Abstract
Purpose
To study the pattern of ocular aberrations in amblyopic children, and evaluate a possible relation to etiology and treatment outcomes of amblyopia.
Methods
The WaveScan Wavefront System (AMO, Santa Ana, CA, USA) aberrometer was used to assess 75 eyes (60 children) after instillation of 1% cyclopentolate eyedrops. There were 29 males and 31 females with a mean age of 9.23 ± 2.55 years (range, 5–14 years). The study sample was subdivided into four groups; 16 emmetropic non-amblyopic eyes (control group); 24 pre-treatment newly diagnosed amblyopic eyes; 16 eyes of treated amblyopes and; 19 eyes with refractory amblyopia.
Results
Amblyopes had statistically significant greater root mean square (RMS) values for whole eye aberrations, 2nd order aberrations, defocus () and astigmatism () compared to emmetropes (P < 0.0001). The refractory amblyopic group showed statistically significant differences in whole eye RMS, 2nd order- aberrations, defocus () and astigmatism () when compared to treated amblyopic groups (P < 0.0001). Apart from a statistically significant difference in 5th order RMS of pre-treated amblyopes versus the control group, no other significant differences were found in higher order aberrations (HOAs: coma, spherical, higher-order astigmatism, trefoil, or 3rd, 4th, 5th or 6th order terms) between emmetropes and any of the amblyopic groups.
Conclusion
Lower order aberrations remain the major factor that affect retinal image quality and hence amblyopia development especially in ametropic eyes. This can be corrected optically. Studying HOA profile in amblyopic eyes failed to explain why refractory amblyopia does not respond to orthoptic treatments. This outcome indicates that theories of central problems in image processing and binocular interaction are likely the main cause of refractory amblyopia.
Keywords: Ocular aberrations, Amblyopia, Wavefront optics, Zernike polynomials
Introduction
Amblyopia is the most common cause of monocular visual impairment in children, with a prevalence of 1.6–3.6% that is higher in medically underserved populations.1–4 It has been related to unequal foveal stimulation at an early age due to form vision deprivation, strabismus or refractive error.2,5,6 The deficit in amblyopia is thought to be cortical in nature, but abnormalities have also been found in the lateral geniculate bodies and in the retina.1,7–13
Amblyopia can be successfully treated in subjects up to 10 years of age.5 However, even if visual acuity returns to normal after successful treatment, several studies have reported that the treated eye as well as the fellow eye behaves abnormally when evaluated for a variety of functions which include reduction in visual acuity, contrast sensitivity and position acuity.1,14–18
Visual acuity (VA) alone does not reflect the visual performance of the amblyopic eye. Studies have shown that VA testing using conventional optotypes is insensitive for detecting subtle defects in visual function.19 The advent of new technology has introduced new tests for evaluating foveal function especially in amblyopic children. These include the subjective visual acuity tests such as TriVA-test and the rarebit fovea test (RFT). However, their dependence on the psychophysical interaction of the children with their short attention span limits their actual benefits.19 Alternately, wavefront aberrometry is a relatively new diagnostic tool used globally to measure ocular aberrations and to study the objective visual performance of human eyes including amblyopic eyes.20
Wavefront aberrations (ocular as well as optical aberrations) referred to the deviation of light, as it enters the eye compared to optically perfect eye, resulting in blurred images and decreased visual performance.21,22 Aberrations may be subdivided into low order aberrations, which can be corrected by sphero-cylindrical lenses, and higher order aberrations, which cannot.23
In the present study we evaluate the ocular aberrations of amblyopic children and test for an association to amblyopia, to further understand the possible etiologies of amblyopia and explain possible outcomes of traditional amblyopia treatment. Photorefractive procedures have been used as a promising treatment of anisometropic amblyopes,20,24,25 yet aberration free custom ablation remains controversial as to whether they can control the remaining portion of refractory amblyopia.
Subjects and methods
Subjects
This prospective, single center, cross sectional study was conducted at ALHOKAMA eye specialist center in Riyadh city, Kingdom of Saudi Arabia, from February–May 2011. The study received the approval of the ethical and scientific committee of the institution and was performed in accordance to the tenets of the Declaration of Helsinki. Each of the participating patients and his/her parents underwent a thorough informed consent procedure that explained the study protocol, investigations and the observational nature of the study. All the parents were required to sign a written consent for participation in the study.
