Abstract
Purpose
To assess the safety and efficacy of the implantable contact lens (ICL™) to treat myopia.
Design
Clinical, retrospective, single center, non-randomized case series.
Participants
Sixty-nine eyes of 46 patients with myopia ranging from −3.00 to 25.00 D were included in this study.
Intervention
Implantation of the ICL™.
Main outcome measures
Uncorrected Visual Acuity (UCVA), refraction, best spectacle corrected visual acuity (BSCVA), adverse events, operative and postoperative complications, subjective assessment and symptoms.
Results
The mean follow-up was 12.35 ± 6.13 (SD) months (range, 6 months–32 months). At the last visit, 49.20% of eyes had 20/20 or better UCVA compared to preoperative 20/20 or better BSCVA of 31.9% of eyes; 69.23% of eyes had postoperative UCVA better than or equal to preoperative BSCVA. The mean manifest refractive cylinder was 1.93 ± 1.21 D at baseline and 1.00 ± 0.92 D postoperatively. The mean manifest refraction spherical equivalent (MRSE) was −11.70 ± 4.24 D preoperatively and −0.69 ± 1.13 D postoperatively. A total of 69.8% of eyes were within ±0.5 D of the predicted MRSE; 84.1% were within ±1.0 D, and 88.90% were within ±2.0 D. BSCVA of 20/20 or better was achieved in 64.6% of eyes postoperatively, compared to 31.9% preoperatively. Mean improvement in BSCVA was 1line. One eye (1.5%) lost ⩾2 lines of BSCVA at the last visit, whereas 20% of eyes improved by ⩾2 lines. A total of 56.92% of cases gained ⩾1 line of BSCVA and 4.62% of cases lost ⩾1 line. Four ICL lenses were removed without significant loss of BSCVA, and 2 eyes with clinically significant lens opacities were observed. Four eyes (5.8%) developed a pupillary block the first day postoperatively. One eye (1.4%) developed a hypotony and AC shallowing.
Conclusion
Implantation of ICL for the correction of myopia was a safe procedure with good visual and refractive results from the early postoperative period to 1 year. Long-term follow-up is required to confirm the long-term safety of this implant.
Keywords: Implantable contact lens, Myopia, Visual acuity
Introduction
Refractive surgery confers visual freedom from the use of spectacles and contact lenses. Spectacles continue to be a natural and simple aid in correcting refractive errors. However, many adults with myopia are unwilling and unable to use spectacles due to discomfort from their refractive error and poor quality of vision. The higher the refractive error, the higher the percentage of people who are dissatisfied with the quality of vision with spectacles. Although spectacles or contact lenses are successful, there are several factors that indicate that a permanent solution from refractive surgery should be considered. These include aging with the development of nuclear sclerosis in the lens, contact lens intolerance, and occupational requirements for high myopes. The surgical correction of varying magnitudes of myopia has progressed over the past decade. Various techniques, some complementary and some overlapping, can predictably, safely and permanently treat low, moderate and high myopia.1–7 Corneal surgery shows the greatest promise but is controversial for higher corrections because of increased tissue removal, small ablation zones, increased aberrations, and poor predictability.9–14 Clear lens extraction for high and extreme myopia exposes the patient to the risk of retinal detachment and cystoid macular edema (CME).15 This is true for cataract patients and younger patients undergoing elective clear lens extraction. In younger patients clear lens extraction eliminates accommodation, and intraocular lens (IOL) power calculations are not as predictable.16,17
The concept of a phakic IOL for myopia was developed in the late 1950s with the design of a single-piece poly(methyl methacrylate) plate-haptic IOL that was fixated in the anterior chamber angle. The long-term data showed a significant incidence of corneal decompensation and uveitis–glaucoma–hyphema syndrome, thus, these IOLs were abandoned.18
The Worst iris-claw lens and Baikoff anterior chamber IOL in the middle 1980s resulted in a resurgence of interest in the phakic IOLs.5–8 In 1986, Fyodorov developed a new posterior chamber IOL made of silicone for phakic, highly myopic patients.19 However, implantation of this lens led to a high incidence of cataract.20 In 1993, Staar Surgical (A.G. Nidau) introduced a modified phakic posterior chamber intraocular lens (PPC IOL), the implantable contact lens (ICL), for the correction of high myopia. ICL has undergone at least 4 designs iterations with several studies reporting good refractive outcomes and optical performance.21–33
The Visian Implantable Collamer Lens (ICL) was granted approval by the United States Food and Drug Administration (FDA) in December 2005 for commercial use in the United States for spherical myopia of 3.00–20.00 diopters (D). The Toric Implantable Collamer Lens (TICL) represents an expansion of the earlier Visian ICL study and is currently awaiting approval in the United States. In this paper, we evaluate the efficacy and safety of the ICL implantation for myopia.
Patients and methods
This retrospective study evaluated 69 eyes of 46 consecutive patients who underwent implantation of a phakic IOL (ICL) to correct myopia from January 2007–31 December 2009 at the King Khaled Eye Specialist Hospital, Riyadh, Kingdom of Saudi Arabia. Study approval was obtained from the institutional review board. The target postoperative spherical (SE) refraction was emmetropia. Inclusion criteria were age between 21 and 45 years, with no restrictions in gender or race, myopia between −3.00 and −25.00 D, best spectacle-corrected visual acuity (BSCVA) of better than or equal to 20/100 for the spherical ICL and 20/40 or better for the toric ICL, no preexisting ocular pathology, no previous surgery except for astigmatic keratotomy, no systemic disease, contact lens intolerance, anterior chamber depth (ACD) of 2.80 mm or more (measured from the corneal endothelium to the anterior lens capsule), and endothelial cell count greater than 2000 cells/mm2.
An ophthalmic examination was performed before surgery and postoperatively at 2–4 h, 1 day and 1, 3, 6 months and the last visit. The examinations included distance uncorrected visual acuity (UCVA) and distance BSCVA using a Snellen chart, manifest and cycloplegic refractions, slitlamp and fundus evaluations, applanation tonometry, corneal topography, central pachymetry, horizontal corneal diameter (white-to-white), anterior chamber depth (ACD) and corneal endothelial cell count (cells/mm2).
