Abstract
Background
In individuals with Down syndrome virtually all structures of the eye have some abnormality which likely diminishes vision. We examined basic vision functions in adults with Down syndrome.
Materials and Methods
Participants completed a battery of psychophysical tests which probed a comprehensive array of visual functions. The performance of adults with Down syndrome was compared to younger and older adults without intellectual disability.
Results
Adults with Down syndrome had significant vision deficits; reduced sensitivity across spatial frequencies and temporal modulation rates, reduced stereopsis, impaired vernier acuity, and anomalies in colour discrimination. The pattern of deficits observed was similar to those seen by researchers examining adults with Alzheimer’s disease.
Conclusions
Our findings suggest that a common mechanism may be responsible for the pattern of deficits observed, possibly the presence of Alzheimer’s disease neuropathology in the visual association cortex. We also showed that individuals with mild to moderate intellectual disability are capable of participating in studies employing state-of-the-art psychophysical procedures. This has wider implications in terms of their ability to participate in research that use similar techniques.
Keywords: Down syndrome, intellectual disability, ageing, vision, vision deficits, Alzheimer’s disease
Vision Deficits in Adults with Down syndrome
Down syndrome is associated with a number of phenotypic characteristics which define the syndrome including distinctive facial and physical features, intellectual disability, health problems, features of atypical aging, earlier-onset Alzheimer’s disease, and shortened life expectancy (Baird & Sadovnick 1987; Devenny et al. 2005; Roizen 2001; Silverman 2007). Individuals with Down syndrome also typically have vision deficits (see Liyanage & Barnes 2008 for a recent review). Virtually all structures of the eye have been reported to show some associated abnormality including the lid, iris, cornea, lens, and retina (Berk et al. 1996; Caputo et al. 1989; Catalano 1990; Evenhuis et al. 2001; Merrick & Koslowe 2001; van Splunder et al. 2003). As a result, nystagmus, strabismus, inaccurate accommodation, amblyopia, astigmatism, and significant refractive errors are prevalent in this population (see Appendix for brief definitions of italicized terms) (Averbuch-Heller et al. 1999; da Cunha & Moreira 1996; Kim & Hwang 2009; Little et al. 2009; Stephen et al. 2007; Woodhouse et al. 1997; Woodhouse et al. 1996). While many individuals with Down syndrome present with a combination of these conditions, none of them uniquely contribute to the phenotype (Courage et al. 1994).
There is extensive documentation of the incidence of optical, oculomotor, and neural abnormalities in individuals with Down syndrome though fewer studies have assessed basic vision capacities that may be affected such as spatial and temporal vision, depth perception, and colour vision. Reduced spatial vision would affect the ability to read signs and watch television (high spatial frequency deficits) and to recognize objects and faces (low spatial frequency deficits) (Evans & Ginsburg 1985; Lovegrove et al. 1980). Depth perception is an integral component to many tasks of daily living and impaired depth perception would then make navigating stairs and escalators difficult as well as participating in sports and other leisure activities. While colour vision may not be essential for negotiating our way around the environment, it facilitates the ability to recognize and discriminate objects from one another and to see objects against a varied background.
Visual impairment is especially prevalent in older adults with Down syndrome (Evenhuis et al. 2001; Van Buggenhout et al. 1999; van Schrojenstein Lantman-de Valk et al. 1994; van Splunder et al. 2004). van Schrojenstein Lantman-de Valk et al. found that visual impairment occurred in 46% of adults with Down syndrome between 50 and 59 years of age compared to 13% of individuals of the same age with intellectual disability from other etiologies. This number increased significantly with age such that by 60 years, 85% of people with Down syndrome and 20% of individuals with intellectual disability from other etiologies had visual impairment (also see Evenhuis et al. 2001; Van Buggenhout et al. 1999; van Splunder et al. 2003). Refractive errors were most prevalent (61%), followed by strabismus (44%) (van Splunder et al. 2004). The prevalence of cataracts in elderly people with Down syndrome was common and increased with age from 16% in the group aged 50–59 years to 63% in those 60 years and older (van Schrojenstein Lantman-de Valk et al. 1994).
The few studies that have examined basic vision capacities in individuals with Down syndrome have observed loss of sensitivity to contrast variation in spatial patterns, reduced stereopsis, and colour vision abnormalities (e.g. Kim & Detterman 1996; Kim et al. 1994). Kim and Detterman (1996) measured the contrast sensitivity function (CSF) of adults with Down syndrome between the ages of 18 and 61 years and found that adults with Down syndrome had lower CSFs compared to adults with other forms of intellectual disability and those without intellectual disability.
Researchers have noted that the pattern of vision deficits seen in adults with Down syndrome is similar to the pattern accompanying Alzheimer’s disease in adults without intellectual disability (Rocco et al. 1997). The similarity has been attributed to the presence of the neuropathology of Alzheimer’s disease which, in adults with Down syndrome is observed as early as their 5th decade of life (Lott & Lai 1982; Mann 1988; Wisniewski et al. 1985; see Zigman & Lott 2007).
Deficits in vision have implications for learning, cognitive functioning, and adaptive behavior. Visually impaired individuals with intellectual disability were found to have significantly more maladaptive behaviors than individuals with intellectual disability without visual impairment (Prasher 1994). Kim et al. (1996) found evidence that a pronounced visual disturbance among older adults with Down syndrome affected their reaction time as well. Evenhuis et al. (2009) examined the effects of visual impairment on adaptive functioning in adults with intellectual disability of varied etiology including individuals with Down syndrome, autism spectrum disorder, and of unknown etiology. Adults with visual impairment showed diminished independent living skills, communication and language, initiative and persistence, and social skills as well as exhibiting insecure movement. Visual impairment then had an additional and substantial effect on the functional status in these populations. In individuals without intellectual disability, Cronin-Golomb et al. (1995) found that performance on vision functions (e.g., pattern masking, low spatial frequency contrast sensitivity and colour discrimination) was a better predictor of performance on cognitive tasks than was dementia severity (as assessed with the Blessed Dementia Rating Scale; Blessed et al. 1968).
Comparatively little is known about basic visual capacities of older adults with Down syndrome, perhaps because, until relatively recently, very few of these adults lived long enough for this to be an issue (Silverman et al. 1998). The present study examined a comprehensive array of visual functions employing a wider range of stimulus values and more precise tests of vision than had been previously used with this population. Overall, our goal was to contribute to a better understanding of the status of basic vision functions of adults with Down syndrome.
Method
Participants
Seven adults with Down syndrome (Mage = 40.29 years, SD = 8.99, range 28–48 years; MIQ =60.86, SD = 8.05, range 53–77) participated. All individuals had full trisomy 21, confirmed by cytogenetic testing. All participants had been followed in a longitudinal study of age-associated changes in functioning in memory and cognition (e.g., Devenny et al. 1996; Krinsky-McHale et al. 2002; Silverman et al. 2004) and none showed cognitive decline indicative of clinical dementia at the time of testing, although one individual has subsequently received a diagnosis. For inclusion, participants had to reside near the Applied Vision Institute and Visual Research Laboratory in the Psychology Department at Brooklyn College/City University of New York where the vision testing took place. The first (SJKM), second (WS), and third (DAD) authors were very familiar with these individuals based on their participation in the longitudinal study and judged that they would be able to complete the tasks in the current study. All individuals had good verbal skills. Participants were individually tested during six sessions within a two-month period. At each visit, testing took approximately 1.5 hours to complete, including breaks to prevent fatigue. All participants with Down syndrome provided data for all of the tests.
Prior to testing, an optometrist assessed participants’ optical and refractive status. The optometric evaluation of visual acuity was assessed with a variety of measures including the Tumbling “E” test which consists of a large white chart with black types of the letter “E” shown in different orientations and at different sizes. Participants had to indicate which direction each “E” was facing. As part of our larger longitudinal study, we examined the medical records of participants regarding clinically significant health problems such as ophthalmic abnormalities and disorders including amblyopia, cataracts, astigmatism, and nystagmus. The medical records of two participants indicated that they had mild cataracts, one participant was myopic, one had astigmatism and was presbyopic; all were optically corrected for the viewing distance of the stimuli used in this study. None of the participants were strabismic or markedly anisometropic. For testing, if needed, participants wore their own corrective lenses or they were provided with the appropriate lenses in a trial frame. Corrected binocular acuity was 20/50 or better in all participants.
To examine the effects of ageing on the visual system, the performance of participants with Down syndrome was compared to adults without intellectual disability who were either younger (N=23, Mage = 20.91 years, SD = 3.69, range 16–28 years) or older (N=6, Mage = 56.00 years, SD = 4.15, range 51–61 years). IQs were unavailable for these participants but almost all were college students or faculty. All had acuities of, or were corrected to, at least 20/25 and were tested with their best optical correction. With the few exceptions noted in the results section, all participants without intellectual disability provided data for all of the tests.
Description of Functions and Procedures
Spatio-temporal contrast sensitivity functions
We measured the spatial and temporal resolution of the visual system to monochromatic targets that varied in contrast, while keeping mean luminance constant. Stimuli were sinusoidal gratings (either horizontal or vertical in orientation) whose Michelson contrasts varied from near zero to near 100%. There were 30 stimuli: 6 spatial frequencies (0.6, 1, 2, 5, 12, and 24.4 cy/deg) and 5 temporal frequencies (1, 4, 8, 15, 24 Hz). The contrast of each spatial frequency (grating) was modulated at each of the temporal frequencies (spectral × temporal frequencies), defining a spatio-temporal surface. Temporal modulation was counterphase with a sinusoidal profile. There was no static condition because eye movements prevent a true static condition. However, at our relatively short presentation duration (see below) the 1 Hz condition approximates a static condition.
Apparatus
The apparatus was a dedicated computer and interface driving a monitor (Vision Works Graphics Displays). The display was monochrome (CIE 1931 coordinates x=0.443 and y=0.533, corresponding to a near-monochromatic light of about 570 nm) with a mean luminance of 55 cd/m2. The display screen was seen through a circular aperture (3.5°) in a white surround screen (13° x 13°) front-illuminated at about 25 cd/m2 (CIE 1931 coordinates, x=0.523 y=0.417). Calibrations were made with a scanning spectra-photometer (Photo Research model 704A). Participants were seated 3.6 m from the display with their heads stabilized in a chinrest. They viewed the stimuli binocularly in a darkened room. They were instructed to look at the center of the screen as there was no fixation target.
Psychophysical procedures
In the first session, the adjustment phase, participants viewed each of 30 stimuli. All participants were told that the grating would be moving either up-down or left-right. Stimuli were presented in random order and randomly horizontal or vertical. To establish an initial approximate contrast threshold for each stimulus, participants adjusted the contrast of each stimulus until its orientation could just be detected. Adults without intellectual disability did this by either increasing (using the “I” key on the computer keyboard) or decreasing (using the “D” key) the visibility until they could barely detect the orientation of the stimulus. Adults with Down syndrome were told that their task was to indicate to the experimenter either verbally or by pointing which way the pattern moved on the screen. If the participant was accurate, the experimenter decreased the visibility or if incorrect, the experimenter increased the visibility (the experimenter was able to see the stimuli). This process continued until the experimenter was satisfied that the participant barely detected the orientation of the stimulus. These adjustment data were then used to set starting points for the next procedure which measured thresholds with greater precision. The adjustment phase then, also served as a session of orientation/training to ensure that all participants understood the requirements for task performance.