Sixty children (75 eyes) with a mean age of 9.23 ± 2.55 years (range, 5–14 years) were included in the study. They were 29 males and 31 females. The subjects are divided into two main groups; a control group (16 eyes), whose refractive error was between −0.50 and +0.50 DS, and an amblyopic group (59 eyes) which was further sub-divided into three sub-groups as described below:
-
a.
Pre-treated amblyopic group (24 eyes), of newly diagnosed amblyopic children whose best corrected visual acuity with Snellen E letter acuity chart was ⩽ 0.7, and refractive error (RE) range was: sphere:+9.00 to −7.00 D, cylinder: −0.75 to −4.50 D.
-
b.
Treated amblyopic group (16 eyes), of children whose best corrected visual acuity was ⩾0.9 after treatment for one year. The range of RE was; sphere:+7.00 to −10.00, cylinder: −0.75 to −3.00.
-
c.
Refractory amblyopic group (19 eyes), of children whose best corrected visual acuity was no better than 0.8 after full standard optical and orthoptic treatment for more than one year (average of 18 ± 3 months). The range of RE was; sphere:+8.00 to −9.00, cylinder: −0.50 to −4.00 (Table 1).
Table 1.
Comparison of optical characteristics of different groups.
| Variables | Control (mean ± SD) | PAG⁎ (mean ± SD) | TAG† (mean ± SD) | RAG‡ (mean ± SD) | P-value |
|---|---|---|---|---|---|
| Sphere | 0.2969 ± 0.2085 | 4.656 ± 2.593 | 4.813 ± 2.544 | 6.250 ± 1.805 | <0.0001 |
| Cylinder | 0.2656 ± 0.2135 | 1.646 ± 1.275 | 1.406 ± 0.8459 | 1.934 ± 0.9995 | <0.0001 |
| Sph. Equive§ | 0.3375 ± 0.1974 | 4.023 ± 2.726 | 4.424 ± 2.465 | 6.283 ± 2.198 | <0.0001 |
| BCVA‖ | 1.000 ± 0.000 | 0.6167 ± 0.1373 | 0.9875 ± 0.03416 | 0.6195 ± 0.1588 | <0.0001 |
PAG: pre-treated amblyopic group.
TAG: treated amblyopic group.
RAG: refractory amblyopic group.
Sph. Equive.: spherical equivalent.
BCVA: best corrected visual acuity.
Only children with strabismus and anisometropic amblyopia were included in this study. Those with deprivation amblyopia, history of corneal or lenticular surgery and associated syndromes were excluded.
Methodology
All children underwent a complete eye examination including the assessment of visual acuity (unaided and best corrected visual acuity using Snellen E letter acuity chart), ocular movements, slit-lamp examination, funduscopy and cycloplegic refraction following instillation of topical cyclopentolate 1%.
Higher-order aberrations (HOAs) were measured at 3 and 6 mm pupil diameters with the Wavescan System aberrometer (AMO, Santa Ana, CA, USA) based on the Hartmann–Shack principle. This aberrometer measures the monochromatic aberrations of the entire eye. Measurements were performed in a dark room after ciliary muscle paralysis by using cyclopentolate (1%) eye drops. The patient was asked to blink once just before the scan and focus on the fixation target. Scans were then performed and the measurements repeated three times for each eye, an average value was calculated for statistical analysis. Image quality, least central displacement and the root mean square (RMS) values were collected.
Statistical analysis
The data were analyzed using INSTAT (GraphPad Software Inc., La Jolla, CA, USA) and Excel (Microsoft Corp., Redmond, WA, USA) statistical software. Differences between groups were analyzed using the unpaired two tailed t-test and a one-way analysis of variance (ANOVA). A P value <0.05 was considered statistically significant.
Results
Comparison of the optical characteristics of the four groups is presented in Table 1. Statistically significant differences were found in best corrected vision, spherical and cylindrical powers (P < 0.05).
Whole eye RMS, 2nd order aberrations, defocus and astigmatism were each statistically significantly different between the four groups (P < 0.05, all comparisons) (Tables 2 and 3).
Table 2.
