Abstract
The interactions between inhibitory fast-spiking (FS) interneurons and excitatory pyramidal neurons contribute to the fundamental properties of cortical networks. An important role for FS interneurons in mediating rapid inhibition in local sensory and motor cortex microcircuits and processing thalamic inputs to the cortex has been shown in multiple reports; however, studies in the prefrontal cortex, a key neocortical region supporting working memory, are less numerous. In the present work, connections between layer 2/3 pyramidal cells and FS interneurons were studied with paired whole-cell recordings in acute neocortical slices of the medial prefrontal cortex from juvenile rats. The connection rate between FS interneurons and pyramidal neurons was about 40% in each direction with 16% of pairs connected reciprocally. Excitatory and inhibitory connections had a high efficacy and a low neurotransmission failure rate. Sustained presynaptic activity decreased the amplitude of responses and increased the failure rate more in excitatory connections than in inhibitory connections. In the reciprocal connections between the FS and pyramidal neurons, inhibitory and excitatory neurotransmission was more efficient and had a lower failure rate than in the unidirectional connections; the differences increased during the train stimulation. These results suggest the presence of distinct preferential subnetworks between FS interneurons and pyramidal cells in the rat prefrontal cortex that might be specific for this cortical area.
Keywords: Cortical circuitry, inhibitory transmission, excitatory transmission, paired recordings, patch clamp
Introduction
The mammalian neocortex is composed of two major classes of cells: glutamatergic excitatory principal neurons and GABAergic inhibitory interneurons (Somogyi et al., 1998). The ratio between excitatory and inhibitory neurons is relatively constant (about 4:1) across different cortical areas (Gabbott & Somogyi, 1986; Fitzpatrick et al., 1987; Ren et al., 1992; Beaulieu, 1993); however, a recent study suggested that the prevalence of inhibitory neurons was previously overestimated (Meyer et al., 2011). Among different types of cortical inhibitory cells (Kawaguchi & Kubota, 1997; Markram et al., 2004; Petilla Interneuron Nomenclature et al., 2008; Zaitsev et al., 2009), parvalbumin-containing fast-spiking (FS) interneurons are the most common type of GABAergic neuron in the rodent neocortex (Uematsu et al., 2008). These neurons comprise two principal morphological varieties, basket and chandelier neurons, both of which fire fast action potentials at high, nonaccommodating frequencies (Kawaguchi, 1995; Angulo et al., 1999a; Povysheva et al., 2008). These FS cells are a major source of perisomatic inhibition in the cortex, powerfully controlling the incidence, pattern and timing of firing in excitatory pyramidal cells (Somogyi et al., 1998; Hull et al., 2009; Inan et al., 2013). In turn, FS interneurons are heavily innervated by excitatory inputs from nearby pyramidal cells (Avermann et al., 2012).
The interactions between FS inhibitory interneurons and excitatory pyramidal neurons contribute to the fundamental properties of cortical networks and can be analyzed in brain slices in vitro through simultaneous recordings of presynaptic and postsynaptic neurons (Thomson & Lamy, 2007). Many studies have described the synaptic connectivity between pyramidal neurons and FS interneurons and the functional properties of these connections in sensory and motor cortical areas from different mammalian species (Tamas et al., 1997; Reyes et al., 1998; Thomson et al., 2002; Wang et al., 2002; Holmgren et al., 2003; Sun et al., 2006; Kapfer et al., 2007; Helmstaedter et al., 2008; Otsuka & Kawaguchi, 2009; Chittajallu & Isaac, 2010; Bock et al., 2011; Fino & Yuste, 2011; Hofer et al., 2011; Packer & Yuste, 2011; Yuan et al., 2011); however, similar data from association areas such as the prefrontal cortex (PFC) are quite limited (Krimer & Goldman-Rakic, 2001; Gonzalez-Burgos et al., 2005; Zaitsev et al., 2007).
Although it is widely accepted that a canonical circuit is conserved across cortical areas and mammalian species (Douglas & Martin, 2004), certain structural differences between different cortices and species (DeFelipe, 1993; Povysheva et al., 2008) suggest that each cortical region could contain specific circuits that mediate the distinctive functional properties associated with that region. For example, the PFC, a key neocortical region supporting working memory (Goldman-Rakic, 1995), features specialized synaptic properties such as a high level of interconnectivity between pyramidal cells (Wang et al., 2006), significantly elevated paired pulse ratios, and a reduced initial release probability (Berger et al., 2009) compared to the other cortical areas. These properties are thought to contribute to the persistent neuronal firing observed during the delay period of working memory tasks.
The connectivity between pyramidal cells and FS neurons in the supragranular layers of the primate PFC is critical for generating gamma oscillations in the PFC that are strongly correlated with working memory function (reviewed in Lewis et al., 2012). However, limited quantitative data are available regarding the properties of synaptic connections between pyramidal cells and FS neurons in the PFC, and specific properties of these connections have not been reported. Thus, in the present study the synaptic connection properties between layer 2/3 pyramidal cells and FS interneurons from medial PFC were studied in detail using paired recordings in acute neocortical slices from 19- to 21-day-old rats.
We found that the connection rate between FS and pyramidal neurons was about 40% in each direction, with 16% of pairs connected reciprocally. In general, excitatory and inhibitory connections had high efficacy, a low failure rate, and a similarly short latency of synaptic transmission. During the repetitive activity of a presynaptic neuron, the amplitude of EPSPs decreased to a greater degree than the amplitude of IPSPs. Inhibitory neurotransmission was more efficient and had a lower failure rate in reciprocal connections than in unidirectional connections, suggesting that feedback inhibition is much stronger than lateral inhibition in the rat PFC. Excitatory neurotransmission was also more effective in reciprocal connections than in unidirectional ones, especially during short-train stimulation. These results suggest the presence of distinct subnetworks between FS interneurons and pyramidal cells in the rat PFC that might be specific for this cortical area.
Materials and Methods
Slice preparation
All studies were conducted in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Male Wistar albino rats (30 animals, 19- to 22-days-old) were deeply anesthetized with halothane and decapitated. The brain was rapidly removed and immersed in ice-cold preoxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (ACSF), and 350-μm-thick coronal slices were cut with a vibratome (Leica VT1000S, Leica, Germany). The slices were maintained in the ACSF at 37°C for 1 h and then stored at room temperature (20– 22°C). Slices were transferred to a recording chamber perfused with ACSF at 32 °C. In the recording chamber, we perfused ACSF at 32°C because we previously found that at this temperature the firing properties of neurons are similar to those recorded at physiological temperature but the lower temperature permits longer-lasting recordings which is critically important in these types of experiments. Through all steps, ACSF with the following composition was used (in mM): 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1 MgSO4, 2 CaCl2, 24 NaHCO3, and 20 dextrose.
Single and paired recordings
Neurons in layer 2/3 of the medial PFC were identified visually using infrared transmitted illumination from an Axioscop 2 microscope (Carl Zeiss) equipped with differential interference contrast optics and a Dage MTI NC-70 video camera (Dage-MTI, Michigan City, IN, USA) for contrast enhancement. Pyramidal cells were recognized by their apical dendrites and triangular somata. Interneurons were identified based on their round or oval cell body and lack of apical dendrite; interneurons with relatively large cell bodies were preferentially selected.