The final thresholds were obtained using a two-alternative forced-choice, adaptive, psychophysical algorithm in which the contrast of the stimulus presented on any given trial depended on the participant’s performance on the preceding series of contrasts (using the QUEST algorithm, a Bayesian estimate of threshold, see Watson & Pelli 1983). On each trial, the participant had to decide whether the orientation of the grating was horizontal or vertical. Participants without intellectual disability entered their responses via a computer keyboard with a plastic cover exposing only the relevant keys (pressing either the “H” or “V” key to record their decision). Participants with Down syndrome indicated their responses either verbally or by pointing to show their decision regarding the orientation of the grating. The experimenter then entered the participant’s response into the computer (the experimenter could not see the stimuli). Feedback was provided for correct answers (the computer beeped once) and was used to motivate participants to continue concentrating on the task. For individuals with Down syndrome, the experimenter reiterated the meaning of the beep with statements like, “good job,” and “right answer.” Performance determined the contrast on the next trial in which that grating appeared. A random subset of 15 of the 30 stimuli was chosen for this phase and presented immediately following the adjustment phase; the remaining stimuli were presented in another session (separated from the first to avoid possible practice and order effects). A trial had a fixed duration of 2 s. On any given trial, a stimulus was “ramped” on and off by increasing contrast linearly from zero for 0.5 s and then remained at full contrast for 1 s. In between trials, the display screen was uniform at the mean luminance level.
Hyperacuities
Visual acuity is usually defined as the ability to read optotypes (letters, numbers, or geometric symbols) whose lines subtend a visual angle of one minute of arc (minarc), this corresponds to a limiting grating value of approximately 30 cy/deg. There are however, tasks for which the visual system can perform appreciably better due to the processing of information at higher cortical centers. These are hyperacuities and include the ability to detect minimally detectable stereoscopic “pop out” and vernier offsets (see below). We tested all participants on stereopsis and vernier acuity. To ensure that participants understood the task, they received a practice trial.
Stereopsis
Stereopsis was quantified using standard clinical tests, the Titmus Fly Book Test and Randot Stereo Acuity Test (both from Stereo Optical Co., Chicago). The stimuli were disparate stereopairs reproduced as polarized vectographs. Participants wore polarized glasses which allowed the presentation of a different image to each eye. Both tests required participants to identify figures that appeared to emerge as 3-dimensional images that is, they appeared to “pop-out” in depth from the test plate. The practice trial consisted of the presentation of a “fly” with the greatest disparity. Participants were asked if they could see the fly, if it stood out from the page, and if the fly appeared to move when they moved their head back and forth. All individuals successfully completed the practice trial. The two tests were then administered and consisted of 10 trials which tested amounts of disparity ranging from 400 to 20 s of arc, beginning with the greatest disparity and continuing until the participant was unable to accurately point to the correct figure. The Titmus Test was administered first followed by the Randot Test. Plates from the test books were front-illuminated and presented on an easel at 40 cm from the participant. Their heads were stabilized with a forehead rest. Binocular function may be affected by inter-pupillary distance (IPD). Therefore, IPD was measured when participants viewed a distant target in an optical pupilometer (Essilor Digital Corp). Stereoacuity thresholds were determined and the scores reflect the smallest disparity at which the participant was able to perceive depth. Stereoacuity thresholds were averaged over the two tests.
Vernier acuity
We measured the minimum detectable displacement between two dark, vertical, abutting lines presented on a bright screen seen through a circular aperture (7°) at a 1.8 m testing distance. The lines were presented with the lower line offset to the left or to the right. A forced-choice procedure was used to find a threshold. Participants were told that they had to judge whether the lower line was offset to the left or to the right. Individuals without intellectual disability were required to record their decision by pressing either the arrow key pointing to the LEFT or to RIGHT. Individuals with Down syndrome were required to indicate the direction either verbally or through pointing. The practice trial consisted of two lines that were grossly misaligned. All participants were able to complete the practice trial. Examination of the data of individuals without intellectual disability showed that for this test, thresholds improve with practice, reaching an asymptote after two or three retests on separate days. This was also the case for the data of individuals with Down syndrome. We tested all participants on these stimuli twice and used the values from the second test for analysis.
Colour vision
The quality of participants’ colour vision was assessed under controlled illumination with standard “panel” tests; the Farnsworth Dichotomous Test for Color blindness, Panel D-15 (referred to as the D-15; Farnsworth 1947), the Lanthony’s Desaturated 15 Hue Test (referred to as L-15; Lanthony 1978) and the Farnsworth-Munsell 100-Hue Test (F-M 100; Farnsworth 1943, 1957). These tests were designed to detect deviation in colour vision from normal trichromacy. The combination of the results from these tests allows recognition of several degrees of chromatic discrimination loss.
Each colour vision test was presented on an easel centered inside a 70 cm cube with matte white walls. Tests were viewed through a large window in front of the cube and a chin rest ensured that all tests were seen at a distance of 0.5 m. Over-head illumination of the tests was provided by tungsten-halogen sources together with mired-shift filters (Roscoe, cinegel). Illuminance was 350 lux at a colour temperature of 6800 K, as measured with a scanning spectrophotometer (Photo Research Model 704A) and a white reflectance standard (Photo Research) on the test easel. The D-15 and L-15 each consist of 15 coloured caps differing in hue, but of equal brightness and saturation and go around the hue colour circle with approximately equal sensory spacing. The F–M 100 consists of 85 caps that form a complete hue circle with incremental hue variation spanning the visible spectrum. The only difference between the D-15 and L-15 is that the latter is more desaturated having a “color circle” that is smaller and more sensitive to mild and moderate losses in chromatic discrimination ability. The F–M 100 is similar to the D-15 and L-15 except that the sensory spacing of adjacent caps is much finer.
All tests were performed monocularly since colour deficits may affect only one eye. An opaque eye-patch was placed over the unused eye. Participants’ responses were entered directly into a computer spreadsheet which randomly generated the order of eye sequences for each test. The materials consisted of a rack made of two hinged wood panels and plastic colour caps containing the pigments on their upper surfaces and the scoring number on their underside. A panel was placed in front of the participant with a fixed reference coloured cap on the left and the rest of the panel empty. The remaining caps were randomly arranged in front of the participant. Instructions for individuals without intellectual disability and those with Down syndrome were similar. They were told that the object of the test was to arrange the caps in order according to color. The experimenter pointed to the fixed reference cap and asked participants to choose a cap of those remaining that looked most similar to it and place it next to the fixed cap (the experimenter indicated where the cap should be placed). Participants were told to continue doing this until all the caps were arranged in order.
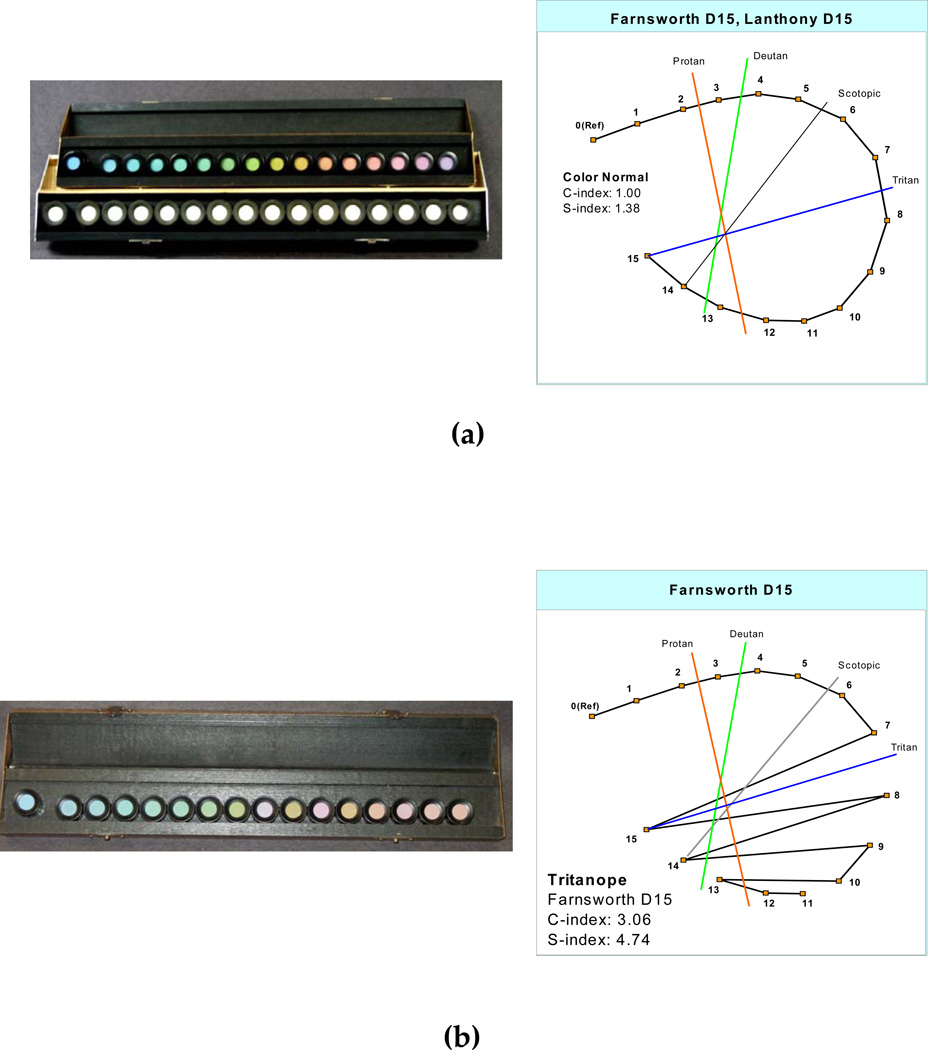
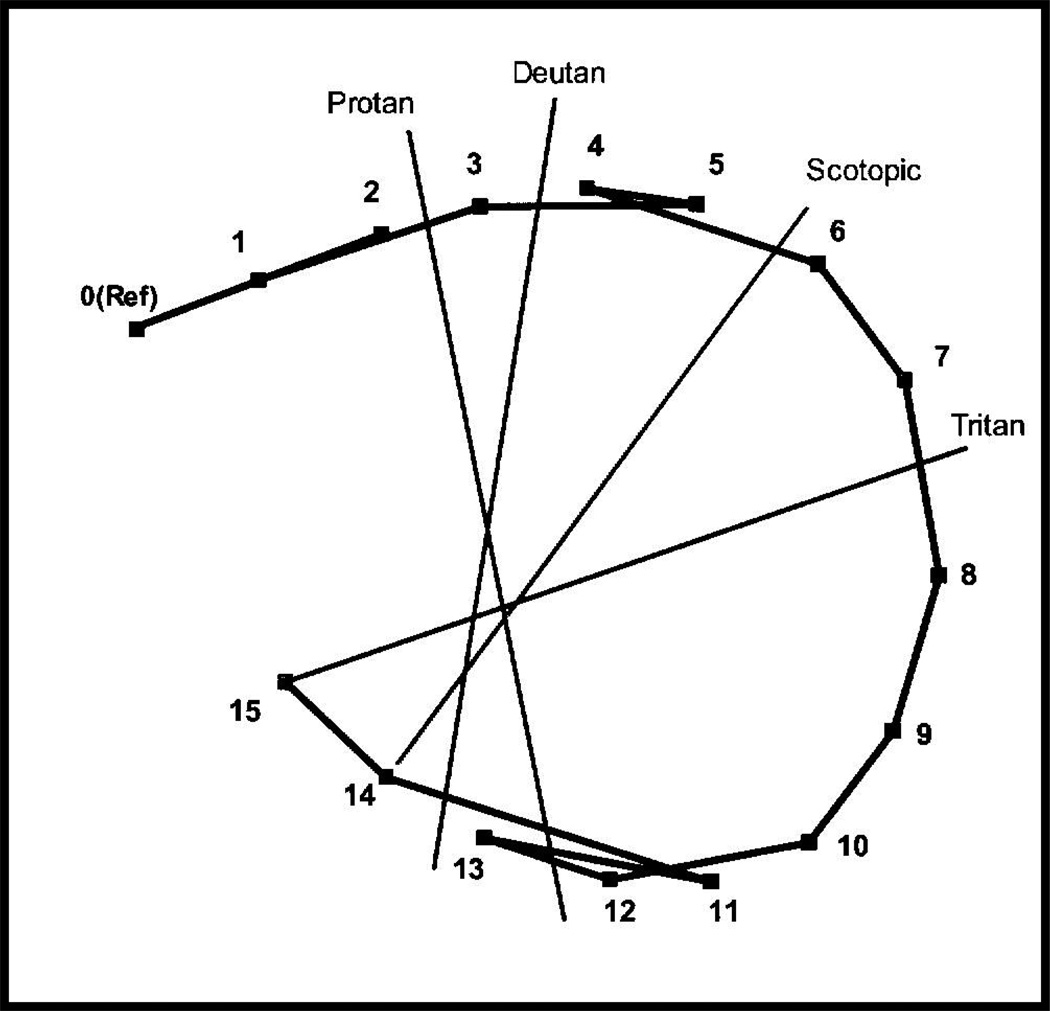
The position of the caps represents coordinates with particular hues and saturation on the CIE color space. One scoring method involves recording the numbers (from the underside of the cap) on the scoring sheet in the order in which they were arranged by the participant (Farnsworth, 1947). Color vision anomalies are diagnosed by specific arrangement errors from the correct arrangement of the cap sequences. These errors are lines that cross the center of the ellipse at a characteristic angle (a confusion axis) specific to a loss of certain retinal cones. The pattern of normal and color-weak participants will follow the circle, that is they will arrange the caps in perfect order or with only one or two transpositions of adjacent caps (Figure 1a). The patterns of color anomalous participants will in general form a series of parallel or crisscrossed lines, with at least two lines crossing the chart in approximately the same direction. The axes of the reversals across the colour circle are diagnostic of which types of retinal cones are involved (Farnsworth 1947). Figure 1b shows the pattern of tritan or blue blindness, the score lines parallel the index line marked “tritan”.