The ICL is a plate-haptic single-piece lens designed for implantation in the posterior chamber with support on the ciliary sulcus. It is made of collamer, a flexible, hydrophilic material derived from collagen that is a copolymer comprising hydrophilic collagen and HEMA. It is 6.0 mm wide and comes in 5 d (11.0, 11.5, 12.0, 12.5, and 13.0 mm). The lens has a central convex–concave optic zone with a diameter of 4.5–5.5 mm, depending on dioptric power. The ICL design has been modified many times. In this study, all patients had implantation of the ICM V4 model, which is presumed to offer better vaulting over the crystalline lens than the ICM V3 because the optical zone has greater convex–concave curvature. Lens power calculations were performed with formulas developed by Staar. The variables in the formula were preoperatively manifested and cycloplegic refractions, average keratometric power, corneal thickness, and central ACD. The length of the implanted ICL was determined based on the patient’s horizontal corneal diameter (white to white).
Statistical analysis
Statistical analysis was performed with Epi info software (Centers for Disease Control and Prevention, Atlanta, GA, USA). Postoperative data were compared with the Student’s t-test. Results were considered statistically significant when P < 0.05.
Results
Patient population
The study cohort comprised, 69 eyes of 46 patients with a mean patient age of 27.5 years ± 5.7 (SD) (range, 21–44 years). Twenty-four of the 46 patients were female (52.2%). The mean follow-up, was 12.35 ± 6.13 months (range 6–32 months). The mean preoperative sphere was −10.85 ± 4.0D (range −4.00 to −23.75). The mean cylinder was −1.93 ± 1.21 D (range, 0.00 to −5.50 D) preoperatively and −1.00 ± 0.92 D (range 0.00 to −4.75 D) postoperatively. The mean SE was −11.70 ± 4.24 D (range −4.75 to −25.25 D) preoperatively and −0.69 ± 1.13 D (range −0.25 to −5.125 D) postoperatively (P = 0.000). Two (2 patients) of the 69 eyes (2.8%) in this study were amblyopic, and 1 (1.40%) eye had an inferior paracentral scar. Fifty-nine eyes (85.4%) had a myopic fundus,1 eye(1.4%) with a tilted disc. One (1.4%) eye had a history of prior intracorneal ring insertion and removal.
UCVA
There was a statistically signficant increase in mean UCVA from 20/400 preoperatively to 20/25 at the last postoperative visit (P < 0.0001). The preoperative UCVA was 20/100 or worse in 98.2%, no eyes (0%) were 20/40 or better, with only one eye (2.10%) 20/50 or better uncorrected at the baseline (Fig. 1).
Figure 1.
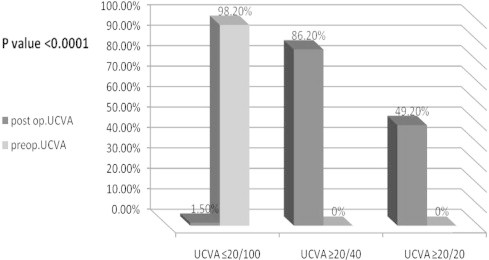
Preoperative (pre-op) uncorrected visual acuity (UCVA) versus last postoperative visit uncorrected visual acuity (UCVA) for 69 eyes that underwent implantable collamer lens surgery for myopia. P < 0.05 indicates statistical significance.
At the last postoperative visit, mean UCVA improved by 12 lines. The UCVA improved to 20/20 or better in 49.20% of patients and the proportion of eyes with 20/40 or better was 86.2%. No eyes lost UCVA. Fig. 2 presents the stability of UCVA (P > 0.05 between each postoperative interval).
Figure 2.
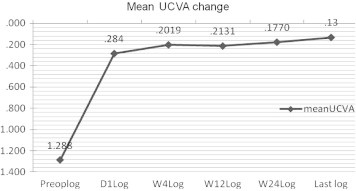
The change in mean uncorrected visual acuity (UCVA) (Stability) at 1 day (55 eyes), 4 weeks (65 eyes), 3 months (34 eyes), 6 months (36 eyes), last visit (65 eyes) postoperatively for implantable collamer lens surgery for myopia. Acuity data are in LogMAR units.
Preoperative best spectacle-corrected acuity (BSCVA) versus postoperative uncorrected visual acuity
Fig. 3 presents last postoperative visit UCVA compared to preoperative BSCVA. Comparing the proportion of eyes with 20/20 or better acuity, only 31.9% of eyes had this level of BSCVA preoperatively, compared with 49.2% of eyes with this level of UCVA at the last postoperative visit. Similarly, at the last examination 64.6% of eyes had 20/25 or better UCVA, compared with 55.1% BSCVA at baseline. Eighty-six percent of eyes achieved 20/40 or better UCVA at the last visit postoperatively, whereas 97% of eyes had 20/40 or better BSCVA preoperatively. Postoperative UCVA in 69.23% of eyes was equal to or better than preoperative BSCVA. The proportion of eyes that presented with preoperative BSCVA < 20/20 yet had postoperative UCVA ⩾ 20/20 was 23.07%. This difference was statistically signficant (P = 0.0012).
Figure 3.
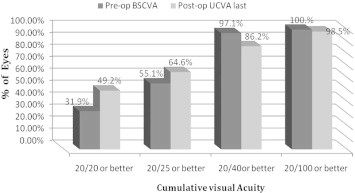
Preoperative (pre-op) best spectacle-corrected visual acuity (BSCVA) versus last postoperative visit uncorrected visual acuity (UCVA) for implantable collamer lens surgery for myopia.
Refractive outcomes
Manifest refractive spherical equivalent (MRSE)
There was statistically signficant decrease in mean MRSE from −11.70 ± 4.24 D (range, −4.75 to −25.25 D) preoperatively to −0.69 ± 1.13 D (range, −0.25 to −5.125 D) postoperatively (P < 0.0001). Only 7.20% of eyes had preoperative myopia ⩽−7.0 D, 73.9% of eyes were between −7 and −15 D and 18.8% of eyes had >−15 D myopia (Fig. 4). At baseline, no eyes (0%) fell within ±1.00 D MRSE compared with 84.1% at the last postoperative visit.
Figure 4.