Patch electrodes (4–7 MΩ) were pulled from borosilicate capillary glass. The internal solution for the interneurons contained the following (in mM): 114 K-gluconate, 6 KCl, 10 HEPES, 4 ATP-Mg, and 0.3 GTP (pH was adjusted to 7.25 with KOH). To reverse and increase the amplitude of GABAA receptor-mediated IPSPs in postsynaptic pyramidal cells at resting membrane potential, an internal solution of high chloride concentration was used containing the following (in mM): 120 KCl, 10 HEPES, 4 ATP-Mg, and 0.3 GTP (pH was adjusted to 7.25 with KOH). The chloride equilibrium potential was estimated to be −2.6 mV using the Nernst equation calculated for a temperature of 32 °C. The decay time course of the GABAergic inhibitory synaptic currents was considerably faster when recorded with physiological internal Cl− concentrations than with symmetrical Cl− solutions (Houston et al., 2009). Thus, the IPSP decay time might be overestimated in the present study.
Whole-cell voltage recordings were made with MultiClamp 700A amplifiers (Axon Instruments, Union City, CA, USA). Somatic bridge balance and pipette capacitance were adjusted using MultiClamp 700A Commander software. Recordings were terminated if the access resistance exceeded 25 MΩ. When necessary, a small bias current was injected to maintain a baseline membrane potential near -70 mV in both types of cells. Signals were filtered at 4 kHz and acquired at a sampling rate of 20 kHz using a 16-bit resolution Power 1401 interface and Signal software (CED, Cambridge, UK).
Intrinsic membrane properties were assessed from the voltage responses to the series of 500-ms hyper- and depolarizing current steps with 5- to 10-pA increments at 0.5 Hz. Synaptically connected pairs were identified during simultaneous dual whole-cell voltage recordings as follows, single spikes were evoked in the presynaptic neurons with the injection of short-duration (2 ms) suprathreshold current steps. The level of stimulation current, injected into presynaptic neurons, was adjusted to elicit action potentials (APs) with little trial-to-trial variability in spike latency. Typically, the stimulation current was in the range of 1.5-2 nA. Seven to ten consecutive postsynaptic voltage responses were averaged on-line, and the start of fast voltage deflection within a 1- to 2-ms delay after the presynaptic spike was interpreted as a monosynaptic unitary IPSP or EPSP. Once the presence of a synaptic connection was established, single stimuli and/or trains of five presynaptic APs at 20 Hz were applied every 10-20 s.
Data analysis and statistics
Data were analyzed using Signal software. Input resistance was determined as the slope from a linear regression fit to the plot of the injected current/voltage response. The linear current/voltage relationship was usually preserved between −50 and −10 pA, and voltage responses were measured at the end of the 500-ms step. The membrane time constant was determined by fitting a single exponential to the on-phase of the averaged voltage responses to −10 to −30-pA current steps. The AP properties were measured using the first spike in a sweep evoked by a nearthreshold current step. The AP amplitude and afterhyperpolarization (AHP) were measured from the threshold of the first AP. Duration of the AP was measured at its half amplitude. The spike threshold was determined as the membrane potential at the point at which the interpolated rate of the voltage rise (dV/dt) reached >10 mV/1 ms. The spike frequency adaptation (the adaptation ratio coefficient) was determined as the ratio between the first interspike interval to the last interspike interval measured at twice the threshold level of the depolarizing current steps.
Synaptic latency (the peak of the presynaptic AP to the onset of the postsynaptic potential (PSP)), PSP amplitude (baseline to the peak of the PSP), rise time (10–90% of the PSP peak amplitude), and decay time (time constant of a monoexponential decay function) were determined on traces obtained by averaging 30–50 consecutive responses, including failures. The coefficient of variation (CV) of the synaptic latency distribution was calculated based on measurements of synaptic latency at the individual traces, the latency of the individual PSP onset was defined as the time from the AP peak to 10% of the PSP amplitude. PSP failures were considered when the membrane potential deflection was <1.5 times the noise amplitude within a 0.5- to 2.5-ms time window of the expected IPSP peak. The noise value was measured in a 1- to 2-ms time window before the IPSP onset (Gonzalez-Burgos et al., 2005). The detected PSP failures were also confirmed by visual inspection of the individual traces.
To characterize PSP dynamics during repetitive stimulation of presynaptic neurons, 30–50 consecutive traces including failures were averaged, and the amplitude of each PSP in the train was measured from the baseline directly preceding the rising phase. The magnitude of the synaptic depression was measured as the amplitude ratio between the fifth and the first PCP (PCP5/PCP1).
Values are given as means ± s.e.m. The error bars in the figures indicate s.e.m. unless otherwise indicated. Statistical tests were performed using Statistica 8 (Statsoft, Tulsa, OK, USA).
Some of the properties (the amplitude and the failure rate) of 12 connected pairs included in this study have previously been reported (Zaitsev et al., 2007). Additional analysis was performed for those recordings, and new parameters and correlations are reported in the present work.
RESULTS
Dual whole-cell recordings were performed in layer 2/3 pyramidal neurons and FS interneurons in the PFC from juvenile rats (19- to 22-days-old). Because axonal cutting during brain slicing may severely affect cell connectivity tested in slices (Holmgren et al., 2003), special efforts were made to minimize this effect. In each experiment, only slices with pyramidal cell apical dendrites parallel to the cut slice surface were used. Recordings were done from neurons located > 40-50 μm from the surface of the slice. The cell bodies of the pyramidal neurons and interneurons were typically located 10-50 μm from each other. Fifty-one pairs of FS-pyramidal cells were tested. Among them, 13 pairs (25%) had only excitatory connections, 12 pairs (24%) had only inhibitory connections, and 8 pairs (16%) had reciprocal connections.
Identification of pyramidal cells and FS interneurons in rat PFC slices
The FS interneurons and pyramidal cells were identified by their characteristic firing patterns (Fig. 1A). FS interneurons were recognized according to previously specified criteria (Kawaguchi, 1995; Krimer et al., 2005; Povysheva et al., 2008), only cells with a spike half-duration <0.51 ms and adaptation ratio coefficient >0.65 were included in the study. FS interneurons displayed fast action potentials (0.46 ± 0.01 ms), followed by fast and deep monophasic afterhyperpolarization (AHP amplitude = 22.3 ± 0.7 mV, AHP latency = 1.9 ± 0.2 ms). These cells had a low input resistance (Ri = 234 ± 13 MΩ) and fast membrane time constant (8.1 ± 0.6 ms), and they did not show significant time-dependent changes in voltage responses during the application of hyperpolarizing or subthreshold depolarizing current steps. At the threshold-level current injections, the FS interneurons showed a flattened depolarization before a spike that occurred with variable onset (191 ± 29 ms, n = 26). In response to the suprathreshold current level, FS interneurons displayed a high-frequency firing pattern without a significant spike frequency adaptation (AR coefficient = 0.91 ± 0.03). In contrast, all pyramidal cells exhibited an adapting firing pattern (AR coefficient = 0.38 ± 0.08; Fig. 1B). The action potentials had slower kinetics (APD = 1.16 ± 0.05). AHPs had smaller amplitudes (8.4 ± 0.9 mV) and usually consisted of fast and medium components. Input resistance was comparable with the FS interneurons (260 ± 16 MΩ), while the membrane time constant was larger (28.0 ± 1.6 ms).