Figure 1.
Examples of possible results from the Farnsworth Dichotomous Test for Color blindness, Panel D-15 and the Lanthony’s Desaturated 15 Hue Test (a) perfect arrangement or normal colour vision and (b) arrangement showing defective colour vision lying along a tritanopic direction. Normal values for the C-index (confusion axis) and S-index (polarity of an individual’s pattern of cap errors) are included.
The results of a participant’s arrangement of the caps can also be quantified in various ways (see Vingrys & King-Smith 1988 for details). For the D-15 and L-15, we report a confusion/severity measure (C-index) which quantified the magnitude of colour loss relative to a perfect arrangement of caps. For the F–M 100-Hue test we report an overall error score (Benzschawel 1985; Smith et al. 1985; Verriest et al. 1982).
Data Analysis
We performed a qualitative analysis of the group CSF data with Exploratory Data Analysis (EDA; Tukey 1977). While EDA provides a variety of tools for summarizing and gaining insight about a data set, proponents of this approach recommend the use of graphical displays to reveal important characteristics of the data that might otherwise go undetected with inferential statistical analyses. For example, examination of the shape of the curves may capture conceptually meaningful patterns and relationships in the data and call attention to observations that deviate greatly from those fundamental patterns (Mosteller & Tukey 1977).
We conducted an analysis of variance on participants’ vernier acuity threshold scores, stereoacuity threshold scores, C-index scores for the D-15 and L-15, and the error score for the F–M 100 using the SYSTAT 12 statistical package. Group (adults with Down syndrome, younger adults without intellectual disability, and older adults without intellectual disability) was the between-subjects factor.
Results
All individuals both with Down syndrome and without intellectual disability were able to complete all the tests in the battery.
Spatio-Temporal Contrast Sensitivity Functions
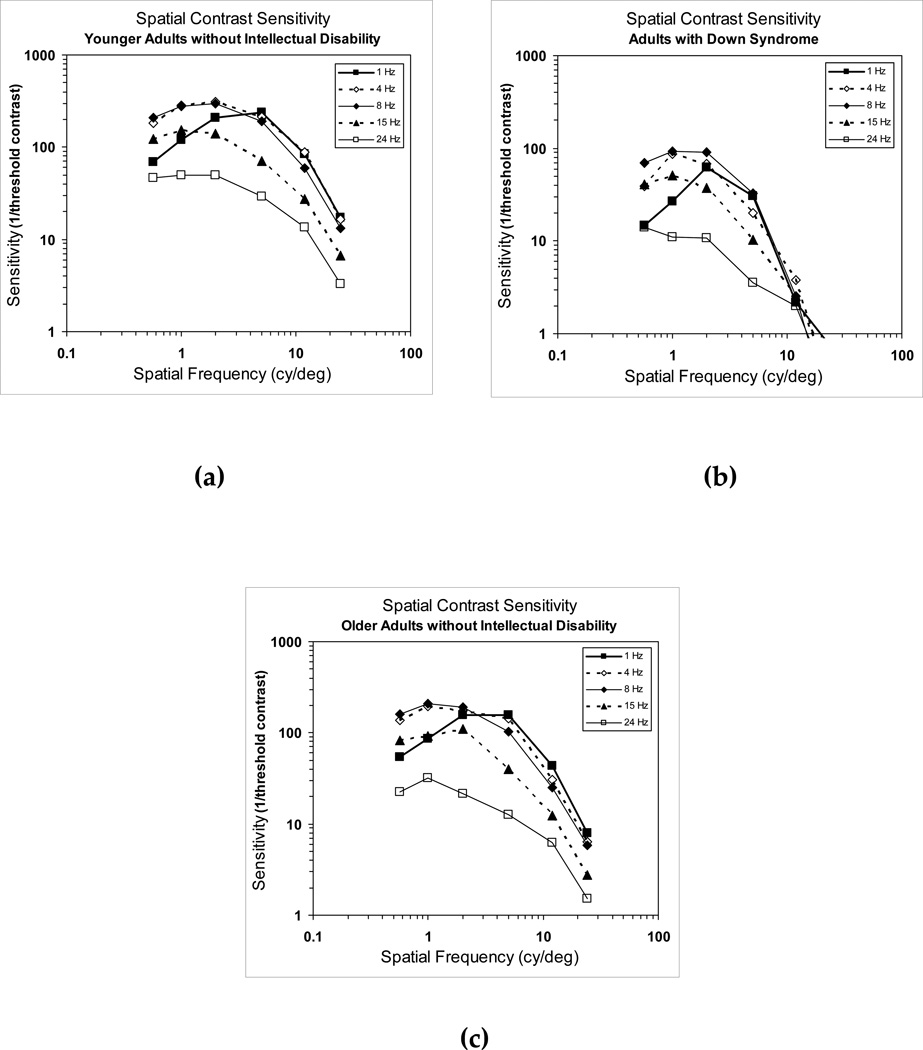
In Figure 2a, we show mean spatial CSFs from younger adults without intellectual disability. The curves show the typical function characteristic of the sensitivity of the visual system; an inverted-U shaped curve with a peak at intermediate spatial frequencies which decreases progressively with increasing or decreasing spatial frequency (Campbell & Robson, 1968). We also observed that with moderate increases in temporal modulation rate, sensitivity to higher spatial frequencies was reduced and sensitivity to low spatial frequencies was enhanced.
Figure 2.
Mean binocular spatial contrast sensitivity functions for, (a) younger adults without intellectual disability, (b) adults with Down syndrome, and (c) older adults without intellectual disability.
In Figure 2b, we show the curves from adults with Down syndrome. The general shape of their curves was similar to those of younger adults without intellectual disability. However, in spite of the overall normality of the curves, individuals with Down syndrome showed lower contrast sensitivity thresholds across the entire spatial frequency range and at all temporal modulation rates. This was especially marked at high spatial frequencies.
Because signs of aging often occur precociously in adults with Down syndrome (e.g., Zigman & Lott 2007) it may be more appropriate to compare their CSFs to older individuals without intellectual disability. In Figure 2c, we present the data from older adults without intellectual disability. In comparison to this older group, participants with Down syndrome still showed sensitivities that were reduced at all spatial frequencies and temporal modulation rates, although the deficits were not as pronounced as seen in comparison to younger individuals without intellectual disability.
