Abstract
Objectives
Evaluate the effects of asymmetric superior laryngeal nerve stimulation on the vibratory phase, laryngeal posture, and acoustics.
Study Design
Basic science study using an in vivo canine model.
Methods
The superior laryngeal nerves were symmetrically and asymmetrically stimulated over eight activation levels to mimic laryngeal asymmetries representing various levels of superior laryngeal nerve paresis and paralysis conditions. Glottal posture change, vocal fold speed, and vibration of these 64 distinct laryngeal activation conditions were evaluated by high speed video and concurrent acoustic and aerodynamic recordings. Assessments were made at phonation onset.
Results
Vibratory phase was symmetric in all symmetric activation conditions but consistent phase asymmetry towards the vocal fold with higher superior laryngeal nerve activation was observed. Superior laryngeal nerve paresis and paralysis conditions had reduced vocal fold strain and fundamental frequency. Superior laryngeal nerve activation increased vocal fold closure speed, but this effect was more pronounced for the ipsilateral vocal fold. Increasing asymmetry led to aperiodic and chaotic vibration.
Conclusions
This study directly links vocal fold tension asymmetry with vibratory phase asymmetry; in particular the side with greater tension leads in the opening phase. The clinical observations of vocal fold lag, reduced vocal range, and aperiodic voice in superior laryngeal paresis and paralysis is also supported.
Keywords: Superior laryngeal nerve, laryngeal asymmetry, high speed video, acoustics, vibration, voice production
Introduction
Regularity of laryngeal vibration is considered essential for optimal voice production and irregular vibration is considered the genesis of poor vocal quality.1 Vibratory irregularity is most commonly caused by asymmetry of vocal fold mass or tension.1-2 Such laryngeal asymmetries often result from vocal fold paresis, which is now recognized as the most common etiology of dysphonia and vocal fatigue.3-4 However, in most instances the diagnosis of paresis is missed because of subtle abnormal laryngeal findings.5-7 The most commonly used tool for clinical assessment of voice disorders is videostroboscopy (VS), which allows assessment of vocal fold pliability as well as the vibratory phase.8 To evaluate vibratory phase the timing of the lateral excursion of the medial edges of the vocal folds at the same phase of the glottal cycle is noted.9 When phase asymmetry is present the timing of the lateral displacement of one vocal fold is different from the other; this finding is attributed to vocal fold mass or tension asymmetry from neurogenic paresis or paralysis.9-11
Sercarz et al. assessed vibratory phase in 3 volunteers before and after lidocaine-induced acute unilateral paralysis of SLN, recurrent laryngeal nerve (RLN), or combined SLN/RLN, and in 12 patients with unilateral laryngeal paralysis.11 They consistently found that the mucosal wave appeared earlier and travelled farther on the non-paralyzed vocal fold surface. Maunsell et al. performed ex vivo phonation of porcine larynges after unilateral cricothyroid (CT) approximation.12 They reported a vibratory phase shift but did not report which vocal fold was leading (i.e., opening earlier).
Although vibratory phase asymmetry is a commonly assessed parameter in the clinical assessment of suspected laryngeal paresis, direct evidence correlating tension asymmetry with phase asymmetry has not been previously reported, especially over a range of paresis/paralysis conditions. Previous studies on vibratory consequences of laryngeal asymmetry have been limited to ex vivo excised larynx experiments, limited number of laryngeal activations conditions (e.g. normal versus paralysis conditions), or clinical scenarios where the grade of paresis cannot be known.13
In this investigation, we evaluated the hypothesis that vocal fold tension asymmetry leads to vibratory phase asymmetry. CT activation level was used as a dependent variable because this is the most effective muscle for controlling vocal fold tension.14-15 We tested our hypothesis in an in vivo larynx model using graded stimulation to test laryngeal activation conditions corresponding to clinical scenarios ranging from complete unilateral SLN paralysis to subtle paresis. Based on clinical experience, we predicted that tension asymmetry would lead to vibratory phase asymmetry with the side with increased tension leading. Additionally, we were also able to evaluate the effects of SLN asymmetry on laryngeal posture, acoustics, and other vibratory parameters. The results further the understanding of the relationship between tension asymmetry and phase asymmetry, and the clinical presentation of a patient with dysphonia from SLN dysfunction.
Methods
A. In vivo canine model
The canine larynx is a close match to the human larynx in terms of its gross, microscopic, and histologic anatomy, and the validity of in vivo canine model is well established in voice research.15-17 This study protocol was approved by the Institutional Animal Research Committee of the University of California, Los Angeles. A mongrel canine was used for this study. Surgical exposure of the larynx and the laryngeal nerves was as described previously.15,18 The nerve branches to the posterior cricoarytenoid (PCA) muscle, Galen's anastomosis, and the internal SLN branches were divided bilaterally to eliminate their effects during nerve stimulation. Appropriately sized tripolar cuff electrodes (Ardiem Medical, Indiana, PA, USA) were applied to the respective motor nerve branches. Graded stimulation was applied to the laryngeal nerves as described previously.15.18 The RLNs were stimulated to achieve symmetric and complete closure at the vocal processes and their activation levels remained same for all conditions, while the SLNs were stimulated separately at 7 levels of graded stimulation (total 8 levels including zero stimulation condition). Neuromuscular stimulation was performed for 1500 ms with 0.1 ms long rectangular unipolar cathodic pulses at repetition rates of 100 Hz. To allow muscle recovery, each stimulation pulse train was followed by a 3.5 seconds pause prior to next stimulation.