Figure 1. Electrophysiological properties of pyramidal cells and FS interneurons from rat medial prefrontal cortex.
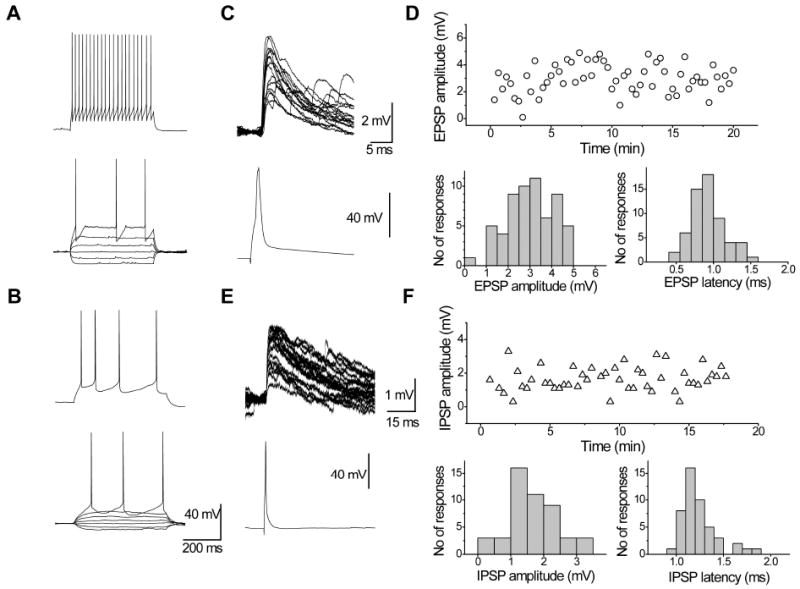
Representative examples of the firing patterns of fast-spiking interneurons (A) and pyramidal cells (B). (C) Representative examples of EPSPs recorded in a FS interneuron (upper traces) evoked by presynaptic APs in a pyramidal cell (bottom traces). (D) This connection had stable amplitude during recording (upper graph); distributions of individual EPSP amplitudes and latencies are shown below. (E) Representative examples of IPSPs recorded in a pyramidal neuron (upper traces) evoked by presynaptic APs in a FS interneuron (bottom traces).
Properties of unitary synaptic responses in FS – pyramidal cells connected pairs
To characterize the functional properties of connections between layer 2/3 pyramidal cells and FS interneurons, the unitary EPSPs in FS interneurons and the unitary IPSPs in pyramidal cells were analyzed by measuring their peak amplitude, failure rate, 10-90% rise time, decay time constant, and latency; correlations between these parameters were also analyzed (Table 1). Unitary postsynaptic potentials were elicited at a low frequency of 0.05-0.10 Hz to avoid a short-term depression (Fig. 1C-F).
Table 1. Significant correlations between the postsynaptic potential properties.
| EPSP (n = 19) | IPSP (n = 18) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Properties | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 1. Latency | ||||||||||||||
| 2. CV of latency | ||||||||||||||
| 3. Amplitude | -0.71 | |||||||||||||
| 4. CV of amplitude | 0.82 | -0.75 | ||||||||||||
| 5. Rise time | 0.68 | 0.66 | 0.60 | |||||||||||
| 6. Decay time | 0.72 | 0.60 | ||||||||||||
| 7. Failure rate | 0.87 | -0.56 | 0.62 | 0.65 | -0.56 | |||||||||
| 8. Ampl2/Ampl1 | 0.56 | 0.56 | ||||||||||||
| 9. Ampl5/Ampl1 | -0.50 | 0.67 | 0.57 | 0.64 | ||||||||||
Statistical significance at P < 0.05.
Efficacy and failure rate of excitatory and inhibitory synaptic connections
The efficacy of the synaptic connections was measured by the average peak amplitude of the unitary postsynaptic responses at the resting membrane potential (FS interneurons: -72 ± 2 mV, pyramidal cells: -69 ± 3 mV). Because a high chloride intracellular solution was used for the pyramidal cells (see Materials and methods), the IPSPs were depolarizing at the resting membrane potential. The mean amplitude of the EPSPs was 3.4 ± 0.7 mV (n = 19) and of the IPSPs was 2.6 ± 0.7 mV (n = 18). The efficacy of the excitatory and inhibitory connections varied substantially among pairs. In some pairs, the average amplitude of the EPSPs/IPSPs reached 8-12 mV, while in others it was less than 0.5 mV; the distributions of mean EPSP and IPSP amplitudes are illustrated in Fig. 2A and B.
Figure 2. Efficacy and failure rate of excitatory and inhibitory synaptic connections.
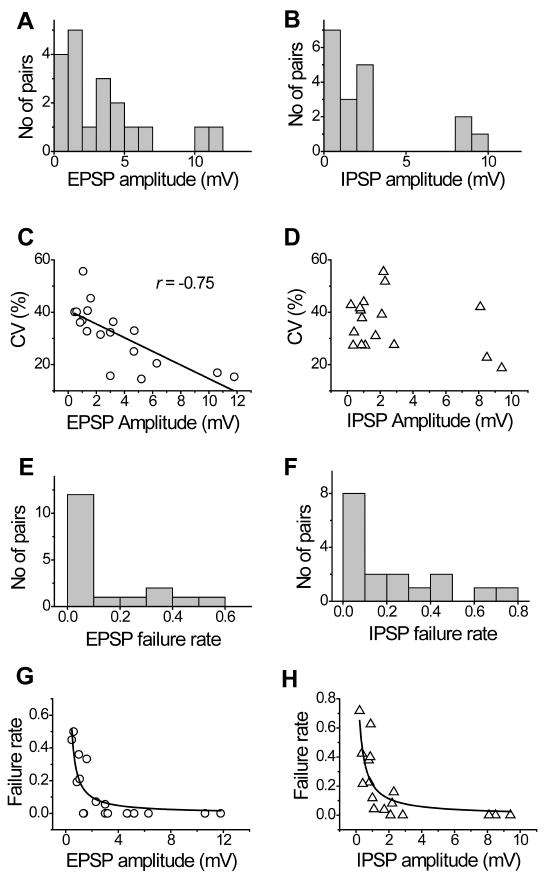
Bar diagrams showing the distributions of mean EPSP (A) and IPSP (B) amplitudes. (C) Plot of peak EPSP amplitude against CV of EPSP amplitudes showing an inverse relationship between these parameters. (D) Plot of peak IPSP amplitude against CV of IPSP amplitudes. Histograms showing the failure rate of excitatory (E) and inhibitory (F) neurotransmission in FS interneuron – pyramids connected pairs. Failure rate is plotted as a function of the unitary EPSP (G) and IPSP (H) peak amplitude.