The RMS (μm) of each aberration parameter for each group.
| Variables | Control (mean ± SD) | PAG⁎ (mean ± SD) | TAG† (mean ± SD) | RAG‡ (mean ± SD) | P-value |
|---|---|---|---|---|---|
| Whole RMS¶ | 0.9288 ± 0.5126 | 7.674 ± 4.330 | 6.554 ± 3.093 | 9.582 ± 2.215 | <0.0001 |
| HOA⁎⁎ RMS | 0.3088 ± 0.1190 | 0.3350 ± 0.1102 | 0.3131 ± 0.1052 | 0.3205 ± 0.1085 | 0.8819 |
| Defocus | 0.6942 ± 0.5532 | 6.957 ± 4.804 | 6.087 ± 3.381 | 9.198 ± 2.403 | <0.0001 |
| Astig. | 0.1610 ± 0.09168 | 0.6950 ± 0.5163 | 0.5552 ± 0.3555 | 0.7985 ± 0.3520 | <0.0001 |
| Coma | 0.1152 ± 0.05071 | 0.1207 ± 0.05133 | 0.1328 ± 0.06220 | 0.1390 ± 0.07120 | 0.6078 |
| Trefoil | 0.09922 ± 0.05493 | 0.09726 ± 0.05025 | 0.09076 ± 0.04132 | 0.08888 ± 0.04824 | 0.9046 |
| Sph. abe | 0.05248 ± 0.03773 | 0.05910 ± 0.04125 | 0.03889 ± 0.02926 | 0.05824 ± 0.03680 | 0.3487 |
| Qudrafoil | 0.04667 ± 0.01984 | 0.03866 ± 0.01958 | 0.04010 ± 0.01835 | 0.04035 ± 0.01857 | 0.6122 |
| Penta | 0.04805 ± 0.02131 | 0.04025 ± 0.02042 | 0.04052 ± 0.02238 | 0.03766 ± 0.01908 | 0.5032 |
| Hexa | 0.04854 ± 0.05229 | 0.02465 ± 0.01290 | 0.02939 ± 0.01826 | 0.02850 ± 0.01380 | 0.0539 |
PAG: pre-treated amblyopic group.
TAG: treated amblyopic group.
RAG: refractory amblyopic group.
RMS: root of mean square.
HOAs: high order aberrations.
Table 3.
Comparison by order between different groups.
| Order | Groups |
||||
|---|---|---|---|---|---|
| Control (mean ± SD) | PAG⁎ (mean ± SD) | TAG† (mean ± SD) | RAG‡ (mean ± SD) | P-value | |
| 2nd order | 0.5441 ± 0.3357 | 4.471 ± 2.394 | 3.838 ± 1.669 | 5.743 ± 1.167 | <0.0001 |
| 3rd order | 0.1666 ± 0.07737 | 0.1851 ± 0.07171 | 0.1807 ± 0.07146 | 0.1882 ± 0.08005 | 0.8415 |
| 4th order | 0.07040 ± 0.02978 | 0.07522 ± 0.03888 | 0.05446 ± 0.02955 | 0.07457 ± 0.03093 | 0.2270 |
| 5th order | 0.04786 ± 0.01538 | 0.03527 ± 0.01426 | 0.04206 ± 0.01573 | 0.03901 ± 0.01707 | 0.0947 |
| 6th order | 0.02685 ± 0.01074 | 0.02351 ± 0.01158 | 0.02540 ± 0.008802 | 0.02721 ± 0.009050 | 0.6371 |
PAG: pre-treated amblyopic group.
TAG: treated amblyopic group.
RAG: refractory amblyopic group.
Comparison of aberrations between individual groups
Each of the three amblyopic groups showed higher whole eye RMS values, 2nd order aberrations, defocus () and astigmatism () compared to the emmetropic (control) group, (P < 0.05 all comparisons) (Figs. 1, 3 and 5). Fifth (5th) order aberrations was statistically different in the pre-treated group only (P = 0.0116) (Fig. 2, Table 4). There were no statistical differences in HOAs between control and pre-treated, treated and refractory amblyopes (Figs. 2, 4 and 6).
Figure 1.
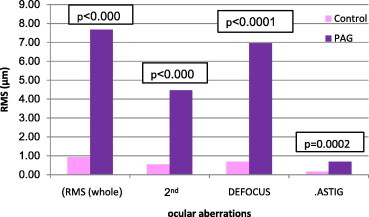
Comparison of whole and low ocular aberrations between control and pre-treated amblyopic group (PAG).
Figure 3.