A subglottal tube to provide rostral airflow for phonation was attached to the trachea at ring 2-3 and connected to an airflow controller (MCS Series Mass Flow Controller, Alicat Scientific, Tucson, Arizona, USA). The airflow rate was increased linearly from 300 to 1600 ml/s from onset to end of nerve stimulation (1500 ms) for each condition such that subglottic pressure (Psub) continuously increased beyond phonation onset pressure (Pth) or until maximum airflow was reached. The airflow at the glottic level was 37.5 degrees Celsius and 100% humidified.
B. Measurement of experimental parameters
A high-speed digital video camera (Phantom v210, Vision Research Inc., Wayne, New Jersey, USA) imaged laryngeal deformation and vibration at 3000 frames per second (fps) for the duration of nerve stimulation. The distance from the camera to the larynx remained constant for all conditions. India ink was used to mark several landmarks on the vocal fold surface, including the vocal processes.
Vocal fold strain (ε) was calculated as the ratio of vocal fold length change, by dividing the difference between vocal fold length (measured from anterior commissure to the vocal process) at phonation onset (Li) and at baseline (L0) by the vocal fold length at baseline: ε = (Li − L0)/L0. The time instance of phonation onset was determined using the acoustic signal and confirmed on HSV.
To calculate vocal fold closure speed the distance travelled by the India ink landmark at the vocal processes from onset of stimulation to the point of first contact of the vocal folds at the vocal processes was divided by the time required to travel that distance. Closure speed was normalized to millimeters per second (mm/s).
To evaluate phase of vibration, the HSV files were randomized and reviewed independently by two voice scientists (D.K.C. and N.J.) using the Phantom Camera Control Application software (PCC 1.3, Vision Research Inc., Wayne, New Jersey, USA). Frame-by-frame analysis of the first few vibratory cycles after phonation onset was performed and the phase of vibration noted as symmetric or asymmetric. If asymmetric the vocal fold that led in phase during opening was noted (left versus right). The excursion of the travelling mucosal wave beyond the visible open edge of the vocal fold on the superior vocal fold surface was also noted. No attempt was made to quantify the degree of phase asymmetry due to lack of standard methodology for this. In clinical practice phase asymmetry is also similarly assessed qualitatively by frame-by-frame analysis of glottal opening phase.
Acoustic and aerodynamic data were recorded using a probe tube microphone (Model 4128, Bruel & Kjaer North America, Norcross, Georgia, USA) and a pressure transducer (MKS Baratron 220D, MKS Instruments, Andover, Massachusetts, USA) mounted flush with the inner wall of the subglottic inflow tube about 7 cm below the inferior border of the glottis. The subglottal acoustic pressure signal was used to determine the fundamental frequency (F0) at phonation onset using Sound Forge acoustic analysis software (Sonic Foundry Sound Forge Version 6.0, Sonic Foundry Inc., Madison, Wisconsin, USA). The acoustic signal was manually examined, correlated with glottal opening on HSV, and the first three to four glottal cycles after onset were used to manually calculate the onset F0. The corresponding mean subglottal pressure (Psub) at phonation onset represented the phonation onset pressure (Pth).
E. Data presentation and interpretation
Muscle activation plots (MAPs) are used to illustrate the data. The MAP contains the left SLN activation levels (0-7) on the y-axis and right SLN activation levels (0-7) on the x-axis. This 8 × 8 plot thus concurrently presents all 64 different SLN activation conditions (8 symmetric, 56 asymmetric) using color coding that allows a visual qualitative interpretation of data. This format has been used previously in voice research where data trends from a large number of laryngeal activation conditions are presented.15 Statistical analysis of data is not appropriate in this setting as data trends are emphasized. The data presented below is robust, consistent with previously reported studies,15,18 and the results are highly relevant towards understanding the vibratory effects of tension asymmetry, and the effects of SLN asymmetry on glottal posture, acoustics and vibration.
Results
Posture changes
Symmetry of glottal adduction to RLN stimulation was established at baseline visually by evaluating the HSV images. Once symmetric glottal closure was established, bilateral RLN stimulation level was kept constant for all activation conditions while the left and right SLN stimulation was varied separately from no stimulation to 7 levels of graded stimulation from threshold to maximal muscle activation. Thus, a total of 64 distinct laryngeal activation conditions were tested.