The coefficient of variation (CV) of individual unitary postsynaptic potentials ranged from 15% to 56%, with a mean of 32 ± 3% in the excitatory connections, and ranged from 19% to 56%, with a mean of 36 ± 2% in the inhibitory connections. In the excitatory connections, the CV was inversely correlated with the EPSP amplitude (r = -0.75, n = 18, P <0.01; Fig. 2C), while in the inhibitory connections the correlation between the CV and the IPSP amplitude was not significant (r = -0.35, n = 17, P >0.05; Fig. 2D).
The low background noise of the recordings permitted reliable detection of the success or failure of the presynaptic AP to evoke a postsynaptic potential. The failure rate in the excitatory connections was 0.12 ± 0.04 (range 0.00-0.50) and in the inhibitory connections was 0.20 ± 0.06 (range 0.00-0.72) (Fig. 2E and F). In both types of connections, the amplitude and the failure rate were inversely correlated (excitatory connections, r = -0.56, n = 18, P <0.05; inhibitory connections, r = -0.56, n = 17, P <0.05; Fig. 2G and H).
Latency and kinetics of excitatory and inhibitory postsynaptic potentials
The average latencies of the EPSPs and the IPSPs were the same (0.87 ± 0.07 ms and 0.86 ± 0.07 ms accordingly, t35 = 0.09, P = 0.93) and ranged similarly from 0.5 to 1.6 ms for individual connections, indicating that the postsynaptic neurons were rapidly recruited in both types of connections (Fig. 3A). The latency fluctuation of unitary EPSPs and IPSPs at an individual synaptic connection was also similar and quite small (CV of EPSP latency = 21 ± 3%, CV of IPSP latency = 18 ± 2%, t33 = 0.94, P = 0.36); the latency histograms were narrow and showed a single peak in both types of connections. The latency fluctuation in the excitatory connections correlates with many parameters of postsynaptic responses. Low latency fluctuation was typical for connections with a larger amplitude of responses, a smaller CV of the amplitude and the failure rate, and a high magnitude of short-term depression (Table 1, Fig. 3B-D).
Figure 3. Latency and kinetics of excitatory and inhibitory postsynaptic potentials.
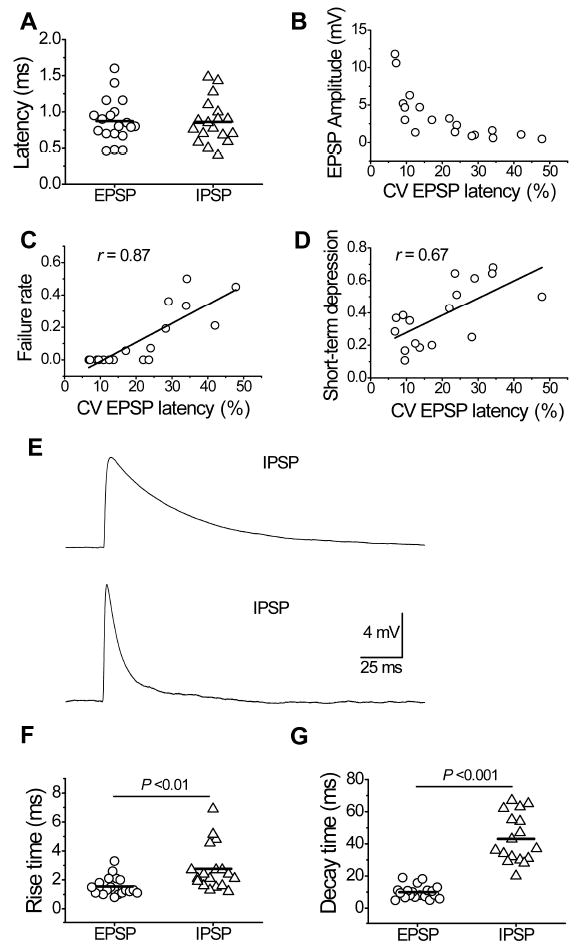
(A) Distribution of mean PSP latencies in excitatory and inhibitory connected pairs. The latency fluctuation in the excitatory connection estimated by CV of EPSP latencies correlates with the EPSP amplitudes (B), the failure rate (C), and magnitude of short-term depression (D). (E) Representative examples of unitary IPSP (upper trace) and EPSP (bottom trace). EPSP has much faster kinetics than IPSP. Diagrams showing the difference between EPSP and IPSP in 10-90% rise time (F) and decay time (G).
At resting membrane potential (FS interneurons: -72 ± 2 mV, pyramidal cells: -69 ± 3 mV), the EPSP kinetics was dramatically faster than that of the IPSPs, showing a shorter 10-90% rise time (1.55 ± 0.14 ms vs. 2.76 ± 0.37 ms, t35 = 3.13, P = 0.003) and decay time (τ = 10.0 ± 0.9 ms vs. 43.1 ± 3.5 ms, t34 = 9.2, P <0.001) (Fig. 3E-G). We found that rise time and decay time were significantly correlated with each other (excitatory connection r = 0.72, P <0.01; inhibitory connections r = 0.60, P <0.05). The EPSP decay time was correlated with the membrane time constant of the interneurons at the trend level of significance (r = 0.50, n = 14, P = 0.07), while the EPSP rise time was not correlated with the membrane time constant (r = -0.01, n = 14, P >0.1).
Short-term EPSP and IPSP dynamics
To determine the effects of repetitive presynaptic firing on the functional properties of the synaptic responses, trains of five suprathreshold current steps were applied to the presynaptic neurons at 20 Hz (Fig. 4A and B). In the majority of the connected pairs, we observed a depression in the EPSP/IPSP amplitude starting from the second PSP. Within the train, the excitatory connections showed a much stronger amplitude depression than the inhibitory connections (Fig. 4C). The fifth IPSP in the train decreased in amplitude to 68 ± 5% of the initial values, while in the excitatory connections amplitudes decreased to 37 ± 4%. The difference in the short-term depression between the excitatory and inhibitory connections was significant for the third to fifth responses.
Figure 4. Effects of sustained presynaptic activity on the efficacy of unitary EPSPs and IPSPs.

Representative examples of trains of unitary EPSPs (A) and IPSPs (B) from connected pairs with different efficacy. Note that the short EPSP duration precludes temporal summation during the train. (C) Changes in EPSP and IPSP amplitudes during repetitive presynaptic firing. Peak PSP amplitude were normalized relative to the first PSP in the train, averaged across neurons and graphed as a function of stimulus number. Excitatory connection exhibited stronger amplitude depression than inhibitory connections; differences between peak EPSP and peak IPSP were significant for 3rd-5th responses (t-test, p<0.01). (D) Changes in failure rates in inhibitory and excitatory connections during repetitive presynaptic firing. Scatter plots showing relationships between amplitudes of the first and the second PSP in excitatory (E) and inhibitory (F) connections. (G) Summary diagram illustrating that the majority of excitatory connection had a significant inverse correlation between the amplitudes of the first and second EPSPs (r=-0.36±0.06), while in inhibitory connections amplitudes of the first and second IPSPs were independent (r=-0.03±0.06, n=17).
