Abstract
OBJECTIVE
Prior studies suggest adverse associations between out-of-hospital advanced airway management (AAM) and patient outcomes after major trauma. This secondary analysis of data from the Resuscitation Outcomes Consortium (ROC) Hypertonic Saline Trial evaluated associations between out-of-hospital AAM and outcomes in patients suffering isolated severe traumatic brain injury (TBI) or hemorrhagic shock.
METHODS
This multicenter study included adults with severe TBI (GCS ≤8) or hemorrhagic shock (SBP ≤70 mmHg, or [SBP 71–90 mmHg and heart rate ≥108 bpm]). We compared patients receiving out-of-hospital AAM with those receiving Emergency Department AAM. We evaluated associations between patient outcomes (28-day mortality, and 6-month poor neurologic or functional outcome) and airway strategy, adjusting for confounders. Analysis was stratified by 1) patients with isolated severe TBI, and 2) patients with hemorrhagic shock with or without severe TBI.
RESULTS
Of 2,135 patients, we studied 1,116 TBI and 528 shock; excluding 491 who died in the field, did not receive AAM or had missing data. In the shock cohort, out-of-hospital AAM was associated with increased 28-day mortality (adjusted OR 5.14; 95% CI: 2.42, 10.90). In TBI, out-of-hospital AAM showed a tendency towards increased 28-day mortality (adjusted OR 1.57; 95% CI: 0.93, 2.64) and 6-month poor functional outcome (1.63; 1.00, 2.68), but these differences were not statistically significant. Out-of-hospital AAM was associated with poorer 6-month TBI neurologic outcome (1.80; 1.09, 2.96).
CONCLUSIONS
Out-of-hospital AAM was associated with increased mortality after hemorrhagic shock. The adverse association between out-of-hospital AAM and injury outcome is most pronounced in patients with hemorrhagic shock.
Keywords: intubation (intratracheal), emergency medical services, traumatic brain injury, shock, trauma
INTRODUCTION
Airway management is one of the most prominent out-of-hospital interventions performed in the treatment of patients with major trauma.1 In North America forms of advanced airway management (AAM) performed by out-of-hospital Emergency Medical Services (EMS) personnel include endotracheal intubation, supraglottic airway insertion, and surgical airway placement (cricothyroidotomy). EMS personnel in North America perform AAM with the intention of preventing and correcting hypoxia. AAM also facilitates controlled ventilation and protects the airway from vomiting and inadvertent aspiration. Some air medical personnel perform AAM for safety during transport. Prior studies evaluating the relationship between out-of-hospital AAM and outcomes after traumatic brain injury have identified adverse associations compared with ED AAM or similarly injured patients not receiving AAM.2–7
It is not clear if there is a causal relationship between out-of-hospital AAM and adverse injury outcomes. Observational studies linking airway management to patient outcomes have important limitations, including the use of retrospective data, biases in the selection of the study population, differences in EMS staffing and configuration, as well as incomplete risk adjustment. An important additional limitation of prior observational studies was the focus on patients with traumatic brain injury without separately analyzing other injury groups, particularly those with hemorrhagic shock. This distinction is important because the outcomes of TBI with and without concomitant shock differ.8 A separate examination of TBI and shock patients could provide importance insights regarding the relationship between out-of-hospital AAM and trauma outcomes.
In this secondary analysis of data from the Resuscitation Outcomes Consortium (ROC) Hypertonic Saline Trial, we sought to determine the association of out-of-hospital AAM with outcomes in patients with 1) isolated severe TBI, and 2) hemorrhagic shock with or without concomitant TBI.
METHODS
Study Design
This study was a secondary analysis of prospectively collected clinical trial data from the ROC Hypertonic Saline (HS) Trial. The study received Institutional Review Board approval from the home institutions of the eleven regional clinical coordinating centers of the ROC consortium. The ROC HS Study was conducted under US regulations for exception from informed consent for emergency research (21 CFR 50.24), and the Canadian Tri-Counsel Policy Statement: Ethical Conduct for Research Involving Humans. Additional reviews and approvals were obtained from the US Food and Drug Administration (FDA) and Health Canada, as well as the institutional review boards and research ethics boards of receiving hospitals in the communities where the research was conducted.