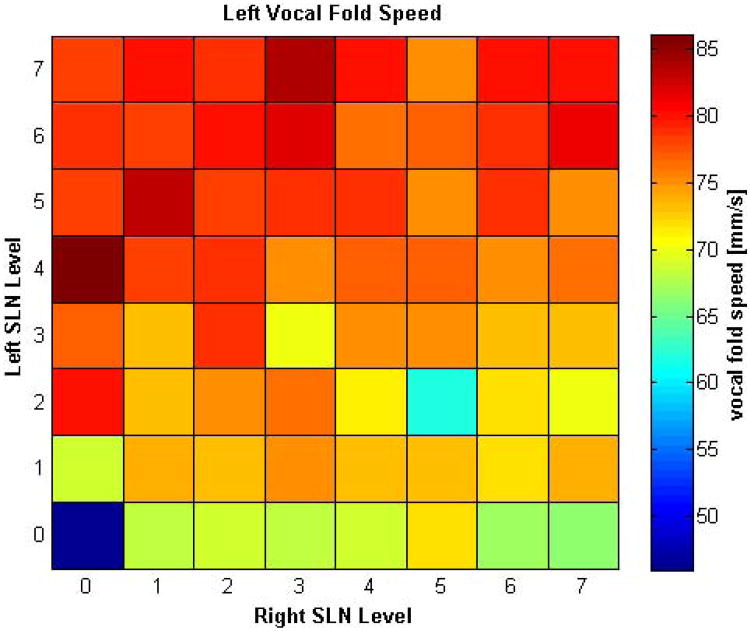
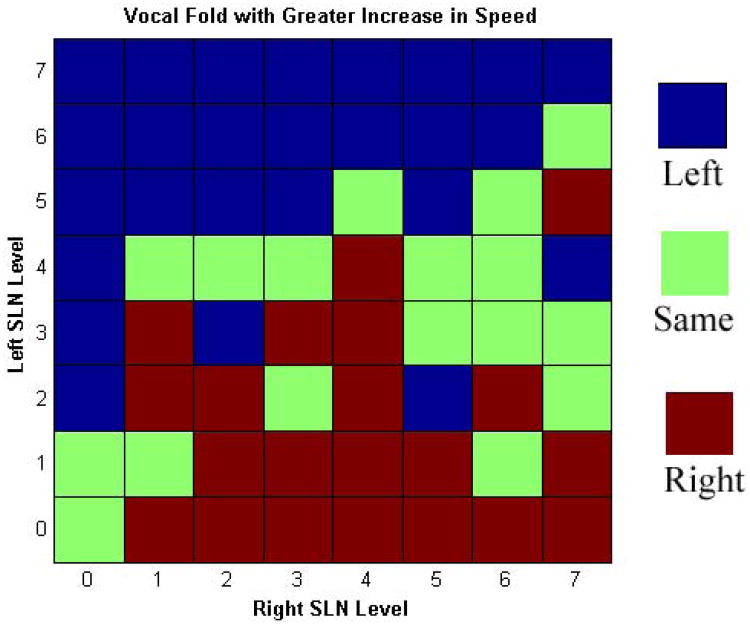
Despite visual symmetry of closure, calculation of vocal fold speed at baseline (zero SLN activation) revealed that the left vocal fold adducted slightly faster (46 mm/s) than the right vocal fold (37 mm/s), but this relationship was maintained throughout because RLN stimulation remained constant for all conditions. SLN stimulation increased the speed of vocal fold closure bilaterally: the left vocal fold speed ranged from 62 to 86 mm/s and the right vocal fold speed ranged from 41 to 72 mm/s. The relative increases in speed were similar for both vocal folds in mirrored conditions. For illustrative purposes the MAP of the left vocal fold speed is shown in Figure 1. It shows that while left vocal fold closure speed increased with either SLN activation, the increase was greater for relative level of left SLN activation. The right vocal fold mirrored this finding. Thus, SLN activation leads to greater increase in ipsilateral vocal fold closure speed than contralateral one. Figure 2 illustrates which vocal fold had a greater relative increase in closure speed (if the speed increase differed by less than 10% then both sides were considered to have the “same” increase). Figures 1 and 2 demonstrate that while SLN activation leads to bilateral increase in vocal fold closure speed, the effects on the ipsilateral vocal fold is greater. This finding was particularly consistent and dramatic in unilateral SLN paralysis conditions.
Figure 1.
Muscle Activation Plot (PLOT) for left vocal fold closure speed as a function of graded levels of left and right SLN activation. The left vocal fold closure speed increased with activation of either CT muscle but the speed increased more when the left SLN activation level was greater. The right vocal fold mirrored this finding.
Figure 2.
Muscle Activation Plot (MAP) illustrating the side with greater relative increase in vocal fold closure speed as a function of graded levels of left and right SLN activation. SLN activation led to greater increase in ipsilateral vocal fold closure speed than the contralateral one. This finding is particularly consistent unilateral SLN paralysis conditions (L SLN levels 2-7 at R SLN level 0; R SLN levels 1-7 at L SLN level 0).