During the short train, we found that the failure rate significantly increased in both types of connections (Fig. 4D). In the excitatory connections, the failure rate increased from 0.16 ± 0.05 for the first EPSP in the train to 0.37 ± 0.06 for the fifth EPSP (repetitive ANOVA test, F4,68 = 11.6, P <0.001). In the inhibitory connections, the failure rate increased within the train from 0.24 ± 0.06 to 0.36 ± 0.07 (repetitive ANOVA test, F4,64 = 6.5, P <0.001).
In the majority of the excitatory connections, we found a significant inverse correlation between the amplitudes of the first and second EPSPs (r = -0.36 ± 0.06, n = 18, ranged from -0.82 to 0.11; Fig. 4E and G). In contrast, we did not observe a correlation between the amplitudes of the first and second IPSPs (r = -0.03 ± 0.06, n = 17; Fig. 4F and G), with the exception of two low-amplitudeconnections (r = -0.60 and r = -0.33).
In the excitatory connections, the magnitude of the synaptic depression, calculated as an amplitude ratio between the fifth and first postsynaptic responses, strongly correlates with the initial failure rate (r = 0.76, P <0.05, n = 16), indicating that connections with a lower proportion of apparent failures of neurotransmission exhibit stronger adaptation (Fig. 5A and B). Indeed, the normalized amplitude of the fifth EPSP in connections without failures was 31 ± 5% of the first EPSP (n = 10), while in connections with failure rates larger than 0.2, it was 52 ± 10% (n = 5; Fig. 5C). In contrast to the excitatory connections, the IPSP short-term dynamics were independent of the initial neurotransmission failure rate (Fig. 5D).
Figure 5. Magnitude of depression in excitatory and inhibitory connections with different failure rate.
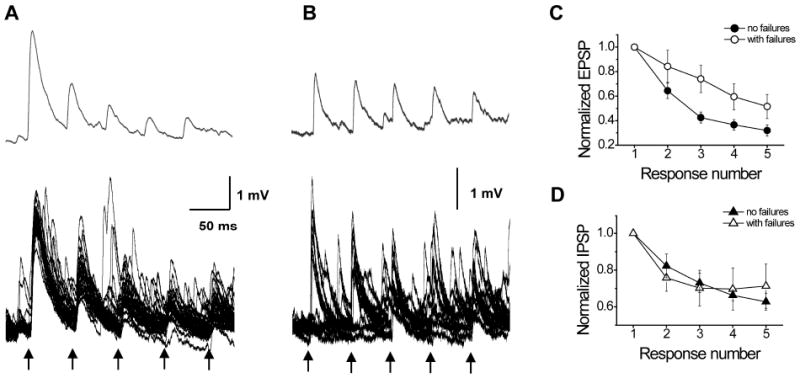
Representative examples of trains of unitary EPSP from connected pairs with different failure rates. Action potentials (shown by arrows) evoked unitary EPSPs (bottom graphs, about 20 consecutive sweeps superimposed) with no failures in the first connection (A) and with high failure rate in the second connection (B). Upper graphs show average unitary EPSPs, including failures. More reliable connection exhibits stronger adaptation. Plots showing short-term dynamics of PSP amplitudes in excitatory (C) and inhibitory (D) connections with different reliability. Normalized amplitude of 5th EPSP in connections without failures was 0.31 ± 0.05 of the 1st EPSP (n=10), while in connections with failure rates larger than 0.2 it was 0.52 ± 0.10 (n=5) (C). In contrast to excitatory connections, short-term dynamics of IPSP amplitudes was independent of reliability on neurotransmission (D).
Differential properties of reciprocal and unidirectional connections between pyramidal cells and fast-spiking interneurons
We further investigated whether reciprocal and unidirectional connections have similar functional properties. We found that the PSP latency and kinetics were the same; however, the amplitudes of responses and failure rates were different (Table 2). Neurotransmission efficacy was higher in the reciprocal connections. The EPSP amplitude was larger in the reciprocal connections (5.6 ± 1.9 mV) than in the unidirectional connections (2.3 ± 0.5 mV, t17 = 2.24, P = 0.04). IPSPs in reciprocal connections also had larger amplitude (4.7 ± 1.4 mV) than in unidirectional connections (1.17 ± 0.23 mV, t16 = 3.07, P = 0.007). Higher efficacy of neurotransmission was also observed during sustained synaptic activity (Fig. 6A and B); after the fifth APs the inhibitory postsynaptic responses were four times larger and the excitatory responses were three times larger in the reciprocal connections compared to the unidirectional connections. The failure rate of excitatory neurotransmission was similar in both reciprocal and unidirectional connected pairs at the beginning of the train stimulation, and was almost three times larger at the end of the short train for both types of connections (Fig. 6C). However, the failure rate in the unidirectional inhibitory connections was always higher than in the reciprocal connections (Fig. 6D).
Table 2. Properties of reciprocal and unidirectional connections.
| Properties | Inhibitory connections | Excitatory connections | ||||||
|---|---|---|---|---|---|---|---|---|
| Unidirectional (n=11) | Reciprocal (n=7) | t-test | p | Unidirectional (n=13) | Reciprocal (n=6) | t-test | p | |
| Latency, ms | 0.90±0.11 | 0.81±0.06 | 0.63 | 0.54 | 0.91±0.09 | 0.79±0.08 | 0.82 | 0.42 |
| Amplitude, mV | 1.2±0.2 | 4.7±1.4 | 3.1 | 0.007 | 2.3±0.5 | 5.59±1.89 | 2.2 | 0.04 |
| Rise time, ms | 3.2±0.6 | 2.0±0.2 | 1.7 | 0.11 | 1.5±0.2 | 1.58±0.26 | 0.16 | 0.86 |
| Decay time, ms | 43±6 | 43±4 | 0.06 | 0.95 | 10.1±1.2 | 9.67±1.52 | 0.22 | 0.83 |
| Failure rate | 0.32±0.07 | 0.04±0.03 | 3.1 | 0.008 | 0.14±0.05 | 0.09±0.07 | 0.57 | 0.58 |
| Failure rate (5th response) | 0.48±0.08 | 0.19±0.10 | 2.3 | 0.04 | 0.46±0.07 | 0.17±0.09 | 2.6 | 0.02 |
| Ampl2/Ampl1 | 0.83±0.06 | 0.76±0.05 | 0.80 | 0.44 | 0.65±0.08 | 0.78±0.05 | 1.0 | 0.32 |
| Ampl5/Ampl1 | 0.74±0.08 | 0.58±0.04 | 1.5 | 0.14 | 0.35±0.07 | 0.41±0.04 | 0.65 | 0.52 |
Figure 6. Differential properties of reciprocal and unidirectional connections between pyramidal cells and fast-spiking interneurons.