Study Setting
The Resuscitation Outcomes Consortium (ROC) is a North American multicenter trial network designed to conduct out-of-hospital interventional and clinical research in the areas of cardiac arrest and severe traumatic injury. ROC has 11 trauma study regional coordinating centers encompassing communities in Birmingham, AL; Dallas, TX; Des Moines, IA; Milwaukee, WI; Pittsburgh, PA; Portland, OR; San Diego, CA; Seattle/King County, WA; British Columbia; Ottawa, Ontario; and Toronto, Ontario. In addition, a data coordinating center is based in Seattle. The ROC network includes over 250 emergency medical services (EMS) agencies, of which 85 participated in the ROC HS trial.9
The ROC HS trial tested the effect of out-of-hospital administration of 250 ml of hypertonic saline, hypertonic saline plus dextran, or normal saline upon outcomes after severe TBI and/or hemorrhagic shock. EMS personnel administered the study drug to eligible patients in a blinded randomized fashion. The methods and results of the ROC HS Trial have been published elsewhere.10–12
Methods of Measurement – Data Collection
As a component of the HS Trial, ROC collected systemic data on all trial patients, including comprehensive information regarding the circumstances and mechanism of the injury, patient demographics, clinical presentation, out-of-hospital and in-hospital interventions, field and hospital course, and clinical outcomes.13 Study personnel obtained data from review of EMS and hospital records as well as through in-person interviews and assessments. Study personnel entered data into a master database managed by the data coordinating center.
Selection of Participants
Inclusion criteria for the ROC HS trial included adult (age ≥15 years) injured patients with either 1) severe TBI, defined as a blunt mechanism of injury with a Glasgow Coma Scale ≤8; or, 2) hemorrhagic shock, defined as a systolic blood pressure of ≤70 mmHg, or a systolic blood pressure of 71–90 mmHg with a concomitant heart rate ≥108 beats per minute. As in the parent trial analysis, patients with both severe TBI and shock were classified in the shock cohort.11,12
Exclusion criteria for the ROC HS trial included known or suspected pregnancy, age <15 years, out-of-hospital cardiopulmonary resuscitation, administration of more than 2000 ml of crystalloid or any colloid or blood products prior to enrollment, severe hypothermia (<28°C), drowning, asphyxia due to hanging, burns of more that 20 percent total body surface area, isolated penetrating head injury, inability to obtain venous access, prisoner status, intra-facility transfers, or >4 hours elapsed time between receipt of dispatched call and study intervention.
In this analysis, we included patients enrolled in the ROC HS trials who had received AAM in the out-of-hospital setting or in the receiving ED. We defined AAM as endotracheal intubation, insertion of supraglottic airway, or surgical airway placement (cricothyroidotomy). In the primary analysis, we included only successfully placed out-of-hospital and ED advanced airways, excluding unsuccessful airway insertion attempts. However, some injured patients may not have received AAM in either the field or ED; for example, an injured but awake individual not intubated until receiving anesthesia in the operating room. Because of their likely different prognoses, we excluded patients who did not receive AAM in the out-of-hospital or ED settings. We excluded patients who were pronounced dead in the field or on arrival to the ED, or who were missing key covariates.
Outcomes and Covariates
The primary outcome for the TBI and hemorrhagic shock groups was death within 28-days of injury (28-day mortality). In the TBI cohort only, the secondary outcomes were 6-month Extended Glasgow Outcome Score (GOSE) as well as 6-month Disability Rating Score (DRS). GOSE and DRS were determined by structured telephone survey with supplemental information provided by a family member or caregiver if the patient was unable to respond to the survey.14 As in the primary study analysis, we dichotomized GOSE into “good” (>4) and “poor” (≤4) outcomes. We similarly dichotomized DRS into “good” (<4) and “poor” (≥4) outcomes.
Clinicians often use serum lactic acid as an indicator of adequacy of cellular perfusion.15 AAM and subsequent post-airway management ventilation patterns may plausibly alter cellular perfusion and lactic acid levels. Therefore, we also examined lactic acid levels collected on ED arrival as an additional secondary outcome in both the TBI and hemorrhagic shock groups.
In the multivariable analysis, the primary exposure was location of AAM (out-of-hospital versus ED). If the data described both successful out-of-hospital and successful ED AAM, we classified the case as an out-of-hospital AAM. EMS personnel reported AAM outcomes; the study did not utilize independent confirmation of airway placement.