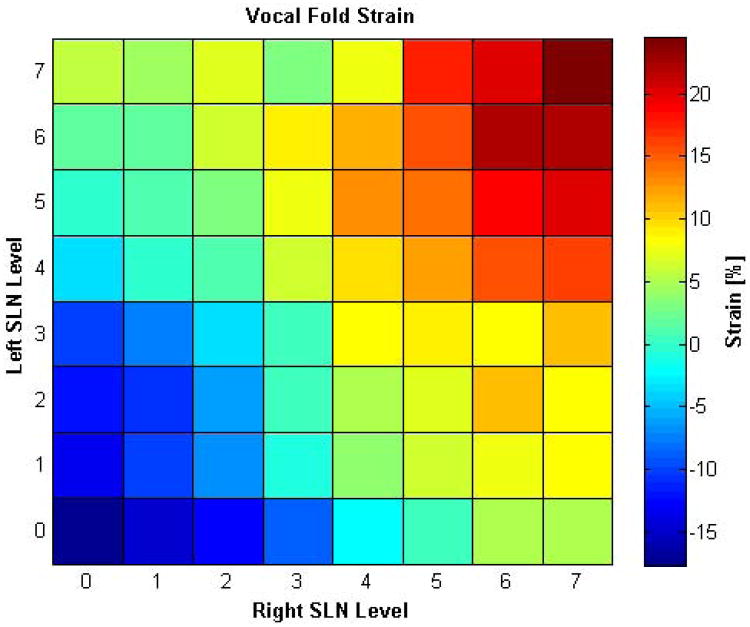
Vocal fold strain varied from -18% at zero SLN activation level to +25% at maximal SLN activation levels (Figure 3). Vocal fold strain was identical (less than 5% variation) for both vocal folds regardless of the level of SLN asymmetry. However, the greatest unilateral SLN activation levels resulted in intermediate strain (+5.8% for L SLN7, R SLN0; +5.3 for L SLN0, R SLN7). SLN activation also caused lateral flaring of the ipsilateral thyroid ala as described previously.18 The degree of lateral flaring correlated with the degree of SLN activation (higher SLN activation caused more robust lateral flaring of the ipsilateral thyroid ala).
Figure 3.
Muscle Activation Plot (MAP) for vocal fold strain as a function of graded levels of left and right SLN activation. The greatest positive strain (+25%) was seen with the highest symmetric bilateral SLN activation and the greatest negative strain (−18%) was seen at bilateral zero SLN activation. Unilateral SLN activation levels resulted in maximal strain that was in between these values (+5.8% for R SLN paralysis; +5.3 for L SLN paralysis).
Vocal fold vibratory pattern
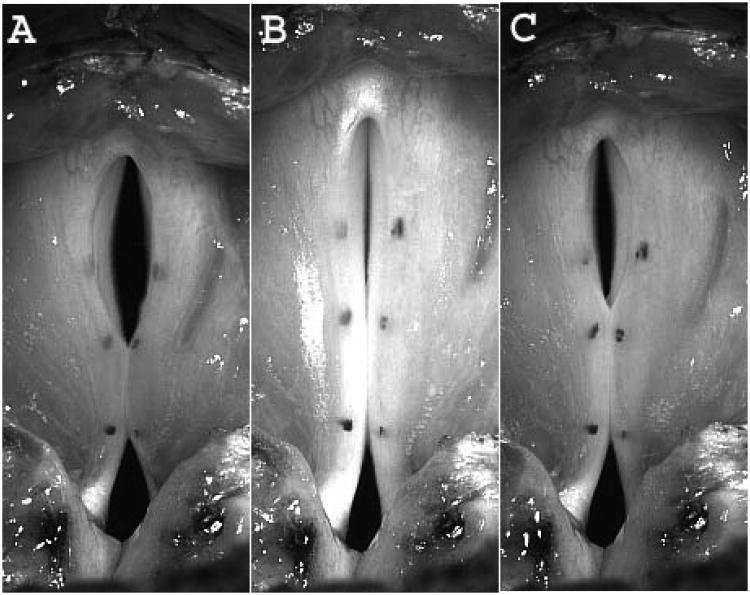
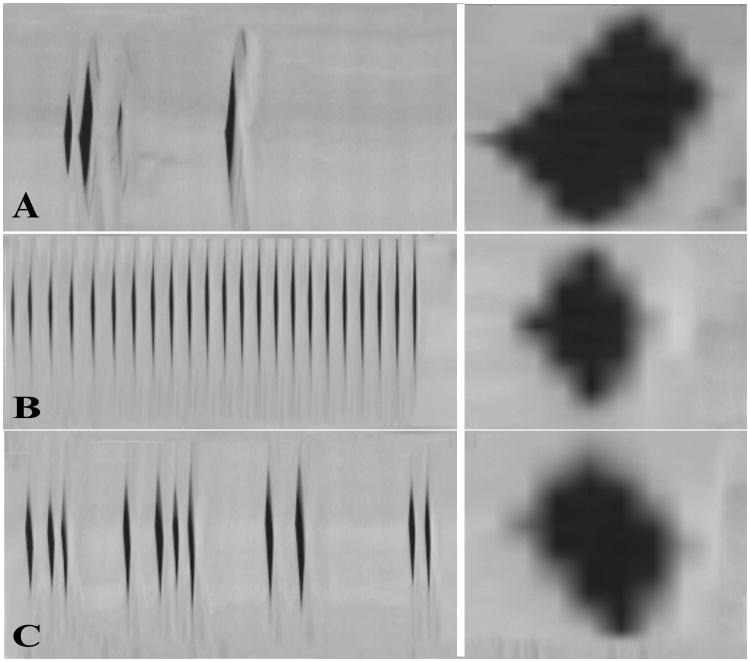
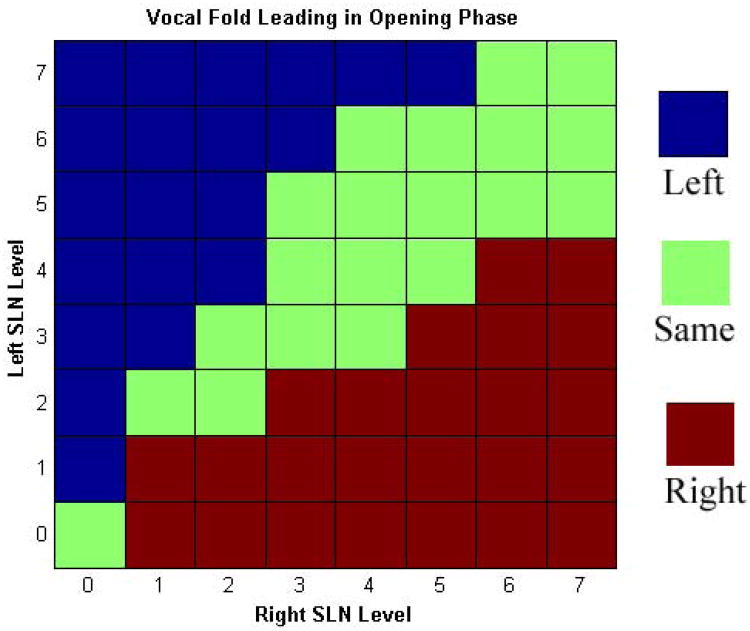
At baseline, the vibratory pattern was periodic with symmetric glottal opening. Glottal opening was mid-membranous at phonation onset for all conditions. As SLN asymmetry increased, glottal opening occurred earlier in the more activated side. Mucosal wave amplitude also followed this phase asymmetry: the mucosal wave consistently travelled farther in the more activated side. Figure 4 displays these findings in three illustrative conditions. Figure 4A represents condition L SLN 4, R SLN 0. The left vocal fold opened earlier and a robust mucosal wave is visible on the left vocal fold while the right vocal fold shows only early hints of mucosal wave. In Figure 4B, L and R SLN Levels 4, mucosal waves are equally robust and symmetric. Finally, in Figure 4C, condition