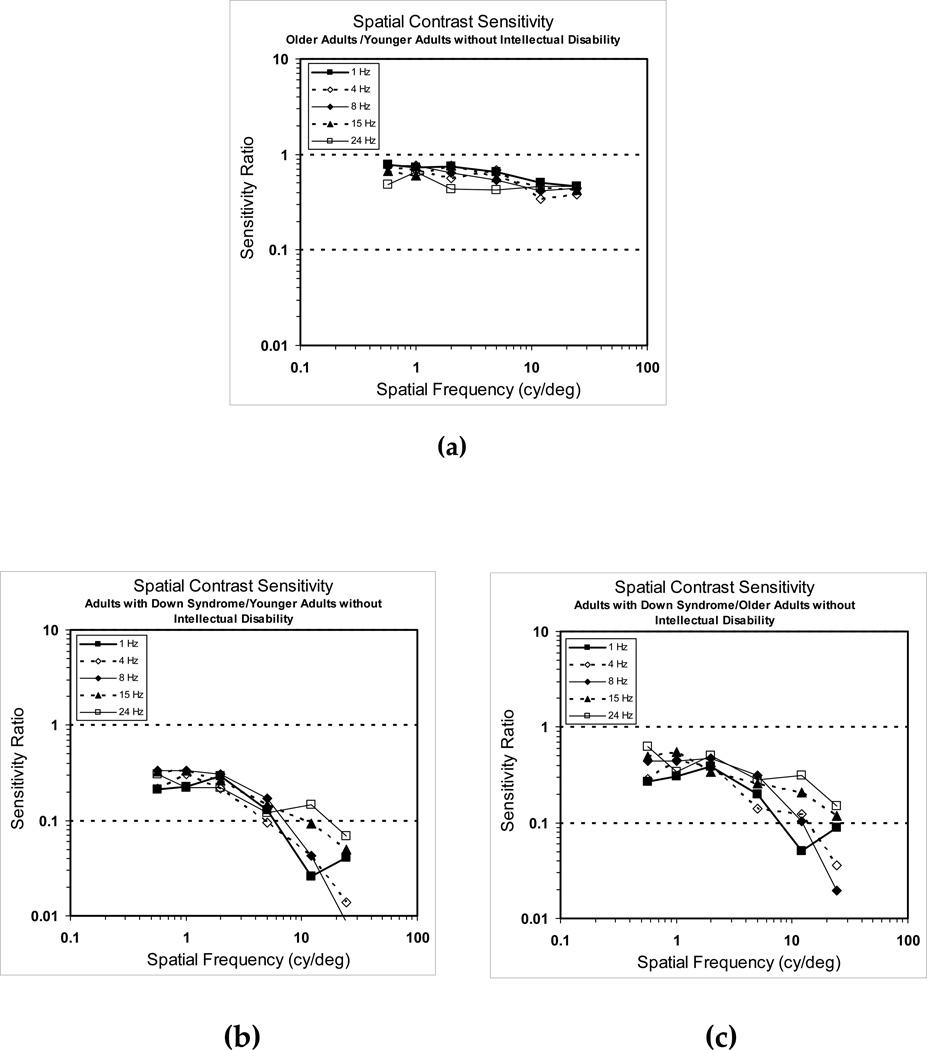
To assess the effects of the aging process per se on CSFs, we computed the ratios of the sensitivities of the older group to the younger group of participants. The graph of these ratios for adults without intellectual disability is shown in Figure 3a. While there was no major distortion in the shape of the CSFs across temporal rates, there was the expected small age-associated loss in overall sensitivity at all spatio-temporal frequency combinations that is typically seen in functions of older adults.
Figure 3.
Group comparisons of binocular spatial contrast sensitivity functions, (a) the ratio of older adults without intellectual disability/younger adults without intellectual disability, (b) the ratio of adults with Down syndrome/younger adults without intellectual disability, and (c) the ratio of adults with Down syndrome/older adults without intellectual disability.
Figures 3b and 3c show the same ratios for adults with Down syndrome compared with younger (Figure 3b) and older (Figure 3c) adults without intellectual disability. For spatial frequencies below 5 cy/deg, the curve values do not deviate from one another and they are relatively flat, implying that the shape of the spatial channels subserving these frequencies appear comparable in individuals with Down syndrome and those without intellectual disability. At the higher spatial frequencies, the reduction in sensitivity is more apparent. However, the magnitude was not the same at all temporal rates as the higher temporal rates (15 and 24 Hz) were somewhat less affected.
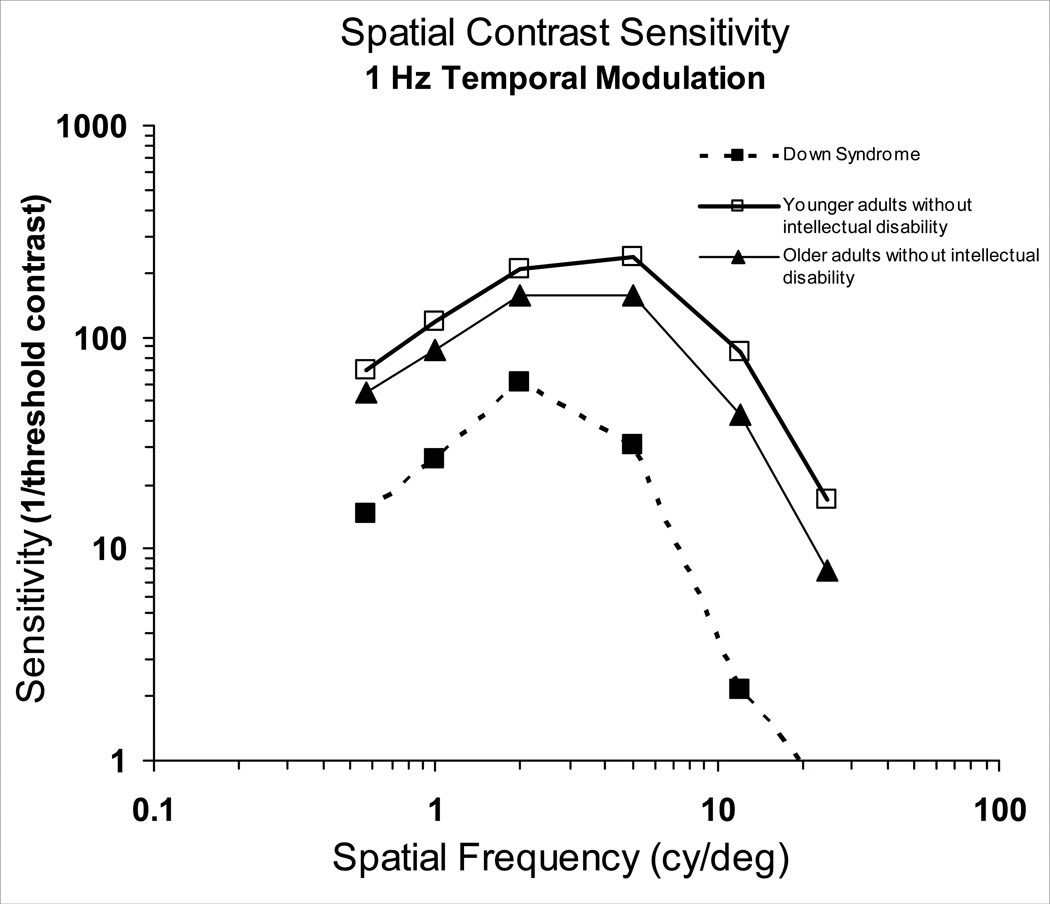
In an attempt to further interpret the deficits seen in the functions of adults with Down syndrome, we compared the CSFs of the different groups, for simplicity at a 1 Hz temporal modulation rate (Figure 4). This figure unambiguously shows the reduced sensitivity across spatial frequencies in the CSF of adults with Down syndrome compared to adults without intellectual disability. (The medical charts of both individuals with cataracts stated that the condition was “mild.” In addition, the exclusion of their data did not appreciably affect the mean results. It is important to note that all individuals with Down syndrome showed a deficit at high spatial frequencies, whether or not they had cataracts.)
Figure 4.
Mean binocular spatial contrast sensitivity functions at 1Hz temporal modulation rate for adults with Down syndrome, younger adults without intellectual disability, and older adults without intellectual disability.
Hyperacuities
Stereopsis
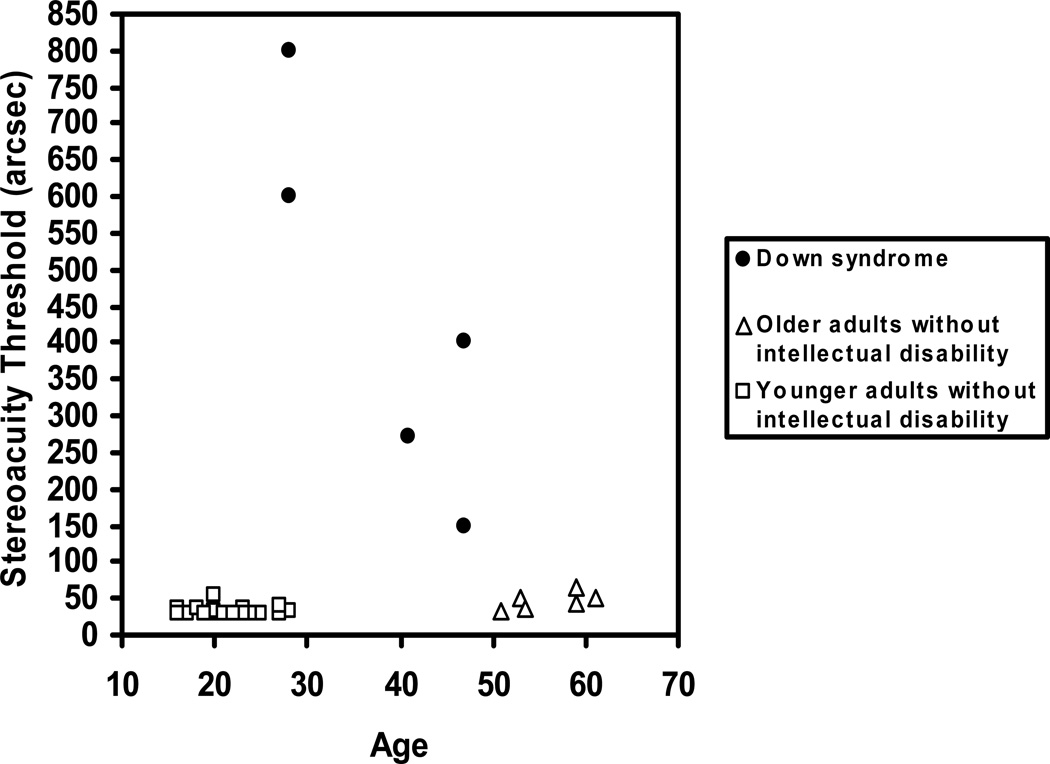
Stereoacuity threshold (in arcsec) for each participant is shown in Figure 5. As expected, both younger and older individuals without intellectual disability had thresholds that were less than 60 arcsec. Of the participants with Down syndrome, two of the older individuals had no demonstrable stereopsis at all. This result was unlikely to be due to a misunderstanding of the task, as none of the participants had difficulty identifying alternatives that seemed to “pop out” for the stimuli with the greatest disparity (the practice trial). The results are for the remaining five participants. These individuals showed elevated stereoacuity thresholds compared to participants without intellectual disability. This finding was confirmed with an ANOVA in which the stereoacuity threshold was the dependent variable and Group was the between-subjects factor, F(2,31)=40.77, p< .001. Post-Hoc analyses showed that individuals with Down syndrome had significantly elevated thresholds compared to younger and older adults without intellectual disability, F(1,31)=79.52, p< .001, Cohen’s d = 2.24 and F(1,31)=49.34, p< .001, Cohen’s d = 2.16, respectively. Therefore, in order to perceive depth, participants with Down syndrome required images which produced greater retinal disparity than adults without intellectual disability.
Figure 5.
Stereoacuity thresholds for adults with Down syndrome, younger adults without intellectual disability, and older adults without intellectual disability.
Stereoacuity thresholds can be influenced by inter-pupillary distance (IPD) since absolute binocular disparity increases with IPD. We found the mean IPD for older individuals without intellectual disability was 62.6 mm (SD = 4.94) while for the five participants with Down syndrome who completed this task, the mean IPD was 56.4 mm (SD = 3.68), F(1,33) = 8.05, p=.008. We computed stereoacuity threshold relative to individual IPD. The pattern of results was identical to the preceding analysis.