Data Analysis
We analyzed the data using multivariable logistic regression. We evaluated separate models for each outcome and patient subgroup. We adjusted all mortality estimates for age, sex, injury severity score, mechanism of injury, initial systolic blood pressure and Glasgow Coma Scale, highest field heart rate, out-of-hospital neuromuscular blockade use, mode of transportation, head and neck abbreviated injury scale (TBI cohort only), parent trial intervention arm (hypertonic saline, hypertonic saline plus Dextran, or normal saline), and ROC study site. We did not adjust the p-values for multiple comparisons.
Of TBI cohort patients surviving to hospital discharge, 6-month GOSE and DRS scores were missing in 12% and 13%, respectively. In order to minimize the risk of bias from missing data, we conducted the analysis of these outcomes using multiple hot deck imputation. These imputations were based upon data on TBI patients discharged alive from the hospital, using either one-month or discharge scores, length of hospitalization, and treatment arm.12,16 We carried out the analyses using 20 imputations.
We performed sensitivity analyses examining the impact of different airway classification definitions. In one, we examined the relationship between 28-day mortality and any attempted (successful or failed) out-of-hospital AAM. The primary analysis also combined all AAM (endotracheal intubation, supraglottic airway and surgical airway). We repeated the sensitivity analysis comparing only out-of-hospital endotracheal intubation with ED AAM. There were inadequate numbers of observations to determine associations with individual supraglottic airway devices (Laryngeal Mask Airway, Combitube, King LT). We also repeated the analyses of 6-month TBI neurological and functional outcome without the use of multiple imputation. We also repeated the analysis excluding prehospital neuromuscular blockade from the multivariable models.
RESULTS
Of 2,135 patients who received fluid in the trials, 1,644 received advanced airway management, including 1,116 TBI (764 out-of-hospital AAM, 352 ED AAM) and 528 hemorrhagic shock (296 out-of-hospital AAM, 232 ED AAM). Most AAM in both settings were endotracheal intubation. (Table 1) We excluded 26 patients who died in the field, 444 who did not receive AAM in the out-of-hospital or ED settings, and 21 who had missing key covariates.
TABLE 1.
Baseline patient characteristics.
| Patient Characteristic | TBI | Shock | ||
|---|---|---|---|---|
| Out-of-hospital AAM N=764 | Emergency Department AAM N=352 | Out-of-hospital AAM N=296 | Emergency Department AAM N=232 | |
| Type of AAM: | ||||
| Endotracheal Intubation | 730 (95.5%) | 351 (99.7%) | 283 (95.6%) | 229 (98.7%) |
| Supraglottic Airway | 36 (4.7%) | 0 (0%) | 11 (3.7%) | 0 (0%) |
| Laryngeal Mask Airway | 6 (0.8%) | 0 (0%) | 2 (0.7%) | 0 (0%) |
| Combitube | 14 (1.8%) | 0 (0%) | 7 (2.4%) | 0 (0%) |
| King LT | 16 (3.5%) | 0 (0%) | 2 (1.6%) | 0 (0%) |
| Surgical Airway | 1 (0.1%) | 1 (0.3%) | 3 (1.0%) | 3 (1.3%) |
| Age – years mean (sd) | 38.3 (18.1) | 40.1 (19.0) | 36.8 (16.8) | 34.9 (15.7) |
| Male - n (%) | 585 (76.6%) | 271 (77.0%) | 223 (75.3%) | 185 (79.7%) |