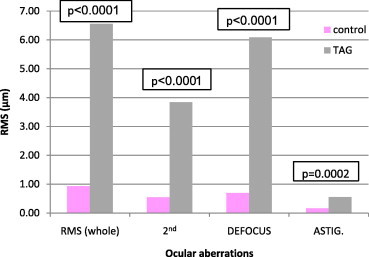
Comparison of whole and low ocular aberrations between control and treated amblyopic group (TAG).
Figure 5.
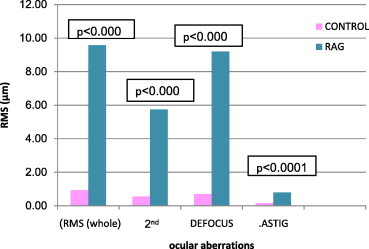
Comparison of whole and low ocular aberrations between control and refractory amblyopic group (RAG).
Figure 2.

Comparison of high ocular aberrations between control and pre-treated amblyopic group (PAG).
Table 4.
Comparison of 5th order aberration between control and amblyopic groups.
| Amblyopic groups 5th order (mean ± SD) | Control group 5th order (mean ± SD) | P-value | |
|---|---|---|---|
| PAG⁎ | 0.03527 ± 0.01426 | 0.04786 ± 0.01538 | 0.0116 |
| TAG† | 0.04206 ± 0.01573 | 0.04786 ± 0.01538 | 0.3001 |
| RAG‡ | 0.03901 ± 0.01707 | 0.04786 ± 0.01538 | 0.1198 |
PAG: pre-treated amblyopic group.
TAG: treated amblyopic group.
RAG: refractory amblyopic group.
Figure 4.
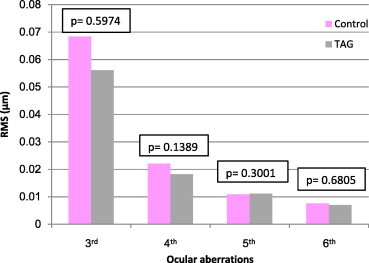
Comparison of high ocular aberrations between control and treated amblyopic group (TAG).
Figure 6.
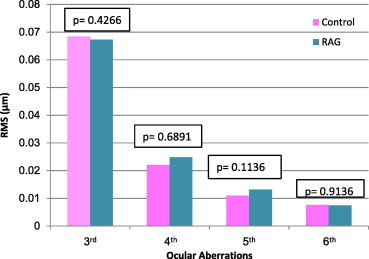
Comparison of high ocular aberrations between control and refractory amblyopic group (RAG).
The aberration values of the refractory amblyopic group were higher than the treated amblyopic group only for whole eye RMS, defocus (), astigmatism () and 2nd order aberrations (P < 0.05, all comparisons) (Figs. 7 and 8). There was a statistically significant difference in 2nd order aberrations between the refractory amblyopic group and the pre-treated amblyopic group (P < 0.05). There were no statistical differences between pre-treated and treated amblyopic groups (P > 0.05).
Figure 7.
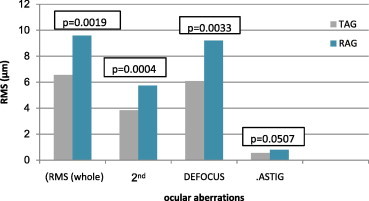
Comparison of whole and low ocular aberrations between treated amblyopes (TAG) and refractory amblyopic group (RAG).
Figure 8.
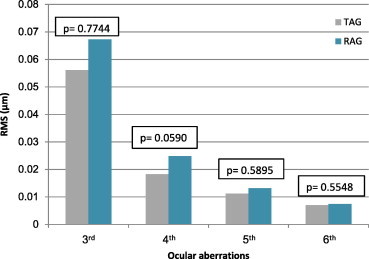
Comparison of high ocular aberrations between treated amblyopic group (TAG) and refractory amblyopic group (RAG).