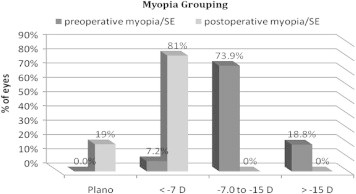
Myopia was stratified based on preoperative (pre-op) versus last visit postoperatively manifest refractive spherical equivalent for 69 eyes that underwent implantable collamer lens surgery for myopia.
Table 1 presents the predictability for all eyes in the study cohort. At the last postoperative visit, 69.8% of eyes were within ±0.50 D and 84.10% were within ±1.0 D.
Table 1.
Predictability of manifest refraction attempted vs. achieved in patients with an implantable collamer lens for myopia.
| Predictability | Frequency | Percent (%) | Cumulative percent (%) |
|---|---|---|---|
| ±0.50 | 44 | 69.80 | 69.80 |
| ±1.00 | 9 | 14.30 | 84.10 |
| ±2.00 | 3 | 4.80 | 88.90 |
| ±3.00 | 4 | 6.30 | 95.20 |
| ±5.00 | 3 | 4.80 | 100.00 |
| Total | 63 | 100.00 | 100.00 |
Predictability outcomes stratified by preoperative MRSE
Table 2 presents predictability stratified according to preoperative MRSE into three groups: ⩽−7 D, −7 to −15 D, and >−15 D. At the last postoperative visit, 100% of eyes in the <−7 D group fell within ±1.0 D of their attempted correction, 89.4% in the −7 to −15 D group, and 54.6% in the >−15−D group. Predictability outcomes within ±0.50 D were: 100% (⩽7 D group), 74.5% (7–15 D group), and 36.4% (>−15 D group).
Table 2.
Predictability of manifest refraction attempted vs. achieved stratified by preoperative MRSE.
| Predictability | Myopia ⩽ −7 | Myopia −7.0 to −15 | Myopia > −15 |
|---|---|---|---|
| ±0.50 | 5/5(100%) | 35/47(74.5%) | 4/11(36.4%) |
| ±1.00 | 0/5(0.00%) | 7/47(89.4%) | 2/11(54.6%) |
| ±2.00 | 0/5(0.00%) | 2/47(93.7%) | 1/11(63.7%) |
| ±3.00 | 0/5(0.00%) | 2/47(98% | 2/11(81.9%) |
| ±5.00 | 0/5(0.00%) | 1/47(100%) | 2/11(100%) |
BSCVA
Mean improvement in postoperative BSCVA was 1 line compared to preoperatively. At the last postoperative visit, 64.6% of eyes had a BSCVA of 20/20 or better compared with only 31.9% preoperatively (Fig. 5). BSCVA of 20/30 or better improved from 79.7% preoperatively to 95.4% at the last postoperative visit. Postoperative BSCVA was higher than or equal to preoperative BSCVA in 95.4% eyes. The proportion of eyes that presented with preoperative BSCVA < 20/20 with an increase in postoperative BSCVA ⩾ 20/20 was 32.3%. This outcome was statistically significant (P = 0.0000471). There was increase of ⩾2 lines in 20% of eyes and one eye (1.5%) lost ⩾2 lines of BSCVA at the last postoperative visit (Fig. 6).
Figure 5.
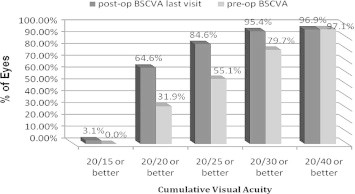
Preoperative (pre-op) versus last visit postoperatively best spectacle-corrected visual acuity (BSCVA) for 69 eyes that underwent implantable collamer lens surgery for myopia.
Figure 6.
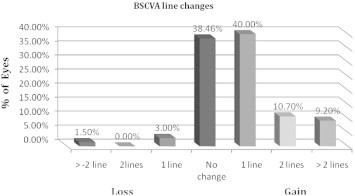
Preoperative versus last visit postoperative change in best spectacle-corrected visual acuity (BSCVA) for 69 eyes that underwent implantable collamer lens surgery for myopia.
Loss of ⩾2 lines of BSCVA occurred in a 34-year-old female with −11.75 D of myopia and 20/30 BSCVA preoperatively. Postoperative examination indicated a decentred ICL with low vault (80 um) that developed an anterior subcapsular catatract. On the last visit her BSCVA was 20/60. Two eyes (3%) lost 1 line of BSCVA 6 months or later. No eyes had vision worse than 20/100 preoperatively or at any postoperative visit.
Complications
Surgical complications
The ICL haptic was torn during surgery in three cases (4.3%). In one additional case (1.4%) the ICL cracked during surgery. None of these cases had a loss of BSCVA. No other significant surgical complications occurred.
Postoperative complications and subjective symptoms
Postoperative complications are presented in Table 3. ICLs were decentred in 3 eyes (4.34%). ICL decentration in 2 eyes was correctable with spectacles, both of these eyes had low vault and developed anterior subcapsular cataract, one eye lost >2 lines of preoperative BSCVA while the other maintained BSCVA. One eye required repositioning of the ICL which increased BSCVA to 20/30 at the last visit compared to preoperative BSCVA of 20/40.One eye with Off axis toric ICL implantation, required repositioning of the ICL. UCVA improved to 20/40 at the last visit from 20/80 one month postoperatively.
Table 3.
Complications in eyes that underwent implantable collamer lens surgery for myopia.
| Complication | Number of eyes (%) | Comment |
|---|---|---|
| ICL decentration | 3(4.34) | 1 eye ICL reposition required, 2 eyes need spectacles |
| Off axis implantation of Toric ICL | 1(1.4) | ICL reposition required |
| Cataract(ASCC) | 3(4.34) | 2 eyes no treatment,1eye ICL removal, Phaco + PC IOL |
| Cyclodialysis cleft | 1(1.4) | ICL removal, Phaco + PC IOL |
| Pupillary block | 4(5.8) | 2 eyes ICL removed,1eye surgical PI,1eye yag-laser PI required |
| Shallow AC with iridocorneal touch | 1(1.45) | ICL removal |
| Incorrect ICL power | 1(1.45) | ICL replacement |
ASCC = anterior subcapsular cataract.