| Head/Neck Abbreviated Injury Score - mean (sd) | 3.8 (1.5) | 3.4 (1.9) | 2.3 (2.1) | 1.4 (1.9) |
| Missing Head/Neck AIS - n (%) | 17 (2.2%) | 5 (1.4%) | 15 (5.1%) | 4 (1.7%) |
| Injury Severity Score - mean (sd) | 29.4 (15.4) | 24.9 (14.8) | 31.0 (16.5) | 25.1 (14.4) |
| Missing Injury Severity Score - n (%) | 22 (2.9%) | 6 (1.7%) | 21 (7.1%) | 6 (2.6%) |
| Mechanism of Injury | ||||
| Blunt injury - n (%) | 750 (98.3%) | 347 (98.6%) | 231 (78.0%) | 133 (57.3%) |
| Penetrating injury - n (%) | 15 (2.0%) | 6 (1.7%) | 68 (23.0%) | 101 (43.5%) |
| Initial SBP – mm Hg mean (sd) | 134.4 (33.5) | 134.6 (30.2) | 78.7 (30.4) | 80.9 (24.2) |
| Initial SBP not detectable - n (%) | 8 (1.0%) | 3 (0.9%) | 80 (27.0%) | 46 (19.8%) |
| Highest Field Heart Rate – bpm mean (sd) | 108.1 (24.9) | 100.2 (26.8) | 119.6 (30.4) | 120.1 (23.1) |
| Initial Glasgow Coma Scale - mean (sd) | 5.0 (2.4) | 5.5 (2.4) | 6.7 (4.5) | 10.3 (4.5) |
| Prehospital Neuromuscular | 531 (69.5%) | 4 (1.1%) | 190 (64.2%) | 1 (0.4%) |
| Blockade Use - n (%) | ||||
| Parent Trial Intervention Arm | ||||
| Normal Saline - n (%) | 334 (43.7%) | 164 (46.6%) | 129 (43.6%) | 106 (45.7%) |
| Hypertonic Saline - n (%) | 209 (27.4%) | 88 (25.0%) | 90 (30.4%) | 66 (28.4%) |
| Hypertonic Saline + Dextran - n (%) | 221 (28.9%) | 100 (28.4%) | 77 (26.0%) | 60 (25.9%) |
| Air medical transport - n (%) | 312 (40.8%) | 19 (5.4%) | 69 (23.3%) | 26 (11.2%) |
| First ED lactate (mmol/L) - mean (sd) | 3.6 (2.8) | 3.6 (2.3) | 6.5 (4.5) | 6.4 (5.4) |
| Survival to 28-days - n (%) | 558 (73.0%) | 259 (73.6%) | 166 (56.1%) | 181 (78.0%) |
| 6-month GOSE - mean (sd) | 4.0 (2.7) | 3.9 (2.7) | - | - |
| Missing 6-month GOSE - n (%) | 84 (11.0%) | 55 (15.6%) | - | - |
| 6-month GOSE ≤ 4 - n (%)1 | 403 (59.3%) | 185 (62.3%) | ||
| 6-month DRS - mean (sd) | 12.2 (13.1) | 12.7 (13.3) | - | - |
| 6-month DRS ≥ 4 - n (%) | 405 (59.6%) | 181 (61.1%) | ||
| Missing 6-month DRS - n (%) | 85 (11.1%) | 56 (15.9%) | - | - |
TBI = Traumatic brain injury. AAM = advanced airway management.
In the TBI cohort, patients receiving out-of-hospital AAM tended to be more severely injured. (Table 1) Mechanism of injury, systolic blood pressure, heart rate and Glasgow Coma Scale were similar between out-of-hospital and ED AAM patients. A large number of patients received air medical transport in the out-of-hospital AAM group. The distribution of the trial interventions drugs (hypertonic saline) was similar between airway groups.
In the shock cohort, injury severity was also worse in the out-of-hospital AAM group. A large proportion of patients in the ED AAM group sustained penetrating injury. Initial blood pressure and heart rate were similar between groups, although Glasgow Coma Scale was lower in the out-of-hospital AAM group. Out-of-hospital TBI and shock AAM patients were more likely to receive air medical transport. The distribution of the trial interventions was similar.
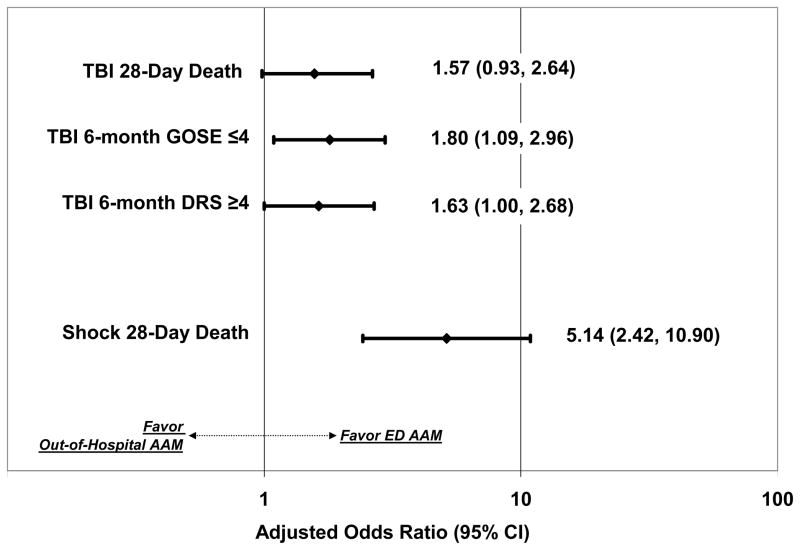
Among shock patients receiving AAM, 28-day mortality was 34.3%. After adjustment for confounders, out-of-hospital AAM was associated with increased 28-day mortality (OR 5.14; 95% CI: 2.42, 10.90). (Figure 1, Appendix 2).