Discussion
Amblyopia is a reduction in corrected visual acuity in the absence of visible organic abnormalities resulting from strabismus, anisometropia, or form deprivation occurring early in visual development.26 It is the most common cause of visual impairment in both children and middle-aged adults and it increases the risk of visual loss of at least 1.2%.4
High degrees of refractive errors, when present in both eyes, may induce isometropic amblyopia or amblyopia secondary to strabismus. This applies to large differences in refractive error between the two eyes where it may induce anisometropic amblyopia. Likewise, due to their potential role in reducing retinal image quality, HOAs in amblyopic eyes may theoretically play a role in inducing amblyopia (Caitriona, 2008).5
The management and treatment of amblyopia may be difficult especially in older children (>10 years), mainly due to compliance issues. It can be successfully treated in subjects up to 10 years of age (Wu & Hunter 2006).2 However, even if visual acuity returns to normal after successful treatment, several studies have reported that the treated eye as well as the fellow eye behaves abnormally when evaluated for a variety of functions (Simons 2005, Pia Agervi, 2009).1,4
Wavefront aberrations comprise several different components termed lower and higher order aberrations. Lower-order aberrations are composed of defocus (myopia, hyperopia) and astigmatism, which may be corrected by sphero-cylindrical lenses and they are responsible for about 90% of the retinal image quality. The remaining 10% is a combination of the effects of the HOA such as 3rd, 4th, 5th, 6th and 7th orders. Each order is composed of several Zernike polynomials and is expressed as RMS values in micrometers (μm).26,27
Although HOAs make a small contribution to the total variance of the eye’s wavefront aberrations, studies21,31 show the deleterious effect of HOAs on image quality and how correction of HOAs can improve visual performance.
In our study we compared ocular aberrations in emmetropic controls versus three amblyopic groups (pre-treated, treated and refractory amblyopes), the results showed that in amblyopic eyes, the whole RMS, defocus, astigmatism and 2nd order-aberrations were higher than the control group and the differences were statistically significant (P < 0.0001). These findings match well with Peng-fei20et al.’s study (2010) of wavefront aberrations in children with amblyopia. However our results are slightly different from Caitriona et al., in 2008,27 who found that only the RMS of the total aberrations was higher among myopes as compared to hyperopes for amblyopic children (P = 0.005).
In our study, there were no statistically significant differences in HOAs (3rd, 4th and 6th order) between controls and the amblyopic groups. These findings concur with Caitriona et al.28 who did not find a statistically significant difference of 3rd order-aberrations between normal and amblyopic children. In children with idiopathic amblyopia, Gaurav et al7 found no statistically significant differences in HOAs between amblyopes and normal children.
The difference in fifth-order aberration in the current study was statistically significant only when pre-treated amblyopic group was compared to the control group (P = 0.0116). Peng-fei et al.3 found that 5th order-aberration was higher in refractory amblyopes compared to controls, pre-treated amblyopes and treated amblyopes. However, Caitriona et al.28 did not find a considerable difference of 5th order-aberration in their study. Caitriona et al. (2006)28 found that variations in HOAs can occur even in normal children. They28 reported that 4th order-aberrations were statistically significantly higher among myopes compared to hyperopes (P = 0.002), whereas differences in Zernike terms were not statistically significant.
Treatment of amblyopia aims at initially correcting the obvious refractive error and optimizing the clarity of the retinal images. Traditional spectacles and contact lenses can correct lower order aberrations such as defocus and astigmatism, but fail to correct the HOA. As improving retinal image quality is a prerequisite to any treatment of amblyopia, residual aberrations can still produce retinal image blur even after ordinary optical correction (lower order correction). Difference in ocular aberrations can affect binocular summation, and visual performance.30 This makes the treatment of amblyopia more difficult and may lead to the development of refractory amblyopia. Refractory amblyopia is defined as any amblyopia that cannot be corrected (VA < 0.8) after stringent patching and vision training; it usually occurs in children under 9 years of age.20
Our study shows the whole eye RMS, defocus, astigmatism and 2nd order aberrations were higher in the refractory amblyopic group compared with treated amblyopic group and the differences were statistically significant. Peng-fei et al.20 also found that the differences in vertical coma () and 5th order aberration values between the refractory amblyopic group and the treated amblyopic group were statistically significantly different (P < 0.05). They20 suggested that the vertical coma () and 5th order-aberrations may play a role in refractory amblyopia. In our study we found a statistically significant higher value of 5th order aberrations in pretreated amblyopes, however, there were no statistical significant differences in and 5th order aberration in refractory or treated amblyopes.
