operative) in this comparison. A single, consistent texture and amount of food was used for each subject to compare pre- and post-operative swallows.
Table 1. Grading of Vallecular and Pyriform Sinus Residue.
| Score | Description |
|---|---|
| 0 | None to Trace Coating |
| 1 | Thick Coating |
| 2 | Up to 50% Full of Residual |
| 3 | Greater than 50% Full of Residual |
| 4 | Overflowing with Residual |
| 5 | Oropharyngeal stasis (Entire bolus stays within vallecula) |
Table 2. Penetration-Aspiration Scale (Rosenbeck JC, et al. A penetration-aspiration scale. Dysphagia. 1996;11:93-8.).
| Scale | Description |
|---|---|
| 1 | Material does not enter the airway. |
| 2 | Material enters the airway, remains above the vocal folds, and is ejected from the airway. |
| 3 | Material enters the airway, remains above the vocal folds, and is not ejected from the airway. |
| 4 | Material enters the airway, contacts the vocal folds, and is ejected from the airway. |
| 5 | Material enters the airway, contacts the vocal folds, and is not ejected from the airway. |
| 6 | Material enters the airway, passes below the vocal folds, and is ejected into the larynx or out of the airway. |
| 7 | Material enters the airway, passes below the vocal folds, and is not ejected from the trachea despite effort. |
| 8 | Material enters the airway, passes below the vocal folds, and no effort is made to eject. |
Suprahyoid epiglottidectomy was performed via a trans-oral approach under general anesthesia. A Dedo or Lindholm laryngoscope was used for exposure. Carbon dioxide laser via a microscope attachment was used to resect the superior one-third to one-half of the epiglottis. Typically the resection was halfway between the tip of the epiglottis and the hyoepiglottic ligament. The laser was used at a setting of 6-10 Watts, continuous mode. The epiglottis was bisected in the midline for ease of resection. Any protruding, de-mucosalized portion of the epiglottic cartilage was gently ablated to prevent necrosis or infection in this area. All patients tolerated the procedure well, and there were no complications in this patient population. Patients were discharged home on the same day on the diet they were previously tolerating.
Office notes and MBSS reports were reviewed to assess patients' subjective responses to surgery. When available, the following information was recorded: presence or absence of subjective improvement in the swallow, presence or absence of weight loss, and any history of aspiration-related pneumonias.
Results
A total of twelve patients with a diagnosis of dysphagia and a history of head neck cancer underwent epiglottidectomy in the specified time frame. Of these, four did not meet the inclusion and/or exclusion criteria, and one had a very limited pre-operative MBSS that could not be objectively compared to his post-operative study.
Of the seven remaining patients, six were male and one was female, with ages ranging from 48 to 77. Primary cancer diagnoses fell into one of the following categories: nasopharynx, oropharynx, or unknown primary. All had undergone radiotherapy as part of their definitive treatment. Six of the seven underwent concurrent or adjuvant chemotherapy as well. Two of the seven underwent partial tongue base resection in addition to chemoradiotherapy (Table 3).
Table 3. Patient Demographics at Presentation.
| Patient | Age | Gender | Primary Cancer Site | Treatment Type | Treatment Completion Year |
|---|---|---|---|---|---|
| 1 | 66 | M | Oropharynx | Chemoradiotherapy | 2012 |
| 2 | 77 | M | Oropharynx | Tonsillectomy + Chemoradiotherapy | 2011 |
| 3 | 70 | M | Nasopharynx | Radiotherapy | 2010 |
| 4 | 63 | M | Oropharynx | BOT Resection + Microvascular Reconstruction + Chemoradiotherapy | 2011 |
| 5 | 51 | M | Oropharynx | Tonsillectomy + Chemoradiotherapy | 2011 |
| 6 | 48 | F | Unknown Primary | Chemoradiotherapy | 2007 |
| 7 | 57 | M | Oropharynx | BOT Resection + Microvascular Reconstruction + Chemoradiotherapy | 2012 |
Pre-operative endoscopies were reviewed to assess the relative pharyngeal diameter occupied by the immobile epiglottis at a pharyngeal rest state by calculating the epiglottic to pharyngeal diameter ratio, as described above (Table 4). In controls, this diameter ratio ranged from 28-40%, with an average of 35%. In irradiated patients, this ranged from 33% to 86%, with an average of 61%. Two radiated patients had epiglottic to pharyngeal diameter ratios that were within the range of the controls. The remaining five patients had ratios of 60% or greater.