One eye, in a 24-year-old patient, developed a hypotony with shallowing of the anterior chamber first day postoperative. (high possibility of cyclodialysis cleft). The AC reformed with an air bubble. The eye was also treated with atropine drops and topical steroid was stopped. Two weeks later IOP build-up was seen, AC became deep and anterior subcapsular cataract developed. The ICL was removed and phacoemulsification with implantation of an AcrySof IOL (Alcon Inc., Fort Worth, TX, USA) in the bag, The IOL implantation was uneventful and at 6 months postoperatively, the BSCVA returned to 20/20.
One eye (1.45%), developed a shallow anterior chamber with iridocorneal touch that required ICL removal at 17 days postoperatively and reimplanted with a smaller diameter ICL (11.5–11 mm) at 5 months after initial surgery. The UCVA was 20/30 at the last visit. One eye (1.45%), with incorrect ICL power was replaced with no loss of BSCVA.
Fourteen patients (20.3%) complained of (halo, glare, diplopia…etc.) postoperatively.
Intraocular pressure
The mean intraocular pressure (IOP) increased statistically significantly from 16.16 ± 2.6 mmHg preoperatively to 21.93 ± 7.45 mmHg 4 h postoperatively (P = 0.001) (Fig. 7). At 1 day postoperatively, the mean IOP was 16.46 ± 9.12 mmHg (P > 0.05). The mean IOP was 16.10 ± 2.6 mmHg at the last postoperative visit (P > 0.05). At 1st day, 10 (14.49%) eyes had high IOP. Pupillary block glaucoma occurred in 4 (5.8%) eyes within the first postoperative day, all of which resolved with secondary surgical intervention. One of the secondary surgical interventions was additional yttrium–aluminum–garnet laser iridotomy to enlarge an existing iridotomy site. Two eyes required ICL removal, and there were two surgical iridectomies. The IOP was controlled with pressure lowering medication in 6 (8.6%) eyes. The IOP remained within normal limits in all eyes after secondary surgical or medical intervention. No patients in this study required long-term glaucoma medication, and there was no significant late elevation of IOP after implantation of the ICL for myopia.
Figure 7.
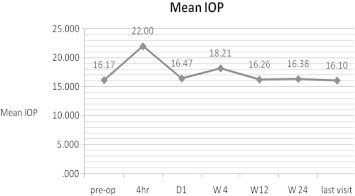
The change in mean intraocular pressure at 4 h (33 eyes) 1 day (67 eyes), 4 week (63 eyes), 3 months (34 eyes), 6 months (36 eyes), last visit (65 eyes).
Endothelial cell density (cells per square millimeter)
Table 4 shows the endothelial cell density. The preoperative endothelial cell density was significantly greater than at the last postoperative visit (P < 0.0001), which implies the expected endothelial cell loss due to surgery.
Table 4.
Endothelial cell density (cells/mm2).
| Exam | Mean ± SD | Eyes | % Loss | P value |
|---|---|---|---|---|
| Preoperative | 2784.7 ± 301.5 | 54 | – | <0.0001 |
| Postoperative | 2614 ± 336.4 | 54 | 5.6% |
When endothelial cell loss was represented as a percentage of the preoperative value, the mean loss was 5.6% (range, 0.15–26.23%).
Discussion
Phakic IOLs are an effective treatment for the correction of myopia and have significant advantages such as reversibility, immediate correction, stability, and relative simplicity. However, some studies describe complications with these lenses.
UCVA, the primary efficacy variable for this study and most refractive surgeries, showed significant improvement over preoperative values. For example, UCVA improved by 12 lines by the last postoperative visit. Additionally vision recovered quickly, with UCVA improving by the first postoperative week and remaining stable throughout the follow-up period. This outcome supported the value of the implantable lens concept. UCVA of 20/40 or better increased from 0% of eyes preoperatively to 86.2% at the last postoperative visit. The proportion of eyes with 20/20 or better also showed a substantial improvement, increasing from 0% preoperatively to 49.20% at the last postoperative visit. These results are similar or better than those reported in previous ICL studies. Vukich et al.,21 reported the proportion of eyes 20/20 or better was 60.1% at 1 year compared to our study (49.2%). Vukich et al.,21 reported (92.5%) of eyes with 20/40 or better, whereas Menezo et al.28 (76.19%), Fernandez et al.24 (44.4%) and Uusitalo et al.25 (52.6%) reported lower UCVA than our study (86.2%) (Table 5).
Table 5.
Summary of safety and effectiveness of previous publications and the current study for implantable collamer lens.
| Parameter procedure | Our study | Vukich et al. | Sander et al. | Lackner | Uusitalo et al. | Fernandez et al. | Menezo et al. |
|---|---|---|---|---|---|---|---|
| Type of study | Retrospective | Prospective | Prospective | Prospective | Prospective | Retrospective | Prospective |
| Myopia range(D) | −4.75 to −25.25 D SE | −3.00 to −20.00 D | −3.00 to −20.00 D | −5.5 to−33.4 D | −7.75 to −29.00 D | −10 to −21.25 D | −11.5 to −28.0 D |
| Mean preoperative myopia | −11.70 ± 4.24 D | −10.046 D | −9.36 ± 2.66 D | −16.5 ± 5.6 D | −15.10 D | −15.27 ± 3.47 D | −16.00 ± 5.05 D |
| Follow-up | 12.35 ± 6.13 months | 16 month | 12 month | 24.0 ± 11.5 month | 13.6 months | 26.6 months ± 11.3 (SD) | 18.3 months |
| Number of cases | 69 | 523 | 210 | 76 | 38 | 18 | 21 |
| Mean postoperative myopia | −0.69 ± 1.13 D | −0.49 ± 0.984 D | 0.05 ± 0.46 D | −1.2 ± 2.0 D | −2.00 ± 2.48 D | −0.62 ± 0.81 D | −1.51 ± 1.11 D |
| Predictability | |||||||
| ±0.50 D | 69.8% | 61.6% | 76.9% | 49.2% | 71.1% | 22.2% | |
| ±1.00 D | 84.1% | 84.7% | 97.3% | 67.7% | 81.6% | 56.9% | NR |
| ±2.00 D | 89% | 96.7% | 100% | 89.2% | NR | NR | |
| UCVA | |||||||
| 20/40 or better | 86.2% | 92.5% | 96% | NR | 52.6% | 44.4% | 76.19% |
| 20/20 or better | 49.2% | 60.1% | 83.1% | 5.3% | 5.5% | 0(0%) | |
| BSCVA | |||||||
| 20/20 or better | 61.6% | 82.4% | 97% | NR | 23.9% | NR | NR |
| 20/40 or better | 97% | 98.1% | 99% | 63.2% | |||
| BSCVA | |||||||
| Loss of ⩾2lines | 1(1.5%) | 1(1.3%) | 3(1.6%) | 1(1.3%) | 0(0%) | 5.5%))1 | 0(0%) |
| Gain ⩾ 2 lines | 13(20%) | 41(9.6%) | 19%)) | NR | 13 (40.6%) | (33.3%) | 10(47.6%) |
BSCVA = best spectacle-corrected visual acuity; D = diopter; SE = spherical equivalent; NR = not reported; UCVA = uncorrected visual acuity.