Apart from the significantly higher values of 5th order aberrations in pretreated amblyopes, there were no significant differences in HOAs in amblyopic eyes, especially between refractory amblyopes, and emmetropic eyes. Our findings indicate that higher order aberrations may not play a role in the development of amblyopia in the presence of strabismus or anisometropia. Hence, it appears that HOAs do not explain why refractory amblyopia does not respond to orthoptic treatments. It seems that other abnormalities in binocular interactions (mostly at the cortical level) account for the poor improvement in the best corrected visual acuity of these eyes. This is supported by Levy et al.,29 who found that eyes with uncorrected visual acuity of 20/15 or better also had significant levels of HOAs.
In conclusion, lower order aberrations remain the major factor that affects retinal image quality and hence, development amblyopia, especially in the ametropes with amblyopia and this can be corrected optically. Investigating HOAs failed to explain the development of refractory amblyopia. This observation leads back to the theories of central problems in image processing and binocular interaction as the main cause of refractory amblyopia.
Further studies with larger sample sizes to further study the effect of HOAs in children with refractory amblyopia are required. Additionally greater discussion may be required regarding the role of refractive surgery in the treatment of refractory amblyopia.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgement
The authors thank Alhokama Eye Specialist Center for their cooperation.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Agervi P., Nilsson M., Martin L. Foveal function in children treated for amblyopia. Acta Ophthalmol. 2009;88(2):222–226. doi: 10.1111/j.1755-3768.2008.01432.x. [DOI] [PubMed] [Google Scholar]
- 2.McKee S., Levi D., Movshon J.A. The pattern of visual deficits in amblyopia. J Vis. 2003;3:380–405. doi: 10.1167/3.5.5. [DOI] [PubMed] [Google Scholar]
- 3.Pandey K.P., Chaudhuri Zia, Kumar Maneesh, Satyabala K., Sharma Pankaj. Effect of levodopa and carbidopa in human amblyopia. J Pediatr Ophthalmol Strabismus. 2002;39(2) doi: 10.3928/0191-3913-20020301-07. [DOI] [PubMed] [Google Scholar]
- 4.Khalaj M., Zeidi M., Gasemi1 M., Keshtkar A. The effect of amblyopia on educational activities of students aged 9–15. J Biomed Sci Eng. 2011;4:516–521. [Google Scholar]
- 5.Wu C., Hunter D.G. Amblyopia: diagnostic and therapeutic options. Am J Ophthalmol. 2006;141:175–184. doi: 10.1016/j.ajo.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 6.Von Noorden G.K., Campos E.C. 6th ed. Mosby; St Louis: 2007. Binocular vision and ocular motility; theory and management of strabismus. [Google Scholar]
- 7.Husk J., Farivar R., Hess R. Amblyopic deficits in processing structure-from-motion. J Vis. 2012;12(4):1–12. doi: 10.1167/12.4.4. [DOI] [PubMed] [Google Scholar]
- 8.Hess R.F., Thompson B., Gole G.A., Mullen K.T. The amblyopic deficit and its relationship to geniculo-cortical processing streams. J Neurophysiol. 2010;104:475–483. doi: 10.1152/jn.01060.2009. [DOI] [PubMed] [Google Scholar]
- 9.Cleland B.G., Crewther D.P., Crewther S.G., Mitchell D.E. Normality of spatial resolution of retinal ganglion cells in cats with strabismic amblyopia. J Physiol. 1982;1982(326):235–249. doi: 10.1113/jphysiol.1982.sp014188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crewther D.P., Crewther S.G., Cleland B.G. Is the retina sensitive to the effects of prolonged blur? Exp Brain Res. 1985;85:427–434. doi: 10.1007/BF00235859. [DOI] [PubMed] [Google Scholar]
- 11.Hess R.F., Baker C.L. Assessment of retinal function in severely amblyopic individuals. Vision Res. 1984;24:1367–1376. doi: 10.1016/0042-6989(84)90192-5. [DOI] [PubMed] [Google Scholar]
- 12.Hess R.F., Baker C.L., Verhoeve J.N., Keesey U.T., France T.D. The pattern evoked electroretinogram: its variability in ormal and its relationship to amblyopia. Invest Ophthalmol Vis Sci. 1985;26:1610–1623. [PubMed] [Google Scholar]
- 13.Muckli L., Kieb S., Tonhausen N., Singer W., Goebel R., Sireteanu R. Cerebral correlates of impaired grating perception in individual, psychophysically. J Vis Res. 2006;46(4):506–526. doi: 10.1016/j.visres.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Simons K. Amblyopia characterization, treatment, and prophylaxis. Surv Ophthalmol. 2005;50:123–166. doi: 10.1016/j.survophthal.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Weger C., Van Den Brom H., Lindeboom R. Termination of amblyopia treatment: when to stop follow-up visits and risk factors for recurrence. J Pediatr Ophthalmol Strabismus. 2010;47:338–346. doi: 10.3928/01913913-20100218-03. [DOI] [PubMed] [Google Scholar]
- 16.Arden G., Wooding S. Pattern ERG in amblyopia. Invest Ophthalmol Vis Sci. 1985;26:88–96. [PubMed] [Google Scholar]
- 17.Ciuffreda, K.J., Levi, D.M., Selenow, A. Amblyopia: basic and clinical aspects. Boston: Butterworth-Heinemann, vol. 64, 1991.