Table 4.
Epiglottis-to-pharyngeal (EP) diameter ratios for radiated versus non-irradiated controls.
| Patient | EP Ratio Subjects | EP Ratio Age-matched Controls |
|---|---|---|
| 1 | 74% | 28% |
| 2 | 33% | 30% |
| 3 | 86% | 30% |
| 4 | 69% | 39% |
| 5 | 60% | 40% |
| 6 | 34% | 38% |
| 7 | 72% | 37% |
Modified barium swallow studies were evaluated to assess the time needed to clear the bolus through the pharynx (Table 5A). Six of the seven studies were performed 2-4 weeks after surgery. One was performed 3.5 months after surgery (patient 6). Consistent textures and amounts were used to compare pre-operative and post-operative evaluations for individual patients. In four cases, one teaspoon of puree was identified as a consistent challenge in both studies, and therefore used as the standardized bolus. Sips of liquids were used for two patients (patients 3 and 7) with considerable dysphagia who were otherwise gatrostomy tube (G-tube) dependent, and a quarter piece of cookie dipped in puree was used for another (patient 4), as this was a consistent challenge used in both of his evaluations. Using these measures, four out of seven subjects demonstrated a decrease in pharyngeal transit time, ranging from a 23% to 85% reduction. One patient did not demonstrate any change in pharyngeal transit time, and two patients increased transit time by 1-2 seconds. The overall change in the time required to clear a bolus through the pharynx showed a 29% decrease.
Table 5.
Pre-operative and Post-operative data from modified barium swallow studies.
| 5A | |||||
|---|---|---|---|---|---|
|
| |||||
| Patient | Pre-op Pharyngeal Phase Time | Post-op Pharyngeal Phase Time | % Change in Pharyngeal Phase Time | Pre-op Tongue Base Contact with Posterior Pharynx | Post-op Tongue Base Contact with Posterior Pharynx |
| 1 | 13 seconds | 2 seconds | 85% decrease | Yes | Yes |
| 2* | 8 seconds | 2 seconds | 75% decrease | No | Yes |
| 3 | 9 seconds | 9 seconds | 0% change | No | No |
| 4 | 12 seconds | 14 seconds | 17% increase | Yes | Yes |
| 5 | 10 seconds | 5 seconds | 50% decrease | Yes | Yes |
| 6 | 13 seconds | 10 seconds | 23% decrease | Yes | Yes |
| 7 | 9 seconds | 10 seconds | 11% increase | No | No** |
| 5B | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Patient No. | Pre-op Vallecular Residue Score | Post-op Vallecular Residue Score | Pre-op Pyriform Sinus Residue Score | Post-op Pyriform Sinus Residue Score | Pre-op Penetration-Aspiration Score | Post-op Penetration-Aspiration Score | Pre-op Diet | Post-op Diet |
| 1 | 3 | 0 | 1 | 0 | 1 | 1 | Soft diet | Soft diet |
| 2* | 5 | 0 | N/A*** | 0 | 1 | 1 | Soft diet | Soft diet |
| 3 | 5 | 1 | 1**** | 4**** | 2 | 2 | G-tube | Full liquid diet |
| 4 | 5 | 3 | 1 | 1 | 1 | 2 | Soft diet | Soft diet |
| 5 | 2 | 0 | 0 | 0 | 1 | 1 | Liquid diet | Chewable diet |
| 6 | 1 | 1 | 3 | 1 | 3 | 2 | G-tube | Sips of liquids |
| 7** | 1 | 0 | 1 | 0 | 8 (trace) | 8 (trace) | G-tube | Full liquid diet |
Patient used behavioral maneuver.
Significant oral cavity backflow seen pre-operatively reduced to trace post-operatively.
Bolus never progressed beyond vallecula.
Copious nasopharyngeal backflow seen pre-operatively reduced to minimal post-operatively.
Presence or absence of tongue base contact with the posterior pharyngeal wall was recorded as an indicator of superior pharyngeal driving force compared to patients with absent tongue base-to-posterior pharyngeal wall apposition (Table 5A). Pre-operatively, four out of the seven demonstrated contact. Post-operatively, this number increased to five out of seven. Of the five who were able to attain tongue base contact with the posterior pharyngeal wall, all but one were able to decrease their pharyngeal transit time. Of the two patients who were not able to make contact, neither improved pharyngeal transit time. Patient 4 demonstrated a 17% increase in transit time despite ability to retract the tongue base to the posterior pharyngeal wall.