In our study, a modified PPC IOL, the ICL, effectively reduced high myopia. We found a different level of efficacy than in other published studies. Limitations in predictability are partly related to the selection of the phakic IOL power based on spectacle refraction. It is well known that refraction may be less reliable in eyes with more extreme levels of myopia. In our study, 69.8% of eyes had an SE within ± 0.50 D of emmetropia and 84.1% had an SE within ± 1.00 D. Vukich et al.21 reported 61.6% of eyes with an SE within ±0.50 D and 84.7% within ±1.00 D, Zaldivar et al.33 report 69% ± 1.00 D and 44% within ±0.50 D, Assetto et al.34 reported 31% within ±1.00 D, and Pesando and coauthors30 report 52.53% within ±1.00 D, while Uusitalo et al.25 and Fernandez et al.24 report 71.1% and 22.2% within ±0.50 D and 81.6% and 56.9% within ±1.00 D, respectively. The wide range of outcomes indicates the need for greater accuracy in ICL power calculations. One alternate is combining phakic IOL implantation with laser in situ keratomileusis or photorefractive keratectomy (bioptics) to improve the final visual outcome.46–48 In our study no eye underwent bioptics for residual refractive error.
Sanders et al.35 reported an improvement in BSCVA after ICL implantation with no accompanying intraoperative or postoperative complications. Preservation of BSCVA, commonly considered the primary criterion for assessing the safety of a refractive surgical procedure, was extremely high in the study cohort presented in this article. Not only maintenance but also an improvement in BSCVA (20/20 or better) was achieved at last visit (64.6%) compared with preoperative levels (31.9%). Only one eye (1.5%) lost more than 2 lines of BSCVA postoperatively. This loss of BSCVA was due to a decentered lens with low vault (80um) that developed and anterior subcapsular cataract. At the last visit her BSCVA was 20/60. Previously published ICL reports have also documented an improvement in BSCVA after ICL implantation. BSCVA was maintained or improved in all eyes in the series published by Gonvers et al,27 Menezo et al.28 and Pesando et al.30 whereas only one eye lost BSCVA in studies by Assetto et al34 and Zaldivar et al.33 Loss of BSCVA (⩾2 lines) in the current study occured in 1 eye (1.5%) for all postoperative visits which was similar to Sanders et al23 who reported three cases (1.6%) that lost ⩾2 lines of BSCVA at 1 year postoperatively. Vukich and coauthors21 reported 7 eyes (1.3%) lost ⩾2 lines of BSCVA 6 months or later. According to our results, PPC IOL (ICL) implantation appears to be safe; BSCVA improved 2 lines in 10.7% of eyes and more than 2 lines in an additional 9.2% of eyes at the last postoperative visit. This gain could be the result of postoperative magnification of the retinal image by eliminating the spectacle-induced minification experienced by high myopes.
Furthermore, as previously reported in the literature, the safety of the ICL procedure is enhanced by the low incidence of postoperative and intraoperative complications.33,35,36 Secondary surgeries, adverse events, and surgical complications, as anticipated, were rare in our study. Intraoperatively there were only 3 cases (4.3%) with haptic tear and only 1 case (1.4%) with a cracked optic during ICL insertion, which could be related to the surgical learning curve.
The complications observed after ICL implantation occurred during the early postoperative period, with an absence of any long-term complications. Postoperative complications included one eye with hypotony and flat anterior chamber with high possiblility of cyclodialysis cleft day one postoperatively which was not previously reported, one with a shallow anterior chamber with iridocorneal touch which was treated by replacing the ICL with a smaller diameter ICL. These complications occurred during the early phase of our experience with ICL implantation at our institute and data from trainees are included in this study. Hence, the surgical learning curve might have been a factor in these complications.
Acute elevation in IOP that required secondary surgical intervention occurred in 4 eyes (5.8%) in the early postoperative period due to small or insufficient laser iridotomies or high vault. These were managed effectively by laser iridotomies, surgical iridectomies or ICL removal due to high vault with shallow anterior chamber. All eyes of elevated IOP returned to normal range after surgical or medical intervention. Of note, no patients in the study cohort required long-term glaucoma medication, and there was no significant late elevation in IOP after implantation of the ICL for myopia. Vukich et al21, reported that 21 eyes (4.0%) required secondary surgical intervention for management of acute pressure rise one month postoperatively. In a report by Zaldivar et al33 of 124 eyes ICL implantation, 14 eyes had IOP spikes greater than 26 mmHg. Of the 6 eyes with pupillary block glaucoma, 4 eyes had not received peripheral iridotomies preoperatively. The iridotomies in the other 2 cases were presumably closed.33 The 6 eyes were treated by postoperative laser iridotomies, but the timing of these postoperative spikes was not provided.33 Fernandez et al24 reported 2 of 18 eyes (11.1%) developed pupillary block with an IOP of 40–50 mmHg at one day postoperatively. In both eyes, 1 of the iridotomies was too small.24 After the iridotomies were enlarged, the IOP returned to preoperative values.24 Uusitalo et al25, reported that pupillary block glaucoma caused by closure or insufficiency of the laser iridotomies requiring surgical intervention occurred in 3 eyes (7.9%) within the first 2 days postoperatively.