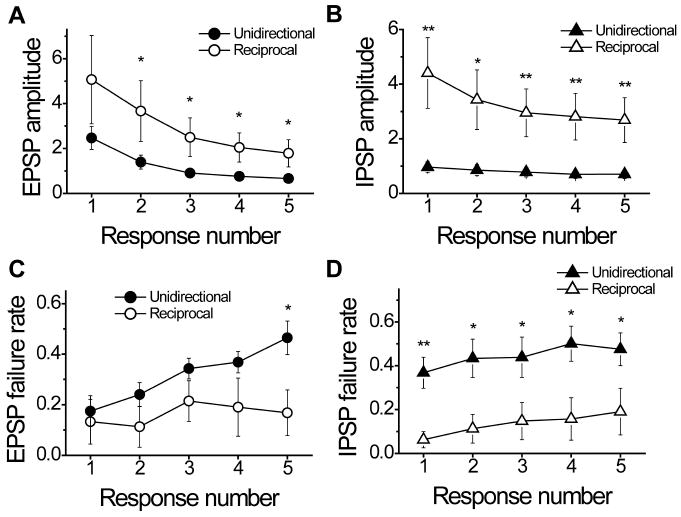
During the sustained synaptic activity, the reciprocal connections compared to the unidirectional connections showed higher efficacy of excitatory (A) and inhibitory (B) neurotransmission and lower failure rate (C and D). *P <0.05; **P<0.01.
Discussion
In the present work, we quantitatively investigated the properties of synaptic connections between FS inhibitory interneurons and pyramidal neurons in layer 2/3 of the rat medial PFC. Layer 2/3 pyramidal cells are among the most abundant cortical cells, and are thought to play a fundamental role in the recurrent excitatory connectivity of the microcircuit (Mountcastle, 1982; Douglas & Martin, 2004). The group of FS interneurons is not homogeneous, and includes two morphologically distinct cell types (more common basket cells and the less frequently encountered chandelier cells, which may have different roles in cortical circuitry) (Szabadics et al., 2006; Bartos et al., 2007; Inda et al., 2007; Woodruff et al., 2009). Although these morphological groups exhibit similar FS firing patterns, differences in the electrophysiological membrane properties were previously described (Krimer & Goldman-Rakic, 2001; Gonzalez-Burgos et al., 2005; Woodruff et al., 2009; Zaitsev et al., 2009). In rodents, the response profile of neurons at the threshold-level current injections provided a reliable means for distinguishing basket and chandelier neurons on a cell-by-cell basis. For example, chandelier cells exhibited a curved membrane potential response before the short spike onset, while basket cells typically showed a flattened depolarizing ramp before a spike that occurred with variable onset (Woodruff et al., 2009). We did not observe a curved membrane potential response in any of the FS cells included in this study. The relatively long delay before the first spike (191 ± 29 ms) and rarity of chandelier neurons in interneuron population suggests that these FS cells most likely were of the basket type.
Connections between pyramidal cells and FS interneurons have been extensively studied in different cortical areas, visual (Buhl et al., 1997; Tamas et al., 1997; Thomson et al., 2002; Holmgren et al., 2003; Bock et al., 2011; Hofer et al., 2011), somatosensory (Thomson et al., 1996; Reyes et al., 1998; Wang et al., 2002; Holmgren et al., 2003; Sun et al., 2006; Mateo et al., 2011; Packer & Yuste, 2011; Avermann et al., 2012), auditory (Oswald et al., 2009), and motor (Angulo et al., 1999b; 2003; Ali & Nelson, 2006; Otsuka & Kawaguchi, 2009; Packer & Yuste, 2011), but rarely in association cortical areas such as the PFC. We found that the connections between the FS interneurons and the pyramidal cells in the rat medial PFC had high efficacy. The average amplitude of unitary EPSPs recorded in FS interneurons (3.4 ± 0.7 mV) was larger than the most typically reported values of 1-2 mV for sensory and motor cortices (Buhl et al., 1997; Reyes et al., 1998; Angulo et al., 1999a; Thomson et al., 2002; Ali & Nelson, 2006; Avermann et al., 2012), and may indicate some regional differences in the properties of the excitatory inputs to FS interneurons. However, the variance in EPSP amplitude is very large in almost all studies. Thus, to make a final conclusion about the differences in synaptic efficacy between cortical areas it will be necessary to compare EPSP recorded in identical experimental conditions from different cortical areas. Direct comparison of IPSP amplitude measured in our study with the published data is difficult due to the different experimental conditions. The low failure rates in excitatory and inhibitory connections in the PFC were comparable with those reported in other neocortical regions.
In agreement with previous studies of local neuronal connectivity (Thomson et al., 2002; Holmgren et al., 2003; Yoshimura & Callaway, 2005; Kapfer et al., 2007; Thomson & Lamy, 2007; Hofer et al., 2011; Avermann et al., 2012), we found that pyramidal-interneuron connectivity in the rat PFC was relatively high; the probability of finding inhibitory and excitatory connections was about 40% in our sample. The exact fraction of local excitatory inputs sampled by FS cells in different cortical area remains unclear, as reports vary from 19% (Yoshimura & Callaway, 2005) to 60% (Holmgren et al., 2003) even in the same cortical area (primary visual cortex) of the same species (rat) and up to 88% in the mouse visual cortex (Hofer et al., 2011). The reported connectivity rates between FS interneurons (or parvalbumin-positive basket cells) and pyramidal cells also vary greatly, from extremely high where every parvalbumin-positive-interneuron is connected to almost every pyramidal cell sampled (Packer & Yuste, 2011) to as low as 16% in layer 3 of the rat cortex (Thomson et al., 2002). Most commonly, findings of connectivity rates between FS interneurons and pyramidal cells obtained with dual-multiple intracellular and whole-cell recordings ranged from 40% to 70% across different cortical areas (Holmgren et al., 2003; Kapfer et al., 2007; Avermann et al., 2012).
In the rat visual cortex, the probability of an inhibitory connection to a pyramidal cell was highly dependent on whether the same cell pair also had an excitatory connection from the pyramidal cell to the inhibitory neuron (Yoshimura & Callaway, 2005). However, no such dependence has been found in the mouse barrel cortex (Avermann et al., 2012) or rat somatosensory and frontal cortices (Holmgren et al., 2003; Packer & Yuste, 2011). In our sample of neuron pairs from the medial PFC, the probability of an inhibitory connection to the pyramidal cell was also independent of the presence of an excitatory connection from the pyramidal cell to the FS interneuron. Moreover, our data on the rate of reciprocal connections (16%) indicate that the formation of connections between neighboring pyramidal cells and FS interneurons is a random process (probability of excitatory connection (0.4) * probability of inhibitory connections (0.4) = probability of reciprocal connections (0.16)).
At the same time, we found that several reciprocal connection properties differed from those of unidirectional connections. Inhibitory neurotransmission was 4 times more efficient and 8 times more reliable in the reciprocal connections than in the unidirectional connections. These data suggest that the inhibition by FS interneurons is biased toward the same layer 2/3 pyramidal cells that provide them with excitatory inputs, which is similar to results obtained in the visual cortex (Yoshimura & Callaway, 2005). Thus, feedback inhibition is much stronger than lateral inhibition in the rat medial PFC. These data are in striking contrast with results obtained in the mouse barrel cortex where unitary IPSP amplitudes were not different between unidirectionally and bidirectionally synaptically coupled pairs of neurons (Avermann et al., 2012).