L SLN 0, R SLN 4, the right vocal fold opened earlier and a robust mucosal wave is present on the right vocal fold surface while only hints of mucosal wave is present on the left vocal fold. In addition, as SLN asymmetry increased the acoustic signal became more aperiodic and chaotic, especially as the Psub increased. Figure 5 shows DKG from a horizontal line over mid-glottal opening of the conditions presented in Figure 4, and illustrates the aperiodicity induced by SLN asymmetry. While the symmetric condition is visually periodic (B), the asymmetric conditions are aperiodic (A and C) or even chaotic (A). The accompanying DKG enlargements of a corresponding glottal cycle clearly demonstrate the phase lead in the asymmetric conditions. Figure 6 shows phase lead over all 64 activation conditions. The phase lead was predominantly to the left with left dominant SLN asymmetry and to the right with right dominant SLN asymmetry.
Figure 4.
Glottal opening phase demonstrating vocal fold lateral excursion and mucosal wave on the superior vocal fold surface. (A) Condition L SLN level 4, R SLN level 0. The left vocal fold opened earlier and a robust mucosal wave is visible on the left vocal fold. (B) Condition L SLN level 4 and R SLN level 4. Mucosal waves are equally robust and symmetric. (C) Condition L SLN 0, R SLN 4, the right vocal fold opened earlier and a robust mucosal wave is present on the right vocal fold surface. (See text for full discussion.)
Figure 5.
Digital kymograms (DKG) from three illustrative cases as shown in figure 4. Enlargements of one glottal cycle from the corresponding condition are in the right subpanel next to its DKG. In the DKG figure top represents the right vocal fold and the bottom represents the left vocal fold. (A) Condition L SLN level 4, R SLN level 0. The vibration is chaotic with phase lead to the left. (B) Condition L SLN and R SLN level 4. Vibration is periodic and glottal opening phase is symmetric. (C) Condition L SLN 0, R SLN 4. Vibration is aperiodic and phase lead is to the right.