Cataract formation is a potential complication in any intraocular procedure. In our study, there were 3 eyes with anterior subcapsular cataracts (4.34%). Two eyes had low vault, while the third case developed hypotony, flat AC with high possibility of cyclodialysis cleft that led to the development of visually significant anterior subcapsular cataract. In the third eye, we elected to explant the ICL and extract the lens with PC IOL implantation and the postoperative BSCVA was 20/20.
Evaluation of ICL-induced cataracts is difficult for several reasons. First, any trauma to the crystalline lens during laser iridotomy or surgical implantation must be ruled out, which we cannot exclude due to the retrospective nature of our study. Secondly a cataract can develop over a long period of time. Gonvers et al42 found that the rate of anterior subcapsular cataracts increased with the duration of follow-up. They found it was 27% (20 of 75 eyes) at one year or more and 33% at 24 months or more, and 38% at 30 months or more.42 This delay between implantation and the development of a cataract may explain why most studies with a short follow-up do not report this complication.32,34,35 In our series with short follow-up (minimum 6 month), we found a rate of 4.34%. Longer term follow-up may be necessary to fully evaluate the real risk of ASCC. Over a span of 3 years, Zaldivar et al37 found an 8.5% rate of cataract development with different generations of ICLs, and in a series of 12 ICLs, Menezo and coauthors28 report 3 anterior subcapsular cataracts occurring at 19, 20, and 21 months, respectively. Vukich et al.,21 reported 14 eyes (2.7%) of ASCCs, 12 eyes (1.9%) occurring early (⩽90 days postoperatively) and 2 eyes (0.40%) occurring late (⩾1 year postoperatively). Sander et al.41 studied 526 eyes, which were followed for an average of 4.7 years to evaluate the incidence of asymptomatic and clinically significant anterior subcapsular opacities and found that approximately 6–7% of eyes develop anterior subcapsular opacities at 7 + years following ICL implantation but only 1% to 2% progress to clinically significant cataract during the same period, especially very high myopes and older patients. Galeana et al43 reported an 8% (14 of 170 eyes) incidence of lens opacities after ICL implantation. Lackner and coauthors44 found that the lens opacifications developed in 25 of 75 eyes (33.3%), 14 eyes(18.7%) showed progressive opacification, and 11 eyes (14.7%) remained stable with 8 eyes (10.7%) requiring cataract surgery. Sanders and Vukich38 compared the STAAR V3 ICL to the V4 lens, which has a more anterior vault. They found significantly fewer anterior subcapsular opacities with the V4 lens (2.9% vs 12.6% with the V3 lens). The older model (V3) was associated with greater late opacities (appearing later than 1 year postoperatively) (9.2% with the V3 vs 0.6% with the V4).
The latest generation of myopic ICLs (model V4) is presumed to completely vault the anterior crystalline lens capsule and to rest on the anterior zonular fibers. This, of course, necessitates correct selection of the overall size of the ICL, which ranges from 11.5 to 13.5 mm. To date, the ICL size can only be estimated and is considered to be appropriate when equal to the horizontal diameter of the cornea (white-to-white distance) plus 0.5 mm. In our study, different methods (Caliper, Orbscan, UBM) were used to measure white-to-white distance. Further studies are required to determine the most accurate method of measuring the horizontal corneal diameter.
It is well known that any intraocular surgical procedure that affects the anterior segment provokes endothelial cell loss, and the decrease of endothelial density is proportional to the surgical time and manipulation.39 Thus one of the main parameters used to evaluate the safety of an anterior segment surgical technique is the endothelial cell density. In our study, the endothelial cell density loss at the last postoperative visit was 5.6%, the progressive cell loss cannot be determined in our study due to the retrospective nature of this study and the single postoperative measurement done at the last visit. Also the long-term effect the ICL on the corneal endothelium requires longer follow-up. Ruhswurm et al40 reported continuous endothelial cell loss after ICL implantation during a 4-year follow-up, with mean endothelial cell loss preoperatively of 1.8% at 3 months, 4.2% at 6 months, 5.5% at 12 months, 7.9% at 2 years, 12.9% at 3 years, and 12.3% at 4 years. Other endothelial cell characteristics remained stable throughout the duration of follow-up. The investigators concluded there was rapid cell loss until 1 year postoperatively, after which the rate of loss was no longer statistically significant. Jimenez-Alfaro et al.45 found the majority of cell loss (4.41%) occurred at 6 months postoperatively. Fernandez et al24 found the endothelial cell density loss at 6 months was 4.91% and did not progress over time, suggesting that the initial loss was related to surgical trauma. Arne49 found that the mean postoperative endothelial cell loss was 2.1% at 3 months, 2.3% at 6 months, 2.0% at 1 year, and 2.0% at 2 years. The variable outcomes indicate that long-term follow up is required to evaluate endothelial cell loss accurately.