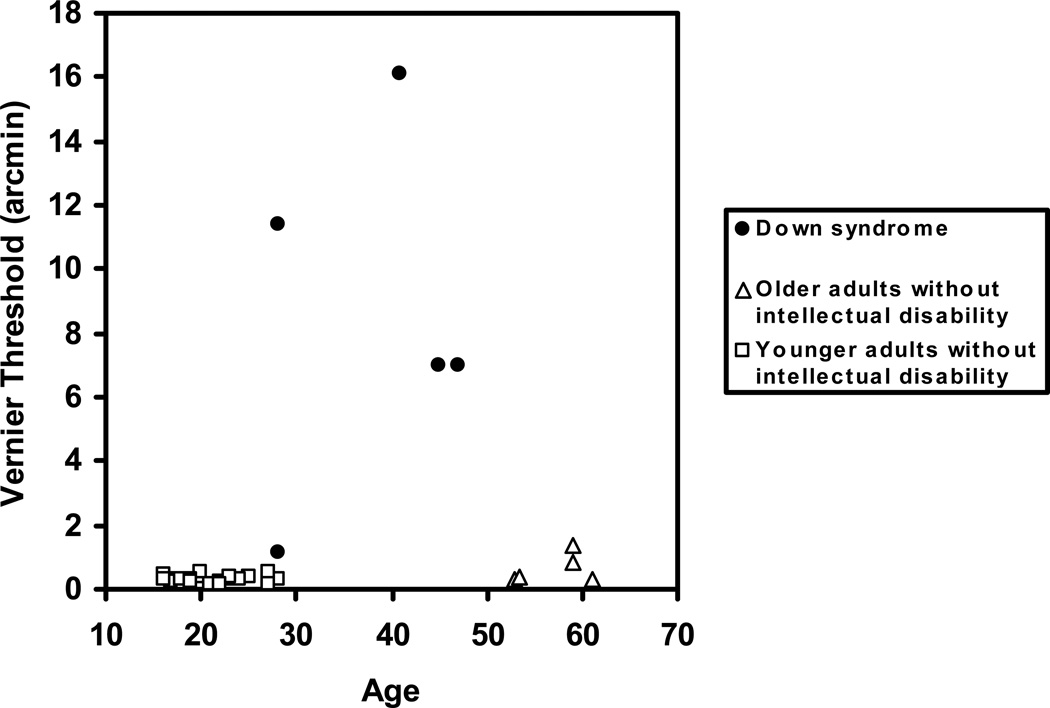
Vernier Acuity
Mean vernier acuity threshold (in arcmin) for each participant group is shown in Figure 6. One older adult without intellectual disability was not tested on vernier acuity due to their time constraints. As expected, the mean threshold for the younger and older participants without intellectual disability was in the hyperacuity range (less than 1 arcmin). We were only able to obtain vernier acuity thresholds for five of the participants with Down syndrome (the same participants for whom we were able to determine stereoacuity thresholds). As a group, individuals with Down syndrome showed greatly elevated thresholds, with the exception of one young female who had a threshold of just over 1 arcmin. An ANOVA with vernier acuity threshold as the dependent variable and Group as the between-subjects factor was significant, F(2,30)=33.55, p< .001. A post-hoc analysis showed that individuals with Down syndrome had significantly higher thresholds compared to both younger, F(1,30)=65.75, p< .001, Cohen’s d= 2.07 and older adults without intellectual disability, F(1,30)=37.08, p< .001, Cohen’s d=1.99.
Figure 6.
Vernier acuity thresholds for adults with Down syndrome, younger adults without intellectual disability, and older adults without intellectual disability.
To test if the high thresholds of the participants with Down syndrome were due to their poor acuity, we estimated their limiting grating acuities by extrapolating their CSFs (at 1 Hz) to the spatial frequency corresponding to 100% contrast. Vernier acuity thresholds were then expressed relative to the grating acuities. The pattern of results in this analysis was identical to the preceding analyses.
Colour Vision
Adults with Down syndrome did not seem to have trouble understanding the requirements of the colour vision panel tests and were able to choose the coloured cap that most closely matched the appearance of the immediately preceding cap. They appeared to pay very careful attention to their choices and often returned to a sequence to make some small adjustment. There were no clear differences found between the eyes for the participants and values obtained for each eye were averaged to give a single score for each participant, for each panel.
Figure 7 presents the cap arrangement for the D-15 completed by one individual with Down syndrome and shows the minors errors the participant made. In general, the errors shown by participants with Down syndrome did not reveal a consistent pattern which would indicate the types of retinal cones that were involved.
Figure 7.
Example of a cap arrangement for the Farnsworth Dichotomous Test for Color Blindness Panel D-15 from an individual with Down syndrome.
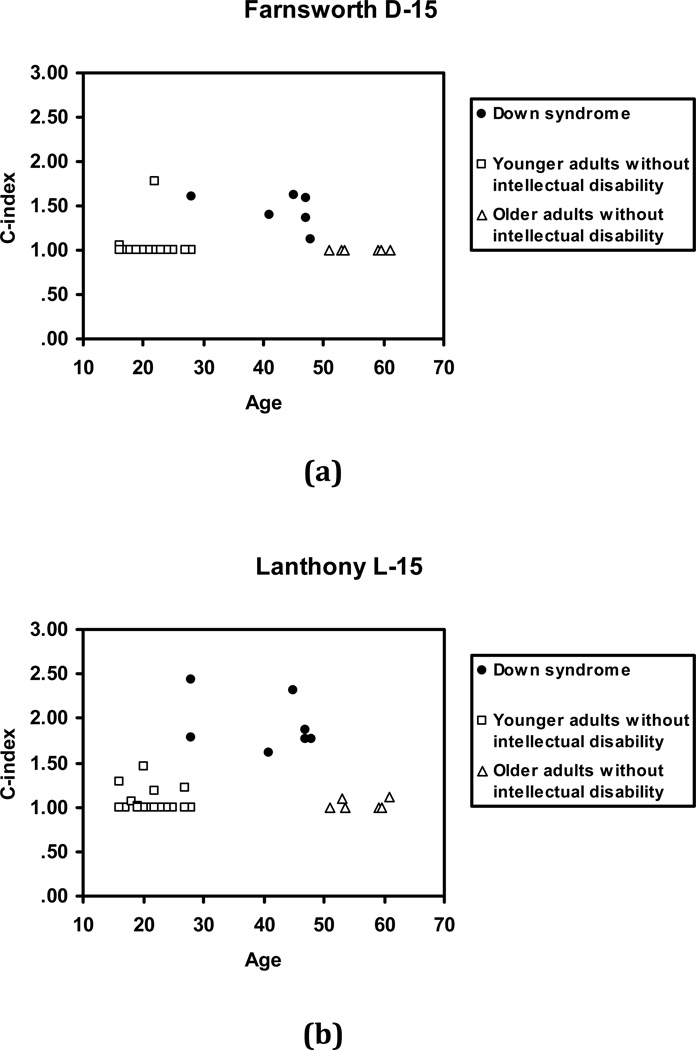
The individual C-index values for each participant for the D-15 and for the L-15 are shown in Figure 8a and 8b, respectively. (For both tests, a C-index value of 1.0, represents a perfect score with no sequence reversals, higher scores indicate greater colour vision defect). Older individuals without intellectual disability had near perfect scores on both the D-15 and L-15 and therefore no appreciable age-associated deficits in colour vision were observed for the limited age range tested in this study. More importantly, for participants with Down syndrome the C-index value on both tests was higher compared to participants without intellectual disability. An ANOVA with the C-index value as the dependent variable and Group as the between-subjects factor was significant for each test; D-15: F(2,33)=12.84, p< .001 and L-15: F(2,32)=78.12, p< .001 (one younger participant without intellectual disability was not tested on the L-15). Post-Hoc analyses showed that individuals with Down syndrome had significantly higher scores compared to both younger, D-15: F(1,33)=23.22, p< .001, Cohen’s d= 1.69 and L-15: F(1,33)=146.66, p< .001, Cohen’s d= 3.72, and older individuals without intellectual disability, D-15: F(1,33)=17.02, p< .001, Cohen’s d= 2.23 and L-15: F(1,34)= 93.35, p< .001, Cohen’s d= 4.01.
Figure 8.
C-index scores on the panel tests of colour vision (a) Farnsworth Dichotomous Test for Color Blindness Panel D-15, and (b) Lanthony Desaturated 15 Hue Test comparing adults with Down syndrome, younger adults without intellectual disability, and older adults without intellectual disability.
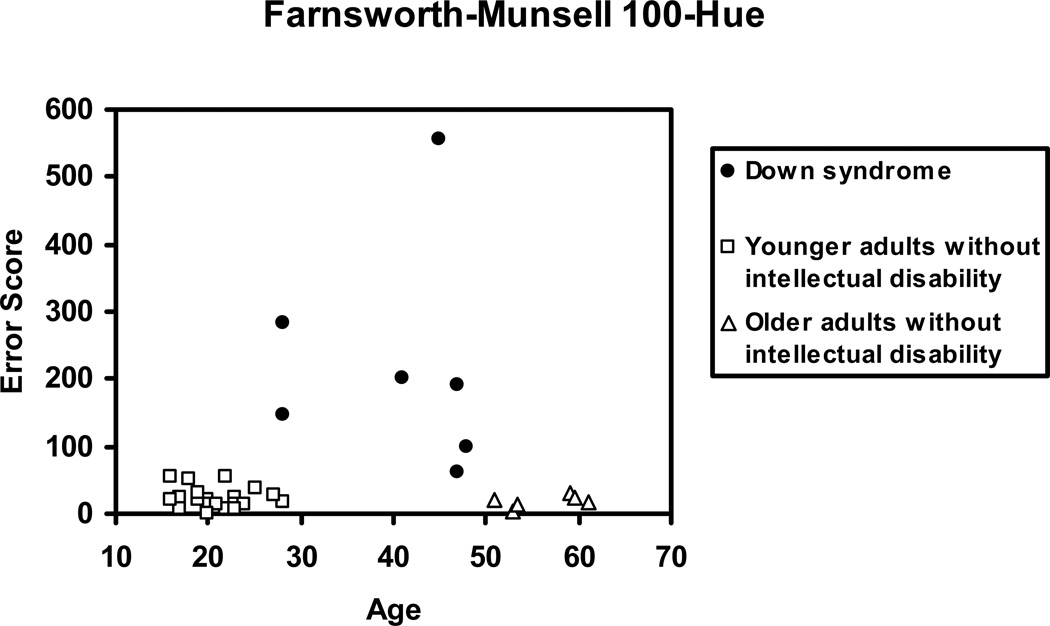
Figure 9 shows the individual error scores for the F–M 100-Hue Test for each participant. Adults with Down syndrome had higher error scores in comparison to adults without intellectual disability, F(2,33)=21.58, p< .001. Post-Hoc analyses showed that individuals with Down syndrome had significantly higher error scores compared to both younger, F(1,33)=40.72, p< .001, Cohen’s d= 1.68, and older individuals without intellectual disability, F(1,33)=25.53, p< .001, Cohen’s d= 1.72. Although adults with Down syndrome had significantly higher error/confusion scores than both younger and older adults without intellectual disability, the overall range of scores are well within what we would expect from an individual who had minor defects with colour discrimination rather than reflecting difficulty with the task per se (see Abramov & Gordon 2009).