FIGURE 1.
Adjusted association of out-of-hospital advanced airway management with traumatic brain injury outcomes (28-day death, and 6-month neurologic and functional outcome) and hemorrhagic shock outcomes (28-day death). AAM = Advanced Airway Management. OR = Odds Ratio. GOSE = Glasgow Outcome Scale Extended. DRS = Disability Rating Scale. (Full models listed in Appendices 1 and 2.)
Among TBI patients receiving AAM, unadjusted 28-day mortality was 26.8%. After adjusting for confounders, out-of-hospital AAM showed a tendency towards increased 28-day mortality (OR 1.57; 95% CI: 0.93, 2.64), but this association was not statistically significant. (Figure 1, Appendix 1) Mean 6-month GOSE and DRS were 4.0 and 12.4. After adjustment for confounders, out-of-hospital AAM showed a tendency towards poorer 6-month DRS (OR 1.63; 95% CI: 1.00, 2.68), but this association was not statistically significant. Out-of-hospital AAM was associated with poorer 6-month GOSE (OR 1.80; 95% CI: 1.09, 2.96).
Out-of-hospital airway management was not associated with elevated initial ED lactate level in either TBI (OR 0.90; 95% CI: 0.48, 1.71) or shock cohorts (1.25; 0.45, 3.42). (Figure 2, Appendix 3).
In a sensitivity analysis, we classified cases receiving any out-of-hospital AAM attempts (successful or failed) as out-of-hospital AAM. The absence of association with TBI mortality persisted. The association with increased shock mortality also persisted. (Appendix 4) We repeated the analysis including only out-of-hospital endotracheal intubations, again finding no association with TBI mortality but identifying increased odds of 28-day death in the shock cohort. In the subgroup of TBI patients, we repeated the analyses of neurologic and functional outcomes without multiple imputation; while the models again showed a tendency toward worsened outcomes, the association between 6-month GOSE and out-of-hospital AAM was not statistically significant. Finally, when repeating the analyses excluding prehospital neuromuscular blockade from the multivariable models, the odds ratios for the associations with out-of-hospital AAM were attenuated but the inferences remained the same.
DISCUSSION
This study offers new insights to clarify the connections between AAM and injury outcomes. Numerous prior studies evaluating the association of out-of-hospital AAM with TBI outcomes have suggested potential harm compared with ED AAM or similarly injured patients not receiving AAM.2–7 For example, in an analysis of over 4,000 TBI patients in Pennsylvania, we observed increased adjusted odds of death and poor neurological outcome among patients receiving endotracheal intubation in the out-of-hospital setting vs. those receiving intubation the ED.2 These studies combined TBI and shock cases in the same analysis, controlling for the confounding effect of hypotension through multivariable adjustment and assuming similar associations with mortality in both groups.2
Our contrasting study examined TBI and shock subgroups separately. We observed that the increased mortality associated with out-of-hospital AAM was limited primarily to patients in shock. If the relationship between AAM and outcomes were due primarily to selection bias, one would expect similar associations when stratified by TBI and shock. Our results are further bolstered by the use of multicenter trial data with subjects prospectively identified using all available out-of-hospital vital signs and Glasgow Coma Scale measurements. This approach better approximated the perspective of the treating paramedic and minimized potential for post hoc misclassification.
If validated, the findings of this study would have important implications for out-of-hospital AAM research and practice. Reasons postulated for the connection between out-of-hospital AAM and poor outcomes in injured patients include suboptimal paramedic training or skill, poor intubation technique, prolonged laryngoscopy, iatrogenic hypotension and bradycardia, or the low rate of out-of-hospital neuromuscular blockade use, among others.1,17–20 More importantly, some experts believe that the worsened AAM outcomes are primarily due to inadvertent hyperventilation.21 In TBI hyperventilation is associated with decreased brain oxygen delivery and perfusion.21–24 In victims of shock, hyperventilation may decrease venous return, mean arterial pressure and cardiac output.21,22 We observed that the physiologic interactions with AAM in the shock state are more closely correlated with mortality than the interactions with TBI. Therefore, clinicians and scientists must strive to better characterize the interaction between airway, ventilation and the shock state. Serum lactate is often used as a marker of cellular perfusion, and differences in serum lactate might indicate airway-related perfusion differences. However, we did not observe associations between out-of-hospital AAM and initial ED lactate in either TBI or shock groups.