The amount of residue in the valleculae and pyriform sinuses was examined post-operatively and compared to pre-operative findings (Table 5B). In all but one patient, there was drastic reduction in the amount of residue in the valleculae, with improvements in vallecular residue score ranging from 1 to 5 points, with an average reduction of 3 points; one patient had a thick coating of residue in both studies. Pyriform sinus residue also decreased in 3 of the 5 patients who had stasis pre-operatively. One patient persisted with a thick coating, and one increased from trace residue (due to complete bolus retention within the valleculae) to considerable residue in the pyriforms. The pre-operative UES opening for this patient was judged adequate (as was the case for the other patients in this study). Interestingly, the increase in pyriform residue in this patient was in conjunction with resolution of nasal backflow, and his penetration and aspiration rates remained stable. In fact, overall, Rosenbeck grading and frequencies of penetration and aspiration were essentially unchanged post-operatively for five patients, although one patient worsened from a score of 1 to 2 (penetration that was not aspirated), and one improved from a score of 3 to 2.
Records were reviewed to determine whether subjects reported improvement in swallowing post-operatively as well as documentation of weight loss or aspiration pneumonia (Table 6). Six out of seven (86%) subjects mentioned that deglutition had become easier in the post-operative follow-up visit. The chart did not mention any subjective patient report regarding swallow in one patient, although his post-operative swallow evaluation enabled advancement from G-tube dependence to a full liquid diet without further need for the G-tube. Of the two remaining G-tube dependent patients, one was advanced to a full liquid diet as well, with recommendations to transition off G-tube feeds. These recommendations were made only after undergoing partial epiglottidectomy, suggesting a direct correlation between the surgical intervention and improved oral intake ability. The patient requiring continued G-tube feeds demonstrated limited hyolaryngeal elevation, and was advised to start with sips of liquid with the additional recommendation of swallow therapy. Of the other four patients, one was advanced from a liquid diet to a chewable one. The other three remain on a soft, chewable diet (Table 5B). The two patients with considerable nasal (patient 3) and oral (patient 7) backflow pre-operatively had resolution of this issue immediately following surgery.
Table 6.
Patient self-reported data.
| Patient | Subjective Improvement | Post-operative Weight Loss | Pre-operative Aspiration Pneumonia | Post-operative Aspiration Pneumonia |
|---|---|---|---|---|
| 1 | Yes | None | No | No |
| 2 | Yes | None | No | No |
| 3 | N/A | None | No | No |
| 4 | Yes | None | No | No |
| 5 | Yes | None | No | No |
| 6 | Yes | None | Yes | No |
| 7 | Yes | None | Yes | No |
Discussion
The role of the epiglottis in swallowing has long been controversial, and over a century of investigations have not led to a consensus14. In early infancy, the epiglottis appears to serve as the primary protector of the upper respiratory tract16. However, the literature for adults presents conflicting data.
Ekberg reviewed the modified barium swallow studies of 250 consecutive patients with dysphagia and found that nineteen of these patients had a totally immobile epiglottis. Sixteen of these patients demonstrated laryngeal penetration, with twelve who developed frank aspiration12. Additionally, on a retrospective review of 330 patients who underwent MBSS evaluation, epiglottic dysfunction was described as an independent predictor of aspiration in patients with oropharyngeal dysphagia13.
Conversely, the experience of supraglottic carcinoma resection suggests that the epiglottis is not necessary to attain a safe and functional swallow in the long-term. Motta et al, reviewed their experience with transoral laser excision of supraglottic carcinoma in 124 patients, stages T1-T3. Fifty-three of these patients underwent naso-gastric tube placement for feeding purposes; these were removed in an average of 1-2 weeks post-operatively17. No patients developed post-operative aspiration. Oeken reported their results with transoral laser supraglottic laryngectomy in fourteen patients with supraglottic carcinomas, all of whom underwent either hemi- or complete epiglottidectomy as part of the surgical resection. Although ten of these patients developed aspiration post-operatively (noted on endoscopy 7-10 days after surgery), leading to PEG placement, these were all removed after 2-9 months of swallowing rehabilitation18.