Figure 9.
Error scores from the Farnsworth-Munsell 100 Hue panel test comparing adults with Down syndrome, older adults without intellectual disability, and younger adults without intellectual disability.
The data presented thus far were based on comparisons of group means. For adults with Down syndrome we also explored relationships among the various psychophysical tests, for each participant. We found no obvious relationships between values on one test and values on any other test. For example, the oldest participant with Down syndrome had spatio-temporal thresholds indicating relatively high ability to make discriminations at all low and middle spatial frequencies and at all temporal modulation rates compared to the group mean, but had no demonstrable stereopsis and no reliable threshold could be obtained for vernier acuity. However, that same participant also exhibited the best colour vision of the group. In contrast, the youngest participant had spatio-temporal thresholds indicating poorer discrimination at all low and middle spatial frequencies and at all temporal modulation rates compared to the group mean but had acceptable colour vision and a vernier acuity threshold comparable to adults without intellectual disability.
Discussion
Vision is a system of parallel neuronal pathways extending from the retina through to the cortex. In this study, we examined several visual functions with a psychophysical battery using state-of-the-art precision and rapid algorithms that avoided confounds with changes in non-sensory factors. In order to contribute to a better understanding of vision in adults with Down syndrome, we looked at the system as a whole and attempted to relate our findings to the best available models of underlying neuronal mechanisms.
Even with the small number of participants tested, we demonstrated that adults with Down syndrome have significant visual deficits including an overall reduction of sensitivity across spatial frequencies and temporal modulation rates, either absent or reduced stereopsis, impairment in vernier acuity, and colour vision anomalies. Their vision was found to be considerably worse than the vision capabilities of older adults without intellectual disability. As with all others aspects of the phenotype associated with Down syndrome, deficits in visual functions showed a high degree of variability in terms of both occurrence and degree of severity. The deficits observed in the vision of adults with Down syndrome warrant the conclusion that the deficits are vision-specific rather than a reflection of a general deficit in performance which is typically expected in any comparison between individuals with intellectual disabilities and their non-disabled peers.
Our test battery examined spatial and temporal vision and covered the entire functional range of the spatio-temporal surface. The general shape of the CSF curve for adults with Down syndrome was identical to that of individuals without intellectual disability that is, an inverted U-shaped curve with a peak at intermediate spatial frequencies which decreases progressively with increasing or decreasing spatial frequency. However, individuals with Down syndrome showed decreased sensitivity at all spatio-temporal frequency combinations in comparison to older adults without intellectual disability, though these were not as substantial as seen in comparison to younger adults without intellectual disability. Because of the gross comparability in the shapes of the curves between the individuals with Down syndrome and the other groups, it is reasonably clear that the deficits in functioning that were observed were not due to their inability to understand the task but, rather to real deficits in spatial and temporal vision. While a small loss in overall sensitivity was also observed in the CSFs of older participants compared to younger participants without intellectual disability, the deficits observed in the CSFs of adults with Down syndrome were considerably greater than expected due to aging alone. The curves were not comparable at all frequencies and therefore this explanation is insufficient in accounting for the deficits observed in adults with Down syndrome. The channels that determine spatio-temporal frequency sensitivity almost certainly reflect properties of neurons in the primary visual cortex (e.g., DeValois et al. 1982; DeValois & DeValois 1988). The pattern of deficits seen in the spatial and temporal vision in participants with Down syndrome then, may be based primarily on differences in cortical functioning.
What are the practical consequences of these deficits in the CSFs for individuals with Down syndrome? The effect of acuity losses is obvious; fine details and discrimination will not be detected unless the objects are very close. However, the more important consequence may be at the lower spatial frequencies. Lowered sensitivity in these regions of the spatio-temporal CSF can greatly compromise the ability to recognize objects and also faces, especially when they are at distances such that their retinal images are relatively small (Ginsburg et al. 1982; Hainline & Abramov 1992)
The image on the retina is a two-dimensional representation of a three-dimensional world. One of vision’s major roles is to extract information about depth, the third dimension. Because the retinal image for each eye has a slightly different view of the world, the brain, then, must coordinate these views into a fused 3-dimensional percept of a single world. For most people, the fused images also provide depth information, or stereopsis. In individuals with Down syndrome, stereopsis was found to be very poor. A deficit in stereopsis is not due to retinal damage to receptors alone, but likely reflects cortical involvement. It is generally agreed that the M-neurons are the major pathway for this visual function. M-neurons are retinal ganglion cells that synapse in the magnocellular layer of the lateral geniculate nucleus (LGN). These cells are thought to be a major input for cortical cells that are sensitive to motion and depth. However, it must be emphasized that once responses reach the cortex of the brain, the distinction between M- and P-neurons (retinal ganglion cells that synapse in the parvocellular area of the LGN and are sensitive to very fine detail and differences in colour, and insensitive to low contrasts) becomes increasingly blurred—there is considerable overlap and interaction.
Vernier acuity in essence examines relative spatial positions of stimuli, a function that most probably involves the “where” or dorsal visual pathway and the parietal lobe. Vernier acuity was also found to be poor in adults with Down syndrome. This was true even when vernier acuity thresholds were expressed relative to the grating acuity. Thus, the poor performance of the adults with Down syndrome was not simply due to worse acuity but may reflect additional deficits in the processing of more extended visual arrays. However, difficulty with the overall demands of the task cannot be ruled out, at least for the two participants for whom vernier acuity thresholds could not be obtained and who showed no demonstrable stereopsis. The fact that they both were able to do the practice trials argues against this explanation, but it cannot be ruled out.
Overall, individuals with Down syndrome showed a diminution of the ability to discriminate colour. There were however, no clear axes of confusion. Rather, discrimination was simply poor everywhere. Colour vision is initially determined by the existence and properties of three types of cones, designated as one of L, M, or S. However, the final result-sensation of hue- is determined by processing of cone responses at several levels in the nervous system, ending high in the cortex (Gordon & Abramov 2001). The sequence of reversals in colour space observed in the data of individuals with Down syndrome were along several directions although, subjectively they might be described as lying along a tritanopic direction, the spectral region of S-cones affecting blue-yellow hue discrimination. Of the three types of retinal cone receptors, S-cones are by far the sparsest in humans (Curcio et al. 1991). It is not surprising to see tritan-like errors given the fact that functions that depend on smaller populations of neurons (such as S-cones) will be more severely affected when aging-related loss occurs. However, the specific deficits that we found in the colour vision of participants with Down syndrome suggest involvement of several areas: some loss of S-cones at the retina, as well as later processing areas, leading to confusion of hues along several axes. The overall poorness in their discrimination of colour, as indicated by performance on the F–M 100, also points to a cortical locus for some of the deficit. It is also possible that the F–M 100 was too difficult for individuals with intellectual disability. Although placement of colour disks was not entirely random, confusions in the concept of colour relatedness may have influenced performance on this more difficult panel test.
Reports that CSFs, stereopsis, vernier acuity, and colour discrimination are all compromised in adulthood suggest that a common mechanism may be responsible (Haegerstrom-Portnoy et al. 1999; Wood & Bullimore 1995). Furthermore, the visual deficits we found in adults with Down syndrome have been similarly found by other researchers in individuals with Alzheimer’s disease without intellectual disability (e.g., Rocco et al 1997). The visual abnormalities associated with Alzheimer’s disease have been attributed to the neuropathology in the visual association cortex (Mendez et al. 1990), rather than changes in the retina, optic nerve, or retino-calcarine pathways (Cronin-Golomb et al. 2000; Rizzo et al. 1992). Other studies have suggested that lesions of cortical regions 17, 18, and 20 may cause the visual dysfunction observed (Cronin-Golomb et al. 1991). Yet it has been found that the number of neurofibrillary tangles and plaques (the neuropathological hallmarks of Alzheimer’s disease) is relatively low in the primary visual cortex but increases 20- to 40-fold in the adjacent visual association cortices (Braak et al. 1989; Lewis et al. 1987; see also Holroyd & Shepherd 2001 for a review). Since adults with Down syndrome are at risk for developing Alzheimer’s disease, perhaps the specific visual deficits we observed in this study may serve as potential “early markers” for the onset of cognitive and behavioral deficits (Kim & Detterman 1996; Rocco et al. 1997). If so, then, the vision changes observed in adults with Down syndrome might be early evidence of Alzheimer disease neuropathology (Kim & Detterman 1996). Unequivocally delineating the mechanism(s) responsible for the observed deficits is difficult since deficits in one function will necessarily affect other functions (Weale 1982) however, all the evidence points to differences and or changes in cortical functioning.
The results of our study should be considered preliminary however, due to the small number of participants. Future studies should include a larger number of participants and evaluate the progression of vision changes over a significant portion of the lifespan in order to determine whether the visual deficits observed in this study are life-long impairments or reflect age-associated declines. That individuals with intellectual disability were able to understand and complete the battery of vision tests used in this study has wider implications in terms of their ability to participate in other studies that use state-of-the-art psychophysical tests and procedures.
Deficits in vision may exacerbate age-associated functional and cognitive declines, since if the processing of information is not properly encoded or categorized initially, “higher” functions will be detrimentally affected thereafter (Rizzo et al. 2000). In fact, Rizzo et al. suggested that visual decline might even contribute to the cognitive deterioration associated with Alzheimer’s disease. It is possible that a better understanding of vision-related dysfunction could aid in the diagnostic interpretation of neuropsychological and cognitive test performance. Also, it is reasonable to assume that the quality of life for older individuals with Down syndrome would be enhanced and functional capacity improved by proper assessment of visual functioning and correction where possible or in appropriately modifying the visual environment to provide more salient visual cues (Kuyk et al. 1998; Lord & Menz 2000).
Acknowledgments
Source of Funding: This work was supported by funds from the City University of New York Collaborative Incentive Grants Program # 919230001 to Israel Abramov, the New York State Office of Mental Retardation and Developmental Disabilities, and NIH grants P01 HD35897, P30 HD024061 and R01 AG14771.
We are grateful to all our participants, their families, and the Guild for Exceptional Children in Brooklyn, NY for their continued support of our research program. We would like to thank Maria Pagano and Theresa Tannazzo for their assistance in data collection. We also thank Gordon Harris OD for conducting all the optometric examinations of participants including, assessment of binocular vision and refractive status.
Appendix
Definitions of Terms
Accommodation: the process of focusing the eye on objects at varying distances in order to see clearly.
Amblyopia: poor vision that is not associated with any specific pathology.
Astigmatism: blurred vision usually caused by the irregular shape of the cornea, which is less curved in one meridian. Occasionally the problem is due to irregular curvature of the lens.
Cataracts: a clouding of the lens of the eye.
Colour vision: the ability to detect changes in the wavelength of light independent of luminance. It is determined by the amounts of light absorbed by each of the three types of cone photoreceptors designated as one of L-, M-, or S-cones (Gordon & Abramov 2001 for a review).
Contrast sensitivity: sensitivity to the difference in the light intensities in two adjacent areas of a pattern in the visual field. It is measured by taking the inverse of the contrast (see Michelson Contrast) required for detection of a pattern.