Observational studies have inherent limitations, including selection bias and incomplete risk adjustment, among others. However, this approach remains one of the best and only available approaches for evaluating the effectiveness of out-of-hospital AAM strategies in injured patients. We note that the association between AAM and mortality was relatively large, supporting the validity of the relationship. While a prospective controlled trial is the optimal approach for evaluating the effectiveness of AAM, clinical trials of out-of-hospital AAM are exceedingly difficult to perform since many patients possess intact airway reflexes, and most EMS providers in North America do not have access to neuromuscular blocking agents.25 To date Bernard, et al. have conducted the only prospective clinical trial evaluating the comparative effectiveness of out-of-hospital intubation, identifying a small benefit in select TBI patients.7 However, this Australian trial utilized paramedics specially trained in neuromuscular blockade use, and thus it is unclear if the results can be extrapolated elsewhere.
Based upon prior studies of TBI patients, some experts have condemned field intubation of injured patients by paramedics. We urge a more restrained interpretation. The techniques of out-of-hospital intubation and advanced airway insertion are likely similar for both TBI and shock patients. Our study highlights differing connections with mortality between TBI and shock patients, pointing to the likely presence of other factors influencing outcomes such as post-intubation ventilatory and hemodynamic management. Additional study is urgently needed to better characterize the physiology of the hemorrhagic shock state, the interactions with airway and ventilatory techniques, and the optimal methods for clinical management.
LIMITATIONS
We conducted a secondary analysis of clinical trial data not intended for evaluating AAM techniques. However, the trial data offered advantages over conventional trauma registries. For example, instead of relying upon ED arrival vital signs (as is customary in trauma registries) and CT results for post hoc identification of TBI, the trial used all available out-of-hospital vital signs and Glasgow Coma Scale scores to prospectively identify patients with TBI or shock. This strategy may more realistically reflect the perspective of treating paramedics. While potentially influencing the results of this analysis, the parent trial found no difference in outcomes between patients receiving hypertonic saline and those receiving normal saline.11,12
Observational studies of airway management are subject to selection bias. To minimize this effect, we narrowed our patient population to those receiving AAM in either the out-of-hospital or ED setting. Our strategy differs from prior studies that included both intubated and non-intubated cases. We also analyzed only successful airway insertions. Due to the limited size of the series, we could not separate out different airway types, nor could we fully account for the different combinations of successful and failed airway insertion efforts. In many cases, AAM in the receiving ED may have been performed primarily for patient agitation and safety and not for ventilatory control; however, given the nature of the data set, we were unable to separate or identify these cases.
There were key differences in the study population; for example, those who received out-of-hospital AAM were more severely injured than the ED AAM group. We attempted to account for the effect of confounders through the use of multivariable adjustment, but incomplete risk adjustment may have amplified the magnitude of the underlying associations. The functional forms of all covariates included in the model were selected after careful examination of the bivariate relationship between each variable and the main outcome. However, unmeasured or unmeasureable confounders (for example, hypoxia during and hyperventilation) may have affected the results.20,21 Other immeasurable factors may have impacted the decision to perform AAM. We chose not to perform a matched analysis due to the potential loss of statistical power. Replication of the analysis with an independent data set (particularly with shock patients) may reinforce the robustness of these results.
CONCLUSIONS
Compared with Emergency Department advanced airway management, out-of-hospital advanced airway management was associated with worsened 28-day mortality in patients with hemorrhagic shock. The associations between out-of-hospital advanced airway management and TBI outcomes were smaller and less certain. The adverse association between out-of-hospital AAM and injury outcome is most pronounced in patients with hemorrhagic shock.