Figure 6.
Muscle Activation Plot (MAP) for phase lead as a function of graded levels of left and right SLN activation. There is a preponderance of phase lead to the left with left dominant SLN asymmetry and phase lead to the right with right dominant SLN asymmetry.
Acoustics and Aerodynamics
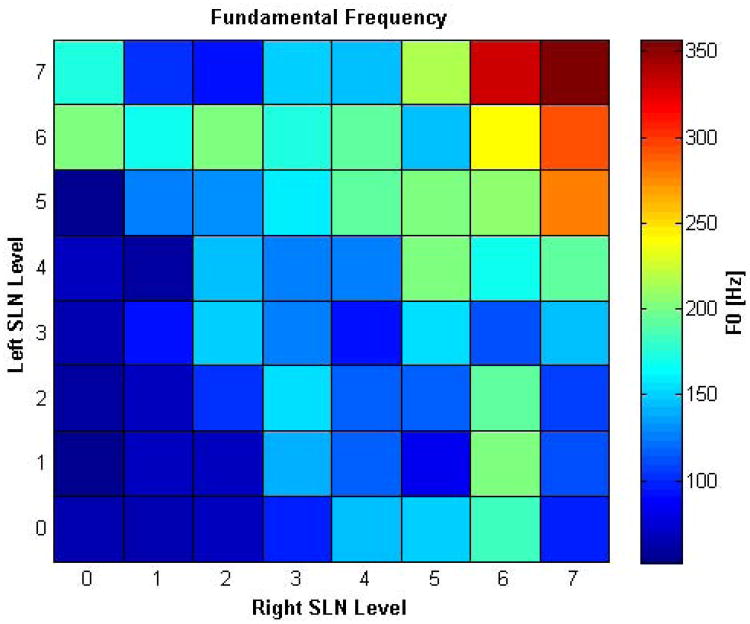
Figure 7 shows onset F0 over all 64 conditions. Higher levels of bilateral SLN activation was needed to achieve the higher frequencies. The highest unilateral SLN activation levels led to F0 values about half that of the highest bilateral SLN activation level. We attempted to calculate perturbation measures from the acoustic data (jitter, shimmer, HNR) but could not find a reliable pattern because perturbation measures could not be calculated accurately in many asymmetric conditions due to vibratory aperiodicity. Aerodynamic measurements revealed that onset Psub and airflow generally increased with increasing levels of asymmetry and decreased somewhat with increasing SLN activation (data not shown).
Figure 7.
Muscle Activation Plot (MAP) for fundamental frequency (F0) at phonation onset, as a function of graded levels of left and right SLN activation. The highest F0 is reached with high bilateral activation levels. SLN paralysis conditions show contracted vocal range. (See text for full discussion.)
Discussion
This study demonstrates that asymmetric SLN stimulation leads to vibratory phase asymmetry. The vibratory phase asymmetry is most likely effected through changes in the overall stiffness and tension of the vocal folds. In particular, the glottal opening leads (occurs earlier) on the side of increased tension and mucosal wave amplitude is also greater on this side (Figure 6). While we tested our hypothesis with SLN stimulation, other mechanisms may exist to change vocal fold tension, such as TA and LCA muscle activation.8,19 Future studies are planned for these questions.
The acoustic-perceptual consequences of laryngeal asymmetry have not been entirely addressed in this study. Several studies have shown that phase asymmetry can be present in normophonic subjects, thus raising the question of the clinically relevant degree of phase asymmetry.9,20 Glottic vibration is an inherently nonlinear phenomenon.21 Thus, some level of perturbation and aperiodicity can still lead to normal sounding voice.22 What degree of asymmetry is necessary to result in a perceptual change in vocal quality is unclear and future studies are needed on systematic evaluation of vibratory asymmetry and its auditory-perceptual consequences.
This study validates some clinical observations made in SLN paresis and paralysis. Eckley et al. found significantly reduced vocal range in patients with SLN paralysis.23 We found that in unilateral SLN paralysis the highest F0 reached is less than half of the F0 reached when both SLNs are fully stimulated (Figure 7). Comparison of MAPs for strain (Figure 3) with F0 (Figure 7) reveals a nearly linear positive relationship, and thus decreased strain in unilateral SLN paralysis and paresis is the most likely culprit leading to limitation of vocal range. Woodson et al. performed unilateral CT contraction in cadaver larynges and found that both vocal folds elongated equally and that bilateral CT contraction led to the most change in vocal fold length.24 We also found identical strain change in both vocal folds in all symmetric and asymmetric conditions, and that bilateral CT activation is needed for maximal strain change. However, this in vivo study shows that unilateral SLN paresis and paralysis do have acoustic consequences, as is also commonly seen in clinical practice. We found reduced vocal range, aperiodicity, and chaotic vibration with SLN asymmetry.