Numerous authors have described performing epiglottidectomies to treat benign supraglottic and hypopharyngeal obstruction as well. These have been performed in cases of obstructive sleep apnea, laryngomalacia, and even globus pharyngeus. These patients experienced improvement in their clinical symptoms without compromising deglutition19-24. Finally, Leder reported a case series of three adults who underwent isolated epiglottidectomy from three diverse causes: trauma, surgical resection for a supraglottic cancer, and malignant erosion of the entire epiglottis. The second patient experienced odynophagia related to post-operative edema, but none of the patients had any other abnormalities of swallow, and none developed aspiration pneumonia. The authors concluded that patients can readily adapt to isolated epiglottidectomy and avoid aspiration25.
Consistent with these findings, post-operative penetration and aspiration were largely unchanged compared to pre-operative findings in our series. One patient (patient 4) worsened slightly by developing deeper penetration, although he did not develop aspiration. Another (patient 8) continued to aspirate trace amounts consistently, both pre- and post-operatively. Patient 2 was initially noted to aspirate infrequently post-operatively, although use of a head turn and chin tuck maneuver eliminated this risk; this maneuver was not beneficial prior to epiglottidectomy. Two patients (6 and 7) had documented pre-operative aspiration pneumonia events. These infections have not recurred as of their latest post-operative follow-up.
Two out of three G-tube dependent patients were able to fully transition off of tube feeds as a result of partial epiglottidectomy, and the remaining individual was advanced to sips of liquid by mouth in addition to tube feeds. One out of four oral feeders was able to advance to a chewable diet from a liquid one. These results suggest that in some patients, partial epiglottidectomy may allow diet advancement.
The thickened appearance of the radiated epiglottis leads to greater pharyngeal area occupation by the epiglottis, which contributes to bolus obstruction. On average, it occupied nearly double the cross-sectional area of the non-radiated epiglottis in the subjects reviewed in this study. Although two patients had nearly normal diameter ratios, the complete lack of epiglottic motion in these individuals still caused it to become a barrier to normal pharyngeal bolus propulsion. Our impression is that the non-deflecting epiglottis obstructs efficient bolus passage.
Patients who were able to retract their tongue base to the posterior pharyngeal wall were most likely to decrease pharyngeal transit time, whereas those who could not either had no change in or increased pharyngeal transit time. Of the two patients who increased transit time (patients 4 and 7), both had undergone partial tongue base resection in addition to chemoradiotherapy. The one patient whose pharyngeal transit time did not change was the only patient with primary nasopharyngeal carcinoma (patient 3). The objective decrease in pharyngeal transit time in the remaining patients is consistent with their subjective report of increased ease of swallowing. The lesser amounts of vallecular and pyriform residue, in addition to reduction of pharyngeal transit time, are also indicative of increased pharyngeal efficiency. Although we did not assess postoperative mealtime changes in our subjects, our head and neck cancer patients often report greatly extended meal times. This study's findings of reduced time to clear material though the pharynx for some subjects suggests that meal times overall may decrease as a consequence of epiglottidectomy.
Tongue base retraction in the presence of vallecular residue with an epiglottis that does not deflect leads to retrograde propulsion of the food bolus. This was demonstrated as nasopharyngeal and oral cavity backflow in patients 3 and 7, respectively. Indeed, patient 3 initially presented to our office with a primary complaint of nasal regurgitation in addition to dysphagia. Backflow disappeared on the post-operative evaluations of both patients.
Reduction in retrograde bolus propulsion into the nasal and/or oral cavity can lead to social embarrassment. In fact, many irradiated head and neck cancer patients eat alone, due to the perceived social unacceptability of their adaptive maneuvers, prolonged meal times, altered diets, frequent coughing, and possibility of choking or regurgitation (based on personal communications with patients). By decreasing meal times and backflow, increasing efficiency and ease of swallow, and potentially advancing diets – particularly when able to transition off of tube feeds – patients may find it easier to consume meals with family and friends. This may help improve quality of life both in regard to swallowing and socialization.
There is clearly some variability in the swallowing results of these patients. Based on patient characteristics in this very small sample, those with oropharyngeal primary cancers appear to have better swallowing outcomes than those with a nasopharyngeal or unknown primary. Inclusion of the nasopharynx and midline pharyngeal structures in the radiation field likely plays a strong role in the development of inadequate pharyngeal driving force following treatment. Ability to contract the base of tongue to the posterior pharyngeal wall also correlated with higher likelihood of success, whereas history of tongue base resection led to less improvement in swallowing efficiency. Surgical resection of the tongue base, in addition to muscular weakness and fibrosis from chemoradiation, likely leads to a bigger challenge to overcome in developing pharyngeal driving force compared to the effects of chemoradiation alone.