- 18.Levi D., Song S., Pelli D.G. Amblyopic reading is crowded. J Vis. 2007;7(2):1–17. doi: 10.1167/7.2.21. [DOI] [PubMed] [Google Scholar]
- 19.Pia Agervi, Maria Nilsson, Lene Martin. Foveal function in children treated for amblyopia. Acta Ophthalmol. 2009:1–6. doi: 10.1111/j.1755-3768.2008.01432.x. [DOI] [PubMed] [Google Scholar]
- 20.Peng-fei Z., Yue-hua Z., Ning-li W., Jing Z. Study of the wavefront aberrations in children with amblyopia. Chin Med J. 2010;123(11):1431–1435. [PubMed] [Google Scholar]
- 21.Lombardo M., Lombardo G. Wave aberration of human eyes and new descriptors of image optical quality and visual performance. J Cataract Refract Surg. 2010;36:313–331. doi: 10.1016/j.jcrs.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 22.Schwiegerling J. Theoretical limits to visual performance. Surv Ophthalmol. 2000;45:139–146. doi: 10.1016/s0039-6257(00)00145-4. [DOI] [PubMed] [Google Scholar]
- 23.Kirwan C., O’keefe M. Higher order aberrations in children with amblyopia. J Pediatr Ophthalmol Strabismus. 2008;45:92–96. doi: 10.3928/01913913-20080301-14. [DOI] [PubMed] [Google Scholar]
- 24.Lin X.M., Yan X.H., Wang Z., Yang B., Chen Q.W., Su J.A. Long-term efficacy of excimer laser in situ keratomileusis in the management of children with high anisometropic amblyopia. Chin Med J. 2009;122:813–817. [PubMed] [Google Scholar]
- 25.Daoud Y.J., Hutchinson A., Wallace D.K., Song J., Kim T. Refractive surgery in children: treatment options, outcomes, and controversies. Am J Ophthalmol. 2009;147:573–582. doi: 10.1016/j.ajo.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 26.Hess R.F., Malin S.A. Threshold vision in amblyopia: orientation and phase. Invest Ophthalmol Vis Sci. 2003;44:4762–4771. doi: 10.1167/iovs.03-0259. [DOI] [PubMed] [Google Scholar]
- 27.Kirwan C., O’keefe M. Higher order aberrations in children with amblyopia. J Pediatr Ophthalmol Strabismus. 2008;2008(45):92–96. doi: 10.3928/01913913-20080301-14. [DOI] [PubMed] [Google Scholar]
- 28.Kirwan C., O’keefe M., Soleldner H. Higher-order aberrations in children. Am J Ophthalmol. 2006;141(1):67–70. doi: 10.1016/j.ajo.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 29.Levy Y., Segal O., Avni I., Zador Zador. Ocular higher order aberrations in supernormal vision. Am J Ophthalmol. 2005;139:225–228. doi: 10.1016/j.ajo.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 30.Fam H.-B., Lim K.-L. Effect of higher-order wavefront aberrations on binocular summation. J Refract Surg. 2004;20:S570–S575. doi: 10.3928/1081-597X-20040901-30. [DOI] [PubMed] [Google Scholar]
- 31.Campbell C.E. Improving visual function diagnostic metrics with the use of higher-order aberration information from the eye. J Refract Surg. 2004;20:S495–S503. doi: 10.3928/1081-597X-20040901-18. [DOI] [PubMed] [Google Scholar]