While reduced vocal range in SLN asymmetry can be explained by the reduced strain, what possible mechanisms could lead to phase asymmetry and vibratory aperiodicity despite identical bilateral vocal fold strain? The exact mechanisms for these differential effects are topics for future systematic investigations. However, there are several possible mechanisms. Vocal fold “tension” includes not only longitudinal tension (measured as strain in this study) but an overall “stress state” that includes transverse (medio-lateral) tension, infero-superior tension, as well as the “stiffness” or stress state of the activated thyroarytenoid muscle. One likely mechanism leading to the phase asymmetry and aperiodicity in the asymmetric conditions is the lateral flaring of the ipsilateral thyroid ala that correlated with SLN stimulation. Lateral flaring of the thyroid ala would be expected to add to the transverse tension of the ipsilateral vocal fold, thus leading to asymmetric stress state between the vocal folds in the asymmetric conditions. Another possible mechanism is flexibility in the cricothyroid joints that may facilitate changes to vocal fold stiffness upon CT contraction. Effects from afferent reflex arc is possible; however, this is unlikely because the electrical stimulation level required to activate the sensory c-fibers is about 90 times required for motor fibers.25 In addition, we carefully controlled the threshold and maximal activation of CT via graded stimulation of the SLN, and we did not notice any reflex activation of other laryngeal muscles visually or by hooked wire electromyography. In addition, the internal SLN, Galen's nerves, and nerve branches to the PCA muscles were divided bilaterally.
Absence of “brisk” abduction and adduction of the vocal fold (“vocal fold lag”) is reported to be a common finding in SLN paresis or paralysis.26 Rubin et al. have recommended that “Repeated Phonatory Tasks” (RPTs) be performed to elicit the signs of subtle paresis.6 Our findings would support this observation. Figure 1 and 2 demonstrate that in unilateral SLN paralysis the vocal fold closure speed in the paralyzed side is less dominant compared to the normal side. Thus the paralyzed side is expected to show a lag compared to the normal side. These illustrations also suggest that the positive predictive value of RPTs in diagnosis of SLN paresis/paralysis would be high for paralysis but may be reduced and variable for paresis conditions.
While this study carefully controlled CT activation levels, in clinical practice patients with dysphonia often present with paresis of multiple intrinsic laryngeal muscles. Further basic science voice research is needed to understand the phonatory consequences of combinations of paresis and paralysis conditions. The ability to experimentally control activation levels of multiple laryngeal muscles may allow the study of these complex neuromuscular interactions.
Conclusions
This in vivo canine study validates the connection between tension asymmetry and phase asymmetry in laryngeal vibration. In particular, the phase of vibration leads on the side of greater tension. This study also validates and supports the clinical observations of vocal fold lag, reduced vocal range, and increased aperiodicity in SLN paresis and paralysis.
Acknowledgments
Funding source: This study was supported by Grant No. RO1 DC011300 from the National Institutes of Health
Footnotes
This article was presented as an oral presentation at The Triological Society Combined Sections Meeting, January 26, 2013, Scottsdale, AZ, USA.
Financial Disclosure: The authors have no other financial disclosures to make.
Conflict of Interest: None
References
- 1.Herzel H, Berry D, Titze I, Steinecke I. Nonlinear dynamics of the voice: Signal analysis and biomechanical modeling. Chaos. 1995;5(1):30–34. doi: 10.1063/1.166078. [DOI] [PubMed] [Google Scholar]
- 2.Eysholdt U, Rosanowski F, Hoppe U. Vocal fold vibration irregularities caused by different types of laryngeal asymmetry. Eur Arch Otorhinolaryngol. 2003;260(8):412–7. doi: 10.1007/s00405-003-0606-y. [DOI] [PubMed] [Google Scholar]
- 3.Koufman JA, Postma GN, Cummins MM, Blalock PD. Vocal fold paresis. Otolaryngol Head Neck Surg. 2000 Apr;122(4):537–41. doi: 10.1067/mhn.2000.102574. [DOI] [PubMed] [Google Scholar]