Contrast sensitivity function: a curve relating contrast sensitivity to spatial frequency of a stimulus (number of repetitions per unit of space of a regularly alternating pattern). It is typically an inverted-U shaped curve with a peak at intermediate spatial frequencies, which decreases progressively with increasing or decreasing spatial frequency. Interpretation of the CSF has proven to be a valuable index of spatial vision and the detection of visual dysfunction. It can be used to predict an individual’s ability to detect and discriminate targets under a wide variety of stimulus conditions that simulate the real world.
Counterphase: a stationary stimulus that is temporally modulated. In a sinusoidal counter phase flicker, the contrast of the pattern is gradually increased then decreased in amplitude, then increased and decreased in amplitude in the opposite phase. Therefore, at any point along the pattern, the light intensity varies sinusoidally in time but the mean luminance across the entire display remains constant.
Dorsal visual pathway: a cortical pathway proposed to be involved in the guidance of actions (e.g. reaching and picking up objects) and the recognition of where objects are in space. It is also known as the “where” or “how” pathway. The dorsal stream begins in the retina with ganglion cells with large cell bodies called M-cells. The axons of these M-cells synapse in layers 1 and 2 of the lateral geniculate nucleus, which together are called the magnocellular layers, and project to the primary visual cortex in the occipital lobe of the cerebral cortex and from there they eventually pass to the parietal cortex.
Hue: the main property of a colour described by terms such as red, yellow etc.
Lateral geniculate nucleus (LGN): the sensory relay nucleus in the thalamus of the brain that receives inputs from the optic nerve and in turn sends axons to the primary visual area of the cortex (visual area 1) in the occipital lobe of the cerebral cortex.
L-cones: one of the three types of cones in the retina responsible for the perception of colour. It is more sensitive than any of the other cones to the longer wavelengths although, like all cones, it is to some degree sensitive to all wavelengths of visible light.
M-cones: one of three types of cones in the retina responsible for the perception of colour. It is more sensitive than any of the others to the middle wavelengths although like all cones, it is to some degree sensitive to all wavelengths of visible light.
Magnocellular pathway: refers to layers 1 and 2 of the LGN.
M-neurons: Retinal ganglion cells that have large-sized cell bodies. These cells synapse in the magnocellular layer of the LGN. These cells are sensitive to low contrast and are probably a major input for cortical cells that are sensitive to motion and depth.
Michelson contrast: used for patterns where both bright and dark features are equal increments and decrements from the space-average of the pattern. Michelson contrast is defined as:
Nystagmus: refers to the rhythmic, repetitive, involuntary eye movements that occur when a large portion of the visual field moves constantly in a horizontal direction. The movements consist of a slow phase in which the moving field is tracked, followed by a rapid “return” movement (saccade). This pattern is repeated until the field stops moving. In pathological cases, nystagmus can occur in the absence of a moving stimulus.
Parvocellular pathway: refers to layers 3, 4, 5, and 6 of the lateral geniculate nucleus. These cells are sensitive to very fine detail and differences in color and insensitive to low contrasts.
P-neurons: Retinal ganglion cells that have small cell bodies (smaller than M-cells). P-cells synapse in the parvocellular area of the LGN. These cells are sensitive to very fine detail and differences in colour, and insensitive to low contrasts. All the cells receive inputs from single ganglion cells from only one eye.
Presbyopia: is the progressively diminishing ability to focus on nearby objects resulting from the loss of elasticity of the crystalline lens that occurs with advancing age.
Refractive errors: errors in the focusing of light.
Retino-calcarine Pathway: major visual pathway going from the retina via the optic nerve, optic chiasm, and the LGN to the primary visual cortex.
S-cones: one of the three types of cones in the retina responsible for the perception of colour. It is more sensitive than any of the others to the shorter wavelengths although, like all cones it is to some degree sensitive to all wavelengths of visible light.
Spatial vision: refers to the visual system’s ability to perceive changes in brightness across space.
Stereopsis: refers to the binocular ability to extract, from an extended visual array, the fine information about depth of targets, independent of cues provided by accommodation and convergence. It is based on the brain’s comparison of the slightly different retinal images of the world obtained by the two eyes.
Strabismus: a condition in which the eyes are not properly aligned with each other. When looking at an object, the images do not fall on corresponding retinal locations.
Temporal vision: refers to the visual system’s ability to perceive changes in brightness over time. An example of a temporal vision task is detecting the flicker of a flashlight. Temporal vision is closely related to the ability to perceive motion.
Trichromacy: refers to the condition of possessing three types of colour receptors (called cones) for conveying colour vision. A person with normal colour vision is called a trichromat.
Tritanopia or Tritan-like deficits: a form of colour blindness caused by a lack of the S-cones. It causes difficulty discriminating blue and yellow hues.
Vernier Acuity: one form of visual acuity, expressed in terms of the minimum visual angle of displacement that can be detected between two bars or lines that are almost, but not quite aligned. In essence, this test examines detection of relative spatial positions of stimuli, a function that most probably involves the “where” (or dorsal) pathway and the parietal lobe.
Footnotes
Conflict of Interest: None.
References
- Abramov I, Gordon J. Colour vision panel tests: a metric for interpreting numeric analytic indices. Optometry and Vision Science. 2009;86:146–152. doi: 10.1097/OPX.0b013e318194e934. [DOI] [PubMed] [Google Scholar]
- Averbuch-Heller L, Dell'Osso LF, Jacobs JB, Remler BF. Latent and congenital nystagmus in Down syndrome. Journal of Neuro-Ophthalmology. 1999;19:166–172. [PubMed] [Google Scholar]
- Baird PA, Sadovnick AD. Life expectancy in Down syndrome. Journal of Pediatrics. 1987;110:849–854. doi: 10.1016/s0022-3476(87)80395-5. [DOI] [PubMed] [Google Scholar]
- Benzschawel T. Computerized analysis of the Farnsworth-Munsell 100-Hue test. American Journal of Optics and Physiological Optics. 1985;62:254–264. doi: 10.1097/00006324-198504000-00004. [DOI] [PubMed] [Google Scholar]
- Berk AT, Saatci AO, Erçal MD, Tunç M, Ergin M. Ocular findings in 55 patients with Down's syndrome. Ophthalmic Genetics. 1996;17:15–19. doi: 10.3109/13816819609057864. [DOI] [PubMed] [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. British Journal of Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J, Lang W. Alzheimer's disease: amyloid plaques in the cerebellum. Journal of Neurological Sciences. 1989;93:277–287. doi: 10.1016/0022-510x(89)90197-4. [DOI] [PubMed] [Google Scholar]
- Campbell FW, Robson JG. Application of Fourier analysis to the visibility of gratings. Journal of Physiology. 1968;13:551–566. doi: 10.1113/jphysiol.1968.sp008574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo AR, Wagner RS, Reynolds DR, Guo SQ, Goel AK. Down syndrome. Clinical review of ocular features. Clinical Pediatrics. 1989;28:355–358. doi: 10.1177/000992288902800804. [DOI] [PubMed] [Google Scholar]
- Catalano RA. Down syndrome. Survey of Ophthalmology. 1990;34:385–398. doi: 10.1016/0039-6257(90)90116-d. [DOI] [PubMed] [Google Scholar]
- Courage ML, Adams RJ, Reyno S, Kwa PG. Visual acuity in infants and children with Down syndrome. Developmental Medicine & Child Neurology. 1994;36:586–593. doi: 10.1111/j.1469-8749.1994.tb11895.x. [DOI] [PubMed] [Google Scholar]
- Cronin-Golomb A, Corkin S, Growdon JH. Visual dysfunction predicts cognitive deficits in Alzheimer's disease. Optometry and Vision Science. 1995;72:168–176. doi: 10.1097/00006324-199503000-00004. [DOI] [PubMed] [Google Scholar]
- Cronin-Golomb A, Corkin S, Rizzo JF, Cohen J, Growdon JH, Banks KS. Visual dysfunction in Alzheimer's disease: relation to normal aging. Annals of Neurology. 1991;29:41–52. doi: 10.1002/ana.410290110. [DOI] [PubMed] [Google Scholar]
- Cronin-Golomb A, Cronin-Golomb M, Dunne TE, Brown AC, Jain K, Cipolloni PB, Auerbach SH. Facial frequency manipulation normalizes face discrimination in AD. Neurology. 2000;54:2316–2318. doi: 10.1212/wnl.54.12.2316. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Allen KA, Sloan KR, Lerea CL, Hurley JB, Klock IB, Milam AH. Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. The Journal of Comparative Neurology. 1991;312:610–624. doi: 10.1002/cne.903120411. [DOI] [PubMed] [Google Scholar]
- da Cunha RP, Moreira JB. Ocular findings in Down's syndrome. American Journal Ophthalmology. 1996;122:236–244. doi: 10.1016/s0002-9394(14)72015-x. [DOI] [PubMed] [Google Scholar]
- De Valois RL, Albrecht DG, Thorell LG. Spatial frequency selectivity of cells in macaque visual cortex. Vision Research. 1982;22:545–559. doi: 10.1016/0042-6989(82)90113-4. [DOI] [PubMed] [Google Scholar]
- De Valois RL, De Valois KK. Spatial Vision. New York: Oxford University Press; 1988. [Google Scholar]
- Devenny DA, Wegiel J, Schupf N, Jenkins E, Zigman W, Krinsky-McHale SJ, Silverman WP. Dementia of the Alzheimer's type and accelerated aging in Down syndrome. Science of Aging Knowledge Environment: SAGE KE. 2005 doi: 10.1126/sageke.2005.14.dn1. [DOI] [PubMed] [Google Scholar]
- Evans DW, Ginsburg AP. Contrast sensitivity predicts age-related differences in highway-sign discriminability. Human Factors. 1985;27:637–642. doi: 10.1177/001872088502700602. [DOI] [PubMed] [Google Scholar]
- Evenhuis HM, Sjoukes L, Koot HM, Kooijman AC. Does visual impairment lead to additional disability in adults with intellectual disabilities? Journal of Intellectual Disability Research. 2009;53:19–28. doi: 10.1111/j.1365-2788.2008.01114.x. [DOI] [PubMed] [Google Scholar]
- Evenhuis HM, Theunissen M, Denkers I, Verschuure H, Kemme H. Prevalence of visual and hearing impairment in a Dutch institutionalized population with intellectual disability. Journal of Intellectual Disability Research. 2001;45:457–464. doi: 10.1046/j.1365-2788.2001.00350.x. [DOI] [PubMed] [Google Scholar]