Acknowledgments
FINANCIAL SUPPORT
The Resuscitation Outcomes Consortium (ROC) is supported by a series of cooperative agreements to nine regional clinical centers and one Data Coordinating Center (5U01 HL077863-University of Washington Data Coordinating Center, HL077866-Medical College of Wisconsin, HL077867-University of Washington, HL077871-University of Pittsburgh, HL077872-St. Michael’s Hospital, HL077873-Oregon Health and Science University, HL077881-University of Alabama at Birmingham, HL077885-Ottawa Hospital Research Institute, HL077887-University of Texas SW Medical Center/Dallas, HL077908-University of California San Diego) from the National Heart, Lung and Blood Institute in partnership with the U.S. Army Medical Research & Material Command, The Canadian Institutes of Health Research (CIHR) - Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, and the Heart, Stroke Foundation of Canada and the American Heart Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the National Institutes of Health.
Footnotes
AUTHOR CONTRIBUTIONS
HEW, SPB and MD conceived the study. SBP obtained the data and performed the analysis. All authors contributed to the critical review of results. HEW drafted and all authors critically reviewed and approved the manuscript. HEW takes responsibility for the paper as a whole.
Contributor Information
Henry E. Wang, Department of Emergency Medicine, University of Alabama at Birmingham, USA.
Siobhan P. Brown, The Clinical Trials Center, Department of Biostatistics, University of Washington, Seattle, Washington, USA.
Russell MacDonald, Ornge Transport Medicine, Mississauga, Ontario, Canada, Division of Emergency Medicine, Department of Medicine, University of Toronto, Toronto, Ontario, Canada.
Shawn K. Dowling, Department of Emergency Medicine, University of Calgary, Calgary, Alberta, Canada.
Steve Lin, Division of Emergency Medicine, Department of Medicine, University of Toronto, Toronto, Ontario, Canada.
Daniel Davis, Department of Emergency Medicine, University of California at San Diego, San Diego, California, USA.
Martin A. Schreiber, Department of Surgery, Division of Trauma, Critical Care and Acute Care Surgery, Oregon Health & Science University, USA.
Judy Powell, The Clinical Trials Center, Department of Biostatistics, University of Washington, Seattle, Washington, USA.
Rardi van Heest, Chief, Trauma Services, Royal Columbian Hospital, Vancouver, British Columbia, Canada.
Mohamud Daya, Department of Emergency Medicine, Oregon Health & Science University, Portland, Oregon, USA.
References
- 1.Wang HE, Yealy DM. Out-of-hospital endotracheal intubation: where are we? Annals of Emergency Medicine. 2006;47:532–41. doi: 10.1016/j.annemergmed.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Wang HE, Peitzman AB, Cassidy LD, Adelson PD, Yealy DM. Out-of-hospital endotracheal intubation and outcome after traumatic brain injury. Annals of Emergency Medicine. 2004;44:439–50. doi: 10.1016/j.annemergmed.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Davis DP, Hoyt DB, Ochs M, et al. The effect of paramedic rapid sequence intubation on outcome in patients with severe traumatic brain injury. The Journal of Trauma. 2003;54:444–53. doi: 10.1097/01.TA.0000053396.02126.CD. [DOI] [PubMed] [Google Scholar]
- 4.Davis DP, Peay J, Sise MJ, et al. The impact of prehospital endotracheal intubation on outcome in moderate to severe traumatic brain injury. Journal of Trauma. 2005;58:933–9. doi: 10.1097/01.ta.0000162731.53812.58. [DOI] [PubMed] [Google Scholar]
- 5.Murray JA, Demetriades D, Berne TV, et al. Prehospital intubation in patients with severe head injury. The Journal of Trauma. 2000;49:1065–70. doi: 10.1097/00005373-200012000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Winchell RJ, Hoyt DB. Endotracheal intubation in the field improves survival in patients with severe head injury. Trauma Research and Education Foundation of San Diego. Archives of surgery. 1997;132:592–7. doi: 10.1001/archsurg.1997.01430300034007. [DOI] [PubMed] [Google Scholar]
- 7.Bernard SA, Nguyen V, Cameron P, et al. Prehospital rapid sequence intubation improves functional outcome for patients with severe traumatic brain injury: a randomized controlled trial. Annals of Surgery. 2010;252:959–65. doi: 10.1097/SLA.0b013e3181efc15f. [DOI] [PubMed] [Google Scholar]