There are a number of limitations in this study. It comprises a small case series, and the data are reviewed in a retrospective manner and thus suffer from the limitations and biases of the study design. Additionally, the small patient sample was heterogeneous in regard to cancer location and treatment. As a result, statistical analyses could not be performed. In addition, follow-up swallow evaluations are short-term, ranging from weeks to months after intervention. However, this is the first report demonstrating the role that the dysfunctional epiglottis may play in dysphagia in this population, as well as the feasibility and utility of partial epiglottidectomy in alleviating dysphagia in patients with epiglottic dysfunction after treatment of head and neck cancer with chemoradiation therapy. It is important to note that none of the patients in this cohort worsened in their swallowing ability or in their ability to protect their airways. A larger, prospective, and long-term study can now be designed using the lessons gleaned from these retrospective data based on our initial experiences.
Conclusion
Based on this preliminary study, it is clear that the epiglottis plays an important role in dysphagia, particularly in the irradiated head and neck cancer population. As such, partial epiglottidectomy can be considered in some patients with pharyngeal dysphagia following radiotherapy with or without chemotherapy for head and neck cancers. Post-treatment MBSS should be carefully reviewed for bolus obstruction due to epiglottic dysfunction, high levels of vallecular residue, and backflow from the vallecular space to the oral and/or nasal cavities in the setting of adequate tongue base contraction. At this time, we speculate that the best candidates for epiglottidectomy are those patients who are able to retract the base of tongue to the posterior pharyngeal wall. Those with a history of nasopharyngeal carcinoma or resection of the base of tongue appear to improve the least. The time required to clear material through the pharynx and pharyngeal residue decreases post-operatively in this population. This results in greater ease and efficiency of swallowing, with the possibility for diet advancement, including the potential to transition off of tube feeds. Of note, in this study, no patient's swallowing ability worsened as a consequence of epiglottidectomy. Further studies should be done to assess the long-term results of this surgery.
Acknowledgments
This study was supported in part by Grant No. RO1 DC011300 from the National Institutes of Health.
Footnotes
Financial Disclosure: The authors have no other financial disclosures to make.
Conflict of Interest: None
This article was presented as an oral presentation at The Triological Society Annual Meeting, April 12, 2013, Orlando, FL, USA.
References
- 1.Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Physics. 2005;61(3):772–8. doi: 10.1016/j.ijrobp.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–8. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 3.Staar S, Rudat V, Stuetzer H, et al. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy: results of a multicentric randomized German trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:1161–71. doi: 10.1016/s0360-3016(01)01544-9. [DOI] [PubMed] [Google Scholar]
- 4.Terrell JE, Ronis DL, Fowler KE. Clinical predictors of quality of life in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130(4):401–8. doi: 10.1001/archotol.130.4.401. [DOI] [PubMed] [Google Scholar]
- 5.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770–6. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 6.Batth SS, Caudell JJ, Chen AM. Practical considerations in reducing swallowing dysfunction following concurrent chemoradiotherapy with intensity-modulated radiotherapy for head and neck cancer. Head Neck. 2013 doi: 10.1002/hed.23246. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Lazarus CL. Effects of chemoradiotherapy on voice and swallowing. Curr Opin Otolaryngol Head Neck Surg. 2009;17:172–8. doi: 10.1097/MOO.0b013e32832af12f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis DO, Weymuller EA, Parvathaneni U, Merati AL, Yueh BY. Dysphagia, stricture, and pneumonia in head and neck cancer patients: does treatment modality matter? Ann Otol Rhinol Laryngol. 2010;119(6):391–7. doi: 10.1177/000348941011900605. [DOI] [PubMed] [Google Scholar]