In conclusion as the follow-up in our study was relatively short, we cannot be certain of the long-term safety of this procedure. We have demonstrated excellent predictability, efficacy, and good visual results with few short-term complications. Hypotony with very shallow AC with high possibility of Cyclodialysis cleft and cataract formation is the most severe complication we have experienced, and we believe long term studies are warranted to determine the long-term safety of ICL implantation.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Verzella F. High myopia: in-the-bag refractive implantation. Ophthalmic Forum. 1985;3:174–175. [Google Scholar]
- 2.Praeger D.L. Phakic myopic intraocular lens—an alternative to kerato-lenticulorefractive procedures (editorial) Ann Ophthalmol. 1988;20:246. [PubMed] [Google Scholar]
- 3.Werblin T.P. Should we consider clear lens extraction for routine refractive surgery? Refract Corneal Surg. 1992;8:480–481. [PubMed] [Google Scholar]
- 4.Goldberg M.F. Clear lens extraction for axial myopia; an appraisal. Ophthalmology. 1987;94:571–582. doi: 10.1016/s0161-6420(87)33425-6. [DOI] [PubMed] [Google Scholar]
- 5.Fechner P.U., Strobel J., Wichmann W. Correction of myopia by implantation of a concave Worst-iris claw lens into phakic eyes. Refract Corneal Surg. 1991;7:286–298. [PubMed] [Google Scholar]
- 6.Praeger D.L., Momose A., Muroff L.L. Thirty-six month follow-up of a contemporary phakic intraocular lens for the surgical correction of myopia. Ann Ophthalmol. 1991;23:6–10. [PubMed] [Google Scholar]
- 7.Fechner P.U., Wichmann W. Correction of myopia by implantation of minus optic (Worst iris claw) lenses into the anterior chamber of phakic eyes. Eur J Implant Refract Surg. 1993;5:55–59. [Google Scholar]
- 8.Baikoff G., Joly P. Comparison of minus power anterior chamber intraocular lenses and myopic epikeratoplasty in phakic eyes. Refract Corneal Surg. 1990;6:252–260. [PubMed] [Google Scholar]
- 9.Stephenson C.G., Gartry D.S., O’Brart D.P.S. Photorefractive keratectomy; a 6-year follow-up study. Ophthalmology. 1998;105:273–281. doi: 10.1016/s0161-6420(98)93055-x. [DOI] [PubMed] [Google Scholar]
- 10.Tuunanen T.H., Tervo T.T. Results of photorefractive keratectomy for low, moderate, and high myopia. J Refract Surg. 1998;14:437–446. doi: 10.3928/1081-597X-19980701-10. [DOI] [PubMed] [Google Scholar]
- 11.Kim J.H., Kim M.S., Hahn T.W. Five year results of photorefractive keratectomy for myopia. J Cataract Refract Surg. 1997;23:731–735. doi: 10.1016/s0886-3350(97)80282-9. [DOI] [PubMed] [Google Scholar]
- 12.Pérez-Santonja J.J., Bellot J., Claramonte P. Laser in situ keratomileusis to correct high myopia. J Cataract refract Surg. 1997;23:372–385. doi: 10.1016/s0886-3350(97)80182-4. [DOI] [PubMed] [Google Scholar]
- 13.Knorz M.C., Wiesinger B., Liermann A. Laser in situ keratomileusis for moderate and high myopia and myopic astigmatism. Ophthalmology. 1998;105:932–940. doi: 10.1016/S0161-6420(98)95040-0. [DOI] [PubMed] [Google Scholar]
- 14.Knorz M.C., Liermann A., Seiberth V. Laser in situ keratomileusis to correct myopia of –6.00 to –29.00 diopters. J Refract Surg. 1996;12:575–584. doi: 10.3928/1081-597X-19960701-09. [DOI] [PubMed] [Google Scholar]
- 15.Colin J., Robinet A., Cochener B. Retinal detachment after clear lens extraction for high myopia; seven-year follow-up. Ophthalmology. 1999;106:2281–2284. doi: 10.1016/S0161-6420(99)90526-2. [discussion by M Stirpe, 2285] [DOI] [PubMed] [Google Scholar]
- 16.Drews R.C. Risk benefit analysis of anterior chamber intraocular lenses for the correction of myopia in phakic patients. Eur J Implant Refract Surg. 1991;3:171–194. [Google Scholar]
- 17.Huber C. Effectiveness of intraocular lens calculation in high ametropia. J Cataract Refract Surg. 1989;15:667–672. doi: 10.1016/s0886-3350(89)80034-3. [DOI] [PubMed] [Google Scholar]
- 18.Barraquer J. Anterior chamber plastic lenses. Results and conclusions from five years’ experience. Trans Ophthalmol Soc UK. 1959;79:393–424. [PubMed] [Google Scholar]
- 19.Fyodorov S.N., Zuyev V.K., Aznabayev B.M. Intraocular correction of high myopia with negative posterior chamber lens [Russian] Oftalmokhirurgiia. 1991;3:57–58. [Google Scholar]
- 20.Brauweiler P.H., Wehler T., Busin M. High incidence of cataract formation after implantation of a silicone posterior chamber lens in phakic, highly myopic eyes. Ophthalmology. 1999;106:1651–1655. doi: 10.1016/S0161-6420(99)90352-4. [DOI] [PubMed] [Google Scholar]
- 21.The implantable contact lens in Treatment of Myopia (ITM) Study Group U.S. Food and Drug Adminstration clinical trial of the implantable contact lens for moderate to high myopia. Ophthalmology. 2003;110:255–266. doi: 10.1016/s0161-6420(02)01771-2. [DOI] [PubMed] [Google Scholar]
- 22.ICL in Treatment of Myopia (ITM) Study Group U.S. Food and Drug Administration clinical trial of the implantable contact lens for moderate to high myopia. Three-year follow-up. Ophthalmology. 2004;111:1683–1692. doi: 10.1016/j.ophtha.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 23.Sanders D.R., Schneider D., Martin R., Brown D., Dulaney D., Vukich J. Toric implantable collamer lens for moderate to high myopic astigmatism. Ophthalmology. 2007;114:54–61. doi: 10.1016/j.ophtha.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 24.Pineda-Fernández A., Jaramillo J., Vargas J., Jaramillo M., Jaramillo J., Galíndez A. Phakic posterior chamber intraocular lens for high myopia. J Cataract Refract Surg. 2004;30:2277–2283. doi: 10.1016/j.jcrs.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 25.Uusitalo R.J., Aine E., Sen N.H., Laatikainen L. Implantable contact lens for high myopia. J Cataract Refract Surg. 2002;28:29–36. doi: 10.1016/s0886-3350(01)01218-4. [DOI] [PubMed] [Google Scholar]