- 8.Chesnut RM, Marshall LF, Klauber MR, et al. The role of secondary brain injury in determining outcome from severe head injury. The Journal of Trauma. 1993;34:216–22. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Davis DP, Garberson LA, Andrusiek DL, et al. A descriptive analysis of Emergency Medical Service Systems participating in the Resuscitation Outcomes Consortium (ROC) network. Prehospital Emergency Care. 2007;11:369–82. doi: 10.1080/10903120701537147. [DOI] [PubMed] [Google Scholar]
- 10.Brasel KJ, Bulger E, Cook AJ, et al. Hypertonic resuscitation: design and implementation of a prehospital intervention trial. Journal of the American College of Surgeons. 2008;206:220–32. doi: 10.1016/j.jamcollsurg.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Bulger EM, May S, Kerby JD, et al. Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial. Annals of Surgery. 2011;253:431–41. doi: 10.1097/SLA.0b013e3181fcdb22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulger EM, May S, Brasel KJ, et al. Out-of-hospital hypertonic resuscitation following severe traumatic brain injury: a randomized controlled trial. JAMA. 2010;304:1455–64. doi: 10.1001/jama.2010.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newgard CD, Sears GK, Rea TD, et al. The Resuscitation Outcomes Consortium Epistry-Trauma: design, development, and implementation of a North American epidemiologic prehospital trauma registry. Resuscitation. 2008;78:170–8. doi: 10.1016/j.resuscitation.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pettigrew LE, Wilson JT, Teasdale GM. Assessing disability after head injury: improved use of the Glasgow Outcome Scale. Journal of Neurosurgery. 1998;89:939–43. doi: 10.3171/jns.1998.89.6.0939. [DOI] [PubMed] [Google Scholar]
- 15.Guyette F, Suffoletto B, Castillo JL, Quintero J, Callaway C, Puyana JC. Prehospital serum lactate as a predictor of outcomes in trauma patients: a retrospective observational study. The Journal of Trauma. 2011;70:782–6. doi: 10.1097/TA.0b013e318210f5c9. [DOI] [PubMed] [Google Scholar]
- 16.Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken, N.J: Wiley-Interscience; 2004. [Google Scholar]
- 17.Wang HE, Kupas DF, Hostler D, Cooney R, Yealy DM, Lave JR. Procedural experience with out-of-hospital endotracheal intubation. Critical care medicine. 2005;33:1718–21. doi: 10.1097/01.ccm.0000171208.07895.2a. [DOI] [PubMed] [Google Scholar]
- 18.Wang HE, Lave JR, Sirio CA, Yealy DM. Paramedic intubation errors: isolated events or symptoms of larger problems? Health Affairs. 2006;25:501–9. doi: 10.1377/hlthaff.25.2.501. [DOI] [PubMed] [Google Scholar]
- 19.Wang HE, Simeone SJ, Weaver MD, Callaway CW. Interruptions in cardiopulmonary resuscitation from paramedic endotracheal intubation. Annals of Emergency Medicine. 2009;54:645–52. e1. doi: 10.1016/j.annemergmed.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Dunford JV, Davis DP, Ochs M, Doney M, Hoyt DB. Incidence of transient hypoxia and pulse rate reactivity during paramedic rapid sequence intubation. Annals of Emergency Medicine. 2003;42:721–8. doi: 10.1016/s0196-0644(03)00660-7. [DOI] [PubMed] [Google Scholar]
- 21.Davis DP, Dunford JV, Poste JC, et al. The impact of hypoxia and hyperventilation on outcome after paramedic rapid sequence intubation of severely head-injured patients. The Journal of Trauma. 2004;57:1–8. doi: 10.1097/01.ta.0000135503.71684.c8. discussion -10. [DOI] [PubMed] [Google Scholar]
- 22.Davis DP, Douglas DJ, Koenig W, Carrison D, Buono C, Dunford JV. Hyperventilation following aero-medical rapid sequence intubation may be a deliberate response to hypoxemia. Resuscitation. 2007;73:354–61. doi: 10.1016/j.resuscitation.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. XIV. Hyperventilation. Journal of Neurotrauma. 2007;24 (Suppl 1):S87–90. doi: 10.1089/neu.2007.9982. [DOI] [PubMed] [Google Scholar]
- 24.Manley GT, Pitts LH, Morabito D, et al. Brain tissue oxygenation during hemorrhagic shock, resuscitation, and alterations in ventilation. The Journal of Trauma. 1999;46:261–7. doi: 10.1097/00005373-199902000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Wang HE, Davis DP, O’Connor RE, Domeier RM. Drug-assisted intubation in the prehospital setting (resource document to NAEMSP position statement) Prehospital Emergency Care. 2006;10:261–71. doi: 10.1080/10903120500541506. [DOI] [PubMed] [Google Scholar]