Excitatory neurotransmission also depended on the presence of inhibitory feedback from targeted interneurons. The difference between the reciprocal and unidirectional connections was even stronger if short train stimulation was applied. Together, these results suggest the presence of preferential subnetworks between FS interneurons and pyramidal cells in the rat PFC.
Both excitatory and inhibitory connections exhibited short-term depression during sustained synaptic activity and a relatively low failure rate. The efficacy of synaptic transmission during repetitive activity largely depends on presynaptic mechanisms (see Zucker & Regehr, 2002 for a review), such as transmitter release machinery or presynaptic calcium dynamics (Rozov et al., 2001). Typically, synapses with low probability of release display a high failure rate and EPSP facilitation, whereas synapses with high release probability exhibit a low failure rate and synaptic depression (Atzori et al., 2001; Rozov et al., 2001). Thus, we suggest that excitatory and inhibitory synaptic connections between pyramidal and basket cells have a high release probability.
Inhibitory and excitatory synapses differ in some properties of short-term dynamics. For instance, we observed a larger magnitude of depression and a larger increase in the failure rate during repetitive stimulation in excitatory synapses than in inhibitory ones. For excitatory connections, we found correlations that were not typical of inhibitory connections: 1) an inverse correlation between the amplitudes of the first and second EPSPs, 2) a positive correlation between the magnitude of the short-term depression and the initial failure rate, and 3) an inverse correlation between the CV and the mean value of the EPSP amplitude.
Inverse correlations between the first and second postsynaptic responses were previously observed in central connections with a relatively low number of release sites (n) and with a high probability of release (p) due to depletion of the immediately available pool of vesicles (Thomson, 2000; Zucker & Regehr, 2002). The depletion of synaptic vesicles model of depression may provide a straightforward accouting for a positive correlation between the magnitude of the short-term depression and the initial failure rate (Zucker & Regehr, 2002). A large CV and a low M (Fig. 2C) can also result from a small n, particularly when q is large as it typically is in connections to interneurons (Beierlein et al., 2003; Bremaud et al., 2007).
These results indicate that there are only a few release sites at excitatory connections and the immediately available pool of vesicles is small and quickly depleted during repetitive stimulation. In contrast, in inhibitory connections the number of release sites and the pool of immediately available vesicles might be larger. These observations are in line with morphological data. Pyramidal cells form a few or a single synaptic contact with postsynaptic inhibitory neurons, and each synaptic contact has one or a few release sites (Gulyas et al., 1993; Buhl et al., 1997; Gulyas et al., 1999; Krimer & Goldman-Rakic, 2001). In contrast, each basket cell forms more than ten synaptic junctions with a pyramidal cell (Tamas et al., 1997). Inhibitory boutons of basket cells are larger than those of pyramidal axons and contain more vesicles and large mitochondria (Kisvarday et al., 1997; Somogyi et al., 1998; Thomson, 2000).
We found that the EPSP latency fluctuation, which reflects the synchrony of synaptic release, correlates with many parameters of postsynaptic responses. Low latency fluctuation is exhibited by the connections showing larger amplitude of responses, smaller CV of amplitude, lower failure rate, and higher magnitude of short-term depression. Together, these data suggest that the connections with a lower latency fluctuation are responsible for the fast and precise activation of FS interneurons during very short time window, while connections with a larger latency fluctuation are less precise and due to smaller synaptic depression may increase their effectiveness during short trains.
Different latency fluctuation may be associated with multiple presynaptic mechanisms, perhaps acting simultaneously. For example, these connections might differ in their proportions of N- and P/Q-type presynaptic voltage-gated Ca2+ channels (Rozov et al., 2001; Reid et al., 2003; Ali & Nelson, 2006). Synapses containing mostly N-type Ca2+ channels exhibit larger latency fluctuation of synaptic release than those containing mostly P/Q-type channels (Armstrong & Soltesz, 2012). N-type channels are predominantly associated with connections displaying larger paired-pulse ratio (Ali & Nelson, 2006).
Regarding the properties of the unitary pyramid – FS interneuron EPSPs reported here, such as their high efficacy, fast temporal characteristics, and short-term depression, several important functional implications emerge. The intrinsic membrane properties and the fast kinetics of the EPSPs of the FS interneurons make these cells poor temporal integrators that require synchronous activation of several presynaptic pyramidal cells to reach their action potential threshold (Angulo et al., 1999a). Temporal integration of converging inputs would therefore be limited to a very narrow time window, during which effective summation can occur. Taking the short-term depression of the unitary synaptic responses into account, it is reasonable to assume that the activation of an interneuron critically depends on synchronous activity in several pyramidal cells.
The efficient reciprocal connections between pools of excitatory pyramidal and inhibitory neurons is thought to be the base for gamma oscillations in different neuronal circuits (Buzsaki & Wang, 2012). In such two-neuron pool models, fast excitation and the delayed feedback inhibition alternate, and with appropriate excitation and inhibition strength, cyclic behavior may persist. Modeling studies also suggest that stronger recurrent synaptic interactions, as a rule, greatly enhance the network’s propensity to exhibit synchronous oscillations (Brunel & Wang, 2003). Thus, the present data indicate that medial PFC circuits fit well for generating gamma oscillations, a prominent form of synchronization in the awake cortex widely believed to play a role in various cognitive functions such as working memory (Fries, 2009).
Acknowledgments
This work was supported by NIH grants MH043784 and MH084053. A.V.Z. was also supported by the RFBR grants 11-04-00912a and 13-04-00244a, by Russian scientific schools grant (6574.2012.4) and by the Program #7 of the Presidium of the RAS.
D.A.L. currently receives investigator-initiated research support from Bristol-Myers Squibb and Pfizer and in 2010-2012 served as a consultant in the areas of target identification and validation and new compound development to Bristol-Myers Squibb.