- 9.Laurell G, Kraepelien T, Mavroidis P, et al. Stricture of the proximal esophagus in head and neck carcinoma patients after radiotherapy. Cancer. 2003;97(7):1693–700. doi: 10.1002/cncr.11236. [DOI] [PubMed] [Google Scholar]
- 10.Ahlberg A, al-Abany M, Alevronta E, et al. Esophageal stricture after radiotherapy in patients with head and neck cancer: experience of a single institution over 2 treatment periods. Head Neck. 2010;32(4):452–61. doi: 10.1002/hed.21201. [DOI] [PubMed] [Google Scholar]
- 11.Garon BR, Huang Z, Hommeyer S, Eckmann D, Stern GA, Ormiston C. Epiglottic dysfunction: abnormal epiglottic movement patterns. Dysphagia. 2002;17:57–68. doi: 10.1007/s00455-001-0102-8. [DOI] [PubMed] [Google Scholar]
- 12.Ekberg O. Epiglottic dysfunction during deglutition in patients with dysphagia. Arch Otolaryngol. 1983;109:376–80. doi: 10.1001/archotol.1983.00800200022007. [DOI] [PubMed] [Google Scholar]
- 13.Perlman AL, Booth BM, Grayhack JP. Videofluoroscopic predictors of aspiration in patients with oropharyngeal dysphagia. Dysphagia. 1994;9(2):90–5. doi: 10.1007/BF00714593. [DOI] [PubMed] [Google Scholar]
- 14.Walton GL. The function of the epiglottis in deglutition and phonation. J Physiol. 1878;1:303–20. doi: 10.1113/jphysiol.1878.sp000025. As cited in: Leder SB, Burrell MI, Van Daele DJ. Epiglottis is not essential for successful swallowing in humans. Ann Otol Rhinol Laryngol. 2010;119(12):795-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbeck JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–8. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki CT, Levine PA, Laitman JT, Crelin ES. Postnatal descent of the epiglottis in man: a preliminary report. Arch Otolaryngol. 1977;103:169–71. doi: 10.1001/archotol.1977.00780200095011. [DOI] [PubMed] [Google Scholar]
- 17.Motta G, Esposito E, Testa D, Iovine R, Motta S. CO2 laser treatment of supraglottic cancer. Head Neck. 2004;26(5):442–6. doi: 10.1002/hed.10395. [DOI] [PubMed] [Google Scholar]
- 18.Oeken J, Hansch U, Thiel S, Bootz F. Swallowing function after endoscopic resection of supraglottic carcinoma with the carbon dioxide laser. Eur Arch Otorhinolaryngol. 2001;258:250–4. doi: 10.1007/s004050100353. [DOI] [PubMed] [Google Scholar]
- 19.Zeitels SM, Vaughan CW, Domanowski GF, Fuleihan NS, Simpson GT., 2nd Laser epiglottectomy: endoscopic technique and indications. Otolaryngol Head Neck Surg. 1990;103(3):337–43. doi: 10.1177/019459989010300301. [DOI] [PubMed] [Google Scholar]
- 20.Catalfumo FJ, Golz A, Westerman ST, Gilbert LM, Joachims HZ, Goldenberg D. The epiglottis and obstructive sleep apnoea syndrome. J Laryngol Otol. 1998;112:940–3. doi: 10.1017/s0022215100142136. [DOI] [PubMed] [Google Scholar]
- 21.Golz A, Goldenberg D, Westerman ST, Catalfumo FJ, Netzer A, Westerman LM, Joachims HZ. Laser partial epiglottidectomy as a treatment for obstructive sleep apnea and laryngomalacia. Ann Otol Rhinol Laryngol. 2000;109(12 pt1):1140–5. doi: 10.1177/000348940010901211. [DOI] [PubMed] [Google Scholar]
- 22.Quesada JL, Lorente J, Quesada P. Partial epiglottectomy as a possible treatment for globus pharyngeus? Eur Arch Otorhinolaryngol. 2000;257(7):386–8. doi: 10.1007/s004059900226. [DOI] [PubMed] [Google Scholar]
- 23.Kanemaru S, Kojima H, Fukushima H, Tamaki H, Tamura Y, Yamashita M, Umeda H, Ito J. A case of floppy epiglottis in adult: a simple surgical remedy. Auris Nasus Larynx. 2007;34(3):409–11. doi: 10.1016/j.anl.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Agada FO, Coatesworth AP, Grace ARH. Retroverted epiglottis presenting as a variant of globus pharyngeus. J Laryngol Otol. 2007;121:390–2. doi: 10.1017/S0022215106003422. [DOI] [PubMed] [Google Scholar]
- 25.Leder SB, Burrell MI, Van Daele DJ. Epiglottis is not essential for successful swallowing in humans. Ann Otol Rhinol Laryngol. 2010;119(12):795–8. doi: 10.1177/000348941011901202. [DOI] [PubMed] [Google Scholar]