- 4.Simpson CB, Cheung EJ, Jackson CJ. Vocal fold paresis: clinical and electrophysiologic features in a tertiary laryngology practice. J Voice. 2009;23(3):396–8. doi: 10.1016/j.jvoice.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Rubin AD, Praneetvatakul V, Heman-Ackah Y, Moyer CA, Mandel S, Sataloff RT. Repetitive phonatory tasks for identifying vocal fold paresis. J Voice. 2005;19(4):679–86. doi: 10.1016/j.jvoice.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Rubin AD, Sataloff RT. Vocal fold paresis and paralysis. Otolaryngol Clin North Am. 2007;40(5):1109–31. doi: 10.1016/j.otc.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Simpson CB, May LS, Green JK, Eller RL, Jackson CE. Vibratory asymmetry in mobile vocal folds: is it predictive of vocal fold paresis? Ann Otol Rhinol Laryngol. 2011;120(4):239–42. doi: 10.1177/000348941112000404. [DOI] [PubMed] [Google Scholar]
- 8.Hirano M, Bless DM. Videostroboscopic examination of the larynx. Singular Publication Group; San Diego, California: 1993. [Google Scholar]
- 9.Bonilha HS, Deliyski DD, Gerlach TT. Phase asymmetries in normophonic speakers: visual judgments and objective findings. Am J Speech Lang Pathol. 2008;17(4):367–76. doi: 10.1044/1058-0360(2008/07-0059). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonilha HS, Deliyski DD. Period and glottal width irregularities in vocally normal speakers. J Voice. 2008;22(6):699–708. doi: 10.1016/j.jvoice.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Sercarz JA, Berke GS, Ming Y, Gerratt BR, Natividad M. Videostroboscopy of human vocal fold paralysis. Ann Otol Rhinol Laryngol. 1992;101(7):567–77. doi: 10.1177/000348949210100705. [DOI] [PubMed] [Google Scholar]
- 12.Maunsell R, Ouaknine M, Giovanni A, Crespo A. Vibratory pattern of vocal folds under tension asymmetry. Otolaryngol Head Neck Surg. 2006;135(3):438–44. doi: 10.1016/j.otohns.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Kendall KA. High-speed laryngeal imaging compared with videostroboscopy in healthy subjects. Arch Otolaryngol Head Neck Surg. 2009;135(3):274–81. doi: 10.1001/archoto.2008.557. [DOI] [PubMed] [Google Scholar]
- 14.Chhetri DK, Berke GS, Lotfizadeh A, Goodyer E. Control of vocal fold cover stiffness by laryngeal muscles: a preliminary study. Laryngoscope. 2009;119(1):222–7. doi: 10.1002/lary.20031. [DOI] [PubMed] [Google Scholar]
- 15.Chhetri DK, Neubauer J, Berry DA. Neuromuscular control of fundamental frequency and glottal posture at phonation onset. J Acoust Soc Am. 2012 Feb;131(2):1401–12. doi: 10.1121/1.3672686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berke GS, Moore DM, Hantke DR, Hanson DG, Gerratt BR, Burstein F. Laryngeal modeling: theoretical, in vitro, in vivo. Laryngoscope. 1987;97(7 Pt 1):871–81. [PubMed] [Google Scholar]
- 17.Garrett CG, Coleman JR, Reinisch L. Comparative histology and vibration of the vocal folds: implications for experimental studies in microlaryngeal surgery. Laryngoscope. 2000;110(5 Pt 1):814–24. doi: 10.1097/00005537-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Chhetri DK, Neubauer J, Berry DA. Graded activation of the intrinsic laryngeal muscles for vocal fold posturing. J Acoust Soc Am. 2010;127(4):EL127–33. doi: 10.1121/1.3310274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirano M. Folia Phoniatr (Basel) 1974. Morphological structure of the vocal cord as a vibrator and its variations; pp. 26pp. 89–94. [DOI] [PubMed] [Google Scholar]
- 20.Haben CM, Kost K, Papagiannis G. Lateral phase mucosal wave asymmetries in the clinical voice laboratory. J Voice. 2003;17(1):3–11. doi: 10.1016/s0892-1997(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 21.Titze Ingo R. Workshop on acoustic voice analysis: Summary statement. National Center for Voice and Speech. 1995 [Google Scholar]
- 22.Giovanni A, Ouaknine M, Guelfucci B, Yu P, Zanaret M, Triglia JM. Nonlinear behavior of vocal fold vibration: the role of coupling between the vocal folds. J Voice. 1999;13(4):465–476. doi: 10.1016/s0892-1997(99)80002-2. [DOI] [PubMed] [Google Scholar]
- 23.Eckley CA, Sataloff RT, Hawkshaw M, Spiegel JR, Mandel S. Voice range in superior laryngeal nerve paresis and paralysis. J Voice. 1998;12(3):340–8. doi: 10.1016/s0892-1997(98)80024-6. [DOI] [PubMed] [Google Scholar]
- 24.Woodson GE, Murry MP, Schweizer V, Hengesteg A, Chen N, Yeung D. Unilateral cricothyroid contraction and glottic configuration. J Voice. 1998;12(3):335–9. doi: 10.1016/s0892-1997(98)80023-4. [DOI] [PubMed] [Google Scholar]
- 25.Castoro MA, Yoo PB, Hincapie JG, et al. Excitation properties of the right cervical vagus nerve in adult dogs. Exp Neurol. 2011;227:62–8. doi: 10.1016/j.expneurol.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Dursun G, Sataloff RT, Spiegel JR, Mandel S, Heuer RJ, Rosen DC. Superior laryngeal nerve paresis and paralysis. J Voice. 1996;10(2):206–11. doi: 10.1016/s0892-1997(96)80048-8. [DOI] [PubMed] [Google Scholar]
- 27.Robinson JL, Mandel S, Sataloff RT. Objective voice measures in nonsinging patients with unilateral superior laryngeal nerve paresis. J Voice. 2005;19(4):665–7. doi: 10.1016/j.jvoice.2005.04.001. Epub 2005 Aug 29. [DOI] [PubMed] [Google Scholar]