References
- Ali AB, Nelson C. Distinct Ca2+ channels mediate transmitter release at excitatory synapses displaying different dynamic properties in rat neocortex. Cereb Cortex. 2006;16:386–393. doi: 10.1093/cercor/bhi117. [DOI] [PubMed] [Google Scholar]
- Angulo MC, Rossier J, Audinat E. Postsynaptic glutamate receptors and integrative properties of fast-spiking interneurons in the rat neocortex. J Neurophysiol. 1999a;82:1295–1302. doi: 10.1152/jn.1999.82.3.1295. [DOI] [PubMed] [Google Scholar]
- Angulo MC, Staiger JF, Rossier J, Audinat E. Developmental synaptic changes increase the range of integrative capabilities of an identified excitatory neocortical connection. J Neurosci. 1999b;19:1566–1576. doi: 10.1523/JNEUROSCI.19-05-01566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo MC, Staiger JF, Rossier J, Audinat E. Distinct local circuits between neocortical pyramidal cells and fast-spiking interneurons in young adult rats. J Neurophysiol. 2003;89:943–953. doi: 10.1152/jn.00750.2002. [DOI] [PubMed] [Google Scholar]
- Armstrong C, Soltesz I. Basket cell dichotomy in microcircuit function. J Physiol. 2012;590:683–694. doi: 10.1113/jphysiol.2011.223669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzori M, Lei S, Evans DI, Kanold PO, Phillips-Tansey E, McIntyre O, McBain CJ. Differential synaptic processing separates stationary from transient inputs to the auditory cortex. Nat Neurosci. 2001;4:1230–1237. doi: 10.1038/nn760. [DOI] [PubMed] [Google Scholar]
- Avermann M, Tomm C, Mateo C, Gerstner W, Petersen CC. Microcircuits of excitatory and inhibitory neurons in layer 2/3 of mouse barrel cortex. J Neurophysiol. 2012;107:3116–3134. doi: 10.1152/jn.00917.2011. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. Numerical data on neocortical neurons in adult rat, with special reference to the GABA population. Brain Res. 1993;609:284–292. doi: 10.1016/0006-8993(93)90884-p. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- Berger TK, Perin R, Silberberg G, Markram H. Frequency-dependent disynaptic inhibition in the pyramidal network: a ubiquitous pathway in the developing rat neocortex. J Physiol. 2009;587:5411–5425. doi: 10.1113/jphysiol.2009.176552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock DD, Lee WC, Kerlin AM, Andermann ML, Hood G, Wetzel AW, Yurgenson S, Soucy ER, Kim HS, Reid RC. Network anatomy and in vivo physiology of visual cortical neurons. Nature. 2011;471:177–182. doi: 10.1038/nature09802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremaud A, West DC, Thomson AM. Binomial parameters differ across neocortical layers and with different classes of connections in adult rat and cat neocortex. Proc Natl Acad Sci U S A. 2007;104:14134–14139. doi: 10.1073/pnas.0705661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunel N, Wang XJ. What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation-inhibition balance. J Neurophysiol. 2003;90:415–430. doi: 10.1152/jn.01095.2002. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Tamas G, Szilagyi T, Stricker C, Paulsen O, Somogyi P. Effect, number and location of synapses made by single pyramidal cells onto aspiny interneurones of cat visual cortex. J Physiol. 1997;500(Pt 3):689–713. doi: 10.1113/jphysiol.1997.sp022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Isaac JT. Emergence of cortical inhibition by coordinated sensory-driven plasticity at distinct synaptic loci. Nat Neurosci. 2010;13:1240–1248. doi: 10.1038/nn.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J. Neocortical neuronal diversity: chemical heterogeneity revealed by colocalization studies of classic neurotransmitters, neuropeptides, calcium-binding proteins, and cell surface molecules. Cereb Cortex. 1993;3:273–289. doi: 10.1093/cercor/3.4.273. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu Rev Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69:1188–1203. doi: 10.1016/j.neuron.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D, Lund JS, Schmechel DE, Towles AC. Distribution of GABAergic neurons and axon terminals in the macaque striate cortex. J Comp Neurol. 1987;264:73–91. doi: 10.1002/cne.902640107. [DOI] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Somogyi P. Quantitative distribution of GABA-immunoreactive neurons in the visual cortex (area 17) of the cat. Exp Brain Res. 1986;61:323–331. doi: 10.1007/BF00239522. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Architecture of the prefrontal cortex and the central executive. Ann N Y Acad Sci. 1995;769:71–83. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Krimer LS, Povysheva NV, Barrionuevo G, Lewis DA. Functional properties of fast spiking interneurons and their synaptic connections with pyramidal cells in primate dorsolateral prefrontal cortex. J Neurophysiol. 2005;93:942–953. doi: 10.1152/jn.00787.2004. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Megias M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci. 1999;19:10082–10097. doi: 10.1523/JNEUROSCI.19-22-10082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Miles R, Sik A, Toth K, Tamamaki N, Freund TF. Hippocampal pyramidal cells excite inhibitory neurons through a single release site. Nature. 1993;366:683–687. doi: 10.1038/366683a0. [DOI] [PubMed] [Google Scholar]
- Helmstaedter M, Staiger JF, Sakmann B, Feldmeyer D. Efficient recruitment of layer 2/3 interneurons by layer 4 input in single columns of rat somatosensory cortex. J Neurosci. 2008;28:8273–8284. doi: 10.1523/JNEUROSCI.5701-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer SB, Ko H, Pichler B, Vogelstein J, Ros H, Zeng H, Lein E, Lesica NA, Mrsic-Flogel TD. Differential connectivity and response dynamics of excitatory and inhibitory neurons in visual cortex. Nat Neurosci. 2011;14:1045–1052. doi: 10.1038/nn.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren C, Harkany T, Svennenfors B, Zilberter Y. Pyramidal cell communication within local networks in layer 2/3 of rat neocortex. J Physiol. 2003;551:139–153. doi: 10.1113/jphysiol.2003.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston CM, Bright DP, Sivilotti LG, Beato M, Smart TG. Intracellular chloride ions regulate the time course of GABA-mediated inhibitory synaptic transmission. J Neurosci. 2009;29:10416–10423. doi: 10.1523/JNEUROSCI.1670-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Isaacson JS, Scanziani M. Postsynaptic mechanisms govern the differential excitation of cortical neurons by thalamic inputs. J Neurosci. 2009;29:9127–9136. doi: 10.1523/JNEUROSCI.5971-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan M, Blazquez-Llorca L, Merchan-Perez A, Anderson SA, DeFelipe J, Yuste R. Dense and overlapping innervation of pyramidal neurons by chandelier cells. J Neurosci. 2013;33:1907–1914. doi: 10.1523/JNEUROSCI.4049-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inda MC, Defelipe J, Munoz A. The distribution of chandelier cell axon terminals that express the GABA plasma membrane transporter GAT-1 in the human neocortex. Cereb Cortex. 2007;17:2060–2071. doi: 10.1093/cercor/bhl114. [DOI] [PubMed] [Google Scholar]
- Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat Neurosci. 2007;10:743–753. doi: 10.1038/nn1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J Neurosci. 1995;15:2638–2655. doi: 10.1523/JNEUROSCI.15-04-02638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kisvarday ZF, Toth E, Rausch M, Eysel UT. Orientation-specific relationship between populations of excitatory and inhibitory lateral connections in the visual cortex of the cat. Cereb Cortex. 1997;7:605–618. doi: 10.1093/cercor/7.7.605. [DOI] [PubMed] [Google Scholar]
- Krimer LS, Goldman-Rakic PS. Prefrontal microcircuits: membrane properties and excitatory input of local, medium, and wide arbor interneurons. J Neurosci. 2001;21:3788–3796. doi: 10.1523/JNEUROSCI.21-11-03788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimer LS, Zaitsev AV, Czanner G, Kroner S, Gonzalez-Burgos G, Povysheva NV, Iyengar S, Barrionuevo G, Lewis DA. Cluster analysis-based physiological classification and morphological properties of inhibitory neurons