- 26.Gimbel H.V., Ziemba S.L. Management of myopic astigmatism with phakic intraocular lens implantation. J Cataract Refract Surg. 2002;28:883–886. doi: 10.1016/s0886-3350(01)01098-7. [DOI] [PubMed] [Google Scholar]
- 27.Gonvers M., Othenin-Girard P., Barnet C., Sickenberg M. Implantable contact lens for moderate to high myopia: short-term follow-up of 2 models. J Cataract Refract Surg. 2001;27:380–388. doi: 10.1016/s0886-3350(00)00759-8. [DOI] [PubMed] [Google Scholar]
- 28.Menezo J.L., Peris-Martinez C., Cisneros A., Martinez-Costa R. Posterior chamber phakic intraocular lenses to correct high myopia: a comparative study between STAAR and Adatomed models. J Refract Surg. 2001;17:32–42. doi: 10.3928/1081-597X-20010101-04. [DOI] [PubMed] [Google Scholar]
- 29.Arne J.L., Lesueur L.C. Phakic posterior chamber lenses for high myopia: functional and anatomical outcomes. J Cataract Refract Surg. 2000;26:369–374. doi: 10.1016/s0886-3350(99)00417-4. [DOI] [PubMed] [Google Scholar]
- 30.Pesando P.M., Ghiringhello M.P., Tagliavacche P. Posterior chamber Collamer phakic intraocular for myopia and hyperopia. J Refract Surg. 1999;15:415–423. doi: 10.3928/1081-597X-19990701-05. [DOI] [PubMed] [Google Scholar]
- 31.Brown D.C., Grabow H.B., Martin R.G. Staar collamer intraocular lens: clinical results from the phase I FDA core study. J Cataract Refract Surg. 1998;24:1032–1038. doi: 10.1016/s0886-3350(98)80095-3. [DOI] [PubMed] [Google Scholar]
- 32.Rosen E., Gore C. Staar collamer posterior chamber phakic intraocular lens to correct myopia and hyperopia. J Cataract Refract Surg. 1998;24:596–606. doi: 10.1016/s0886-3350(98)80253-8. [DOI] [PubMed] [Google Scholar]
- 33.Zaldivar R., Davidorf J.M., Oscherow S. Posterior chamber phakic intraocular lens for myopia of −8 to −19 diopters implantation. J Refract Surg. 1998;14:294–305. doi: 10.3928/1081-597X-19980501-13. [DOI] [PubMed] [Google Scholar]
- 34.Assetto V., Benedetti S., Pesando P. Collamer intraocular contact lens to correct high myopia. J Cararact Refract Surg. 1996;22:551–556. doi: 10.1016/s0886-3350(96)80007-1. [DOI] [PubMed] [Google Scholar]
- 35.Sanders D.R., Brown D.C., Martin R.G. Implantable contact lens for moderate to high myopia: phase I FDA clinical study with 6 month follow-up. J Cataract Refract Surg. 1998;24:607–611. doi: 10.1016/s0886-3350(98)80254-x. [DOI] [PubMed] [Google Scholar]
- 36.Kaya V., Kevser M.A., Yilmaz O.F. Phakic posterior chamber plate intraocular lenses for high myopia. J Refract Surg. 1999;15:580–585. doi: 10.3928/1081-597X-19990901-11. [DOI] [PubMed] [Google Scholar]
- 37.Zaldivar R., Oscherow S., Ricur G. The STAAR posterior chamber phakic intraocular lens. Int Ophthalmol Clin. 2000;40(3):237–244. doi: 10.1097/00004397-200007000-00024. [DOI] [PubMed] [Google Scholar]
- 38.Sanders D.R., Vukich J.A. Incidence of lens opacities and clinically significant cataracts with the Implantable Contact Lens: comparison of two lens designs; the ICL in Treatment of Myopia (ITM) Study Group. J Refract Surg. 2002;18:673–682. doi: 10.3928/1081-597X-20021101-03. [DOI] [PubMed] [Google Scholar]
- 39.Rao G.N., Acquavella J.V., Goldberg S.H., Berk S.L. Pseudophakic bullous keratopathy. Ophthalmology. 1984;91:1135–1140. [PubMed] [Google Scholar]
- 40.Dejaco-Ruhswurm I., Scholz U. Et al.Long-term endothelial changes in phakic eyes with posterior chamber intraocular lenses. J Cataract Refract Surg. 2002;28:1589–159341. doi: 10.1016/s0886-3350(02)01210-5. [DOI] [PubMed] [Google Scholar]
- 41.Sanders D.R., MD, PhD Anterior subcapsular opacities and cataracts 5 years after surgery in the visian implantable collamer lens FDA trial. J Refract Surg. 2008;24:566–570. doi: 10.3928/1081597X-20080601-04. [DOI] [PubMed] [Google Scholar]
- 42.Gonvers M., Bornet C., Othenin-Girard P. Implantable contact lens for moderate to high myopia-relationship of vaulting to cataract formation. J Cataract Refract Surg. 2003;29:918–924. doi: 10.1016/s0886-3350(03)00065-8. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez-Galeana C.A., Smith R.J., Sanders D.R. Lens opacities after posterior chamber phakic intraocular lens implantation. Ophthalmology. 2003;110:781–785. doi: 10.1016/s0161-6420(02)01973-5. [DOI] [PubMed] [Google Scholar]
- 44.Lackner B., Pieh S., Schmidinger G., Hanselmayer G., Dejaco-Ruhswurm I., Funovics M., Skorpik C. Outcome after treatment of ametropia with implantable contact lenses. Ophthalmology. 2003;110:2153–2161. doi: 10.1016/S0161-6420(03)00830-3. [DOI] [PubMed] [Google Scholar]
- 45.Jimenez-Alfaro I. Benitez del Castillo JM, Garcia-Feijoo J, et al. Safety of posterior chamber phakic intraocular lenses for the correction of high myopia: anterior segment changes after posterior chamber phakic intraocular lens implantation. Ophthalmology. 2001;108:90–99. doi: 10.1016/s0161-6420(00)00403-6. [DOI] [PubMed] [Google Scholar]
- 46.Zaldivar R., Davidorf J.M., Oscheo S. Combined posterior chamber phakic intraocular lens and laser in situ keratomileusis: bioptics for extreme myopia. J Refract Surg. 1999;15:299–308. doi: 10.3928/1081-597X-19990501-04. [DOI] [PubMed] [Google Scholar]
- 47.Sánchez-Galeana C.A., Smith R.J., Rodríguez X. Laser in situ keratomileusis and photorefractive keratectomy for residual refractive error after phakic intraocular lens implantation. J Refract Surg. 2001;17:299–304. doi: 10.3928/1081-597X-20010501-02. [DOI] [PubMed] [Google Scholar]
- 48.Gu¨ell J.L., Vazquez M., Gris O. Combined surgery to correct high myopia: iris claw phakic intraocular lens and laser in situ keratomileusis. J Refract Surg. 1999;15:529–537. doi: 10.3928/1081-597X-19990901-05. [DOI] [PubMed] [Google Scholar]
- 49.Arne Jean L. Phakic posterior chamber lenses for high myopia: functional and anatomical outcomes. J Cataract Refract Surg. 2000;26:369–374. doi: 10.1016/s0886-3350(99)00417-4. [DOI] [PubMed] [Google Scholar]