- Farnsworth D. The Farnsworth-Munsell 100-Hue and dichotomous tests for color vision. Journal of the Optical Society of America. 1943;33:568. [Google Scholar]
- Farnsworth D. The Farnsworth Dichotomous Test for Color Blindness Panel D-15 Manual. New York, NY: The Psychological Corporation; 1947. pp. 1–8. [Google Scholar]
- Farnsworth D. The Farnsworth-Munsell 100-Hue Test for the Examination of Color Discrimination Manual. Munsell Color Co: Baltimore, MD; 1957. pp. 1–7. [Google Scholar]
- Ginsburg AP, Evans DW, Sekule R, Harp SA. Contrast sensitivity predicts pilots' performance in aircraft simulators. American Journal of Optics and Physiological Optics. 1982;59:105–109. doi: 10.1097/00006324-198201000-00020. [DOI] [PubMed] [Google Scholar]
- Gordon J, Abramov I. Colour Vision. In: Goldstein B, editor. The Blackwell Handbook of Perception. Blackwell: Oxford UK; 2001. pp. 92–127. [Google Scholar]
- Graham NV. Visual Pattern Analyzers. New York: Oxford University Press; 1989. [Google Scholar]
- Haegerstrom-Portnoy GS, chneck ME, Brabyn JA. Seeing into old age: vision function beyond acuity. Optometry and Vision Science. 1999;76:141–158. doi: 10.1097/00006324-199903000-00014. [DOI] [PubMed] [Google Scholar]
- Hainline L, Abramov I. Eye movement-based measures of development of spatial contrast sensitivity in infants. Optometry and Vision Science. 1997;74:790–799. doi: 10.1097/00006324-199710000-00018. [DOI] [PubMed] [Google Scholar]
- Holroyd S, Shepherd ML. Alzheimer's disease: a review for the ophthalmologist. Survey of Ophthalmology. 2001;45:516–524. doi: 10.1016/s0039-6257(01)00193-x. [DOI] [PubMed] [Google Scholar]
- Kim LY, Detterman DK. Contrast sensitivity in adults with Down syndrome and its effects on cognitive abilities and age; Paper presented at the Gatlinburg Conference on Research and Theory in Mental Retardation and Developmental Disabilities; Gatlinburg TN. 1996. [Google Scholar]
- Kim LY, Detterman DK, Gilmore GC. Contrast sensitivity in adults with Down syndrome; Paper presented at the Gatlinburg Conference on Research and Theory in Mental Retardation and Developmental Disabilities; Gatlinburg TN. 1994. [Google Scholar]
- Kim U, Hwang J-M. Refractive errors and strabismus in Asian patients with Down syndrome. Eye. 2009;23:1560–1564. doi: 10.1038/eye.2008.309. [DOI] [PubMed] [Google Scholar]
- Krinsky-McHale SJ, Devenny DA, Silverman WP. Changes in explicit memory associated with early dementia in adults with Down's syndrome. Journal of Intellectual Disability Research. 2002;46:198–208. doi: 10.1046/j.1365-2788.2002.00365.x. [DOI] [PubMed] [Google Scholar]
- Kuyk T, Elliott JL, Fuhr PS. Visual correlates of mobility in real world settings in older adults with low vision. Optometry and Vision Science. 1998;75:538–547. doi: 10.1097/00006324-199807000-00023. [DOI] [PubMed] [Google Scholar]
- Lanthony P. The desaturated panel D-15. Documenta Ophthalmologica . Advances in Ophthalmology. 1978;46:185–189. doi: 10.1007/BF00174107. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Terry RD, Morrison JH. Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer's disease: a quantitative study of visual and auditory cortices. Journal of Neuroscience. 1987;7:1799–1808. doi: 10.1523/JNEUROSCI.07-06-01799.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little JA, Woodhouse JM, Saunders KJ. Corneal power and astigmatism in Down syndrome. Optometry and Vision Science. 2009;86:748–754. doi: 10.1097/OPX.0b013e3181a59d5d. [DOI] [PubMed] [Google Scholar]
- Liyanage S, Barnes J. The eye and Down's syndrome. British Journal of Hospital Medicine. 2008;69:632–634. doi: 10.12968/hmed.2008.69.11.31686. [DOI] [PubMed] [Google Scholar]
- Lord SR, Menz HB. Visual contributions to postural stability in older adults. Gerontology. 2000;46:306–310. doi: 10.1159/000022182. [DOI] [PubMed] [Google Scholar]
- Lott IT, Lai F. Dementia in Down’s syndrome: Observations from a neurology clinic. Applied Research for the Mentally Retarded. 1982;3:233–239. doi: 10.1016/0270-3092(82)90017-0. [DOI] [PubMed] [Google Scholar]
- Lovegrove WJ, Heddle M, Slaghuis W. Reading disability: Spatial frequency specific deficits in visual information store. Neuropsychologia. 1980;18:111–115. doi: 10.1016/0028-3932(80)90093-7. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory: A User’s Guide. New York: Cambridge University Press; 1991. [Google Scholar]
- Mann DMA. Alzheimer’s disease and Down’s syndrome. Histopathology. 1988;13:125–137. doi: 10.1111/j.1365-2559.1988.tb02018.x. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Mendez MA, Martin R, Smyth KA, Whitehouse PJ. Complex visual disturbances in Alzheimer's disease. Neurology. 1990;40:439–443. doi: 10.1212/wnl.40.3_part_1.439. [DOI] [PubMed] [Google Scholar]
- Merrick J, Koslowe K. Refractive errors and visual anomalies in Down syndrome. Down’s Syndrome, Research and Practice. 2001;6:131–133. doi: 10.3104/reports.105. [DOI] [PubMed] [Google Scholar]
- Mosteller F, Tukey J. Data Analysis and Regression. Reading, MA: Addison-Wesley; 1977. [Google Scholar]
- Prasher V. Screening for ophthalmic pathology and its associated effects on adaptive behaviour in adults with Down's syndrome. The European Journal of Psychiatry. 1994;8:197–204. [Google Scholar]
- Rizzo JF, 3rd, Cronin-Golomb A, Growdon JH, Corkin S, Rosen TJ, Sandberg MA, Chiappa KH, Lessell S. Retinocalcarine function in Alzheimer's disease. A clinical and electrophysiological study. Archives of Neurology. 1992;49:93–101. doi: 10.1001/archneur.1992.00530250097023. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Anderson SW, Dawson J, Nawrot M. Vision and cognition in Alzheimer's disease. Neuropsychologia. 2000;38:1157–1169. doi: 10.1016/s0028-3932(00)00023-3. [DOI] [PubMed] [Google Scholar]
- Rocco FJ, Cronin-Golomb A, Lai F. Alzheimer-like visual deficits in Down syndrome. Alzheimer Disease and Associated Disorders. 1997;11:88–98. doi: 10.1097/00002093-199706000-00005. [DOI] [PubMed] [Google Scholar]
- Roizen NJ. Down syndrome: progress in research. Mental Retardation and Developmental Disabilities Research Reviews. 2001;7:38–44. doi: 10.1002/1098-2779(200102)7:1<38::AID-MRDD1006>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Silverman W. Down syndrome: Cognitive phenotype. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13:228–236. doi: 10.1002/mrdd.20156. [DOI] [PubMed] [Google Scholar]
- Silverman W, Schupf N, Zigman W, Devenny D, Miezejeski C, Schubert R, Ryan R. Dementia in adults with mental retardation: assessment at a single point in time. American Journal of Mental Retardation. 2004;109:111–125. doi: 10.1352/0895-8017(2004)109<111:DIAWMR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Silverman W, Zigman W, Kim H, Krinsky-McHale SJ, Wisniewski HM. Aging and dementia among adults with mental retardation and Down syndrome. Topics in Geriatric Rehabilitation. 1998;13:49–64. [Google Scholar]
- Smith VC, Pokorny J, Pass AS. Colour-axis determination on the Farnsworth-Munsell 100-hue test. American Journal of Ophthalmology. 1985;100:176–182. doi: 10.1016/s0002-9394(14)75002-0. [DOI] [PubMed] [Google Scholar]
- Stephen E, Dickson J, Kindley AD, Scott CC, Charleton PM. Surveillance of vision and ocular disorders in children with Down syndrome. Developmental Medicine and Child Neurology. 2007;49:513–515. doi: 10.1111/j.1469-8749.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- Tukey JW. Exploratory Data Analysis. Reading, MA: Addison-Wesley; 1977. [Google Scholar]
- Van Buggenhout GJ, Trommelen JC, Schoenmaker A, De Bal C, Verbeek JJ, Smeets DF, Ropers HH, Derriendt K, Hamel BC, Fryns JP. Down syndrome in a population of elderly mentally retarded patients: genetic-diagnostic survey and implications for medical care. American Journal of Medical Genetics. 1999;85:376–384. doi: 10.1002/(sici)1096-8628(19990806)85:4<376::aid-ajmg14>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- Van Schrojenstein Lantman-de Valk H, Haveman MJ, Maaskant MA, Kessels AGH, Urlings HFJ, Sturmans F. The need for assessment of sensory functioning in ageing people with mental handicap. Journal of Intellectual Disability Research. 1994;38:289–298. doi: 10.1111/j.1365-2788.1994.tb00396.x. [DOI] [PubMed] [Google Scholar]
- van Splunder J, Stilma JS, Bernsen RM, Arentz TG, Evenhuis HM. Refractive errors and visual impairment in 900 adults with intellectual disabilities in the Netherlands. Acta Ophthalmologica Scandinavica. 2003;81:123–129. doi: 10.1034/j.1600-0420.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- van Splunder J, Stilma JS, Bernsen RM, Evenhuis HM. Prevalence of ocular diagnoses found on screening 1539 adults with intellectual disabilities. Ophthalmology. 2004;111:1457–1463. doi: 10.1016/j.ophtha.2003.12.051. [DOI] [PubMed] [Google Scholar]
- Verriest G, Van Laethem J, Uvijls A. A new assessment of the normal ranges of the Farnsworth-Munsell 100-hue test scores. American Journal of Ophthalmology. 1982;93:635–642. doi: 10.1016/s0002-9394(14)77380-5. [DOI] [PubMed] [Google Scholar]
- Vingrys AJ, King-Smith PE. A quantitative scoring technique for panel tests of colour vision. Investigative Ophthalmology & Visual Science. 1988;29:50–63. [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Perception & Psychophysics. 1983;33:113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- Weale RA. A Biography of the Eye. Development Growth and Age. London: H.K. Lewis; 1982. [Google Scholar]
- Wisniewski K, Wisniewski HM, Wen GY. Occurrence of Alzheimer’s neuropathology and dementia in Down Syndrome. Annuals of Neurology. 1985;17:278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- Wood JM, Bullimore MA. Changes in the lower displacement limit for motion with age. Ophthalmic & Physiological Optics. 1995;15:31–36. [PubMed] [Google Scholar]
- Woodhouse JM, Pakeman VH, Cregg M, Saunders KJ, Parker M, Fraser WI, Sastry P, Lobo S. Refractive errors in young children with Down syndrome. Optometry and Vision Science. 1997;74:844–851. doi: 10.1097/00006324-199710000-00023. [DOI] [PubMed] [Google Scholar]
- Woodhouse JM, Pakeman VH, Saunders KJ, Parker M, Fraser WI, Lobo S, Sastry P. Visual acuity and accommodation in infants and young children with Down's syndrome. Journal of Intellectual Disability Research. 1996;40:49–55. doi: 10.1111/j.1365-2788.1996.tb00602.x. [DOI] [PubMed] [Google Scholar]
- Zigman WB, Lott IT. Alzheimer's disease in down syndrome: Neurobiology and risk. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13:237–246. doi: 10.1002/mrdd.20163. [DOI] [PubMed] [Google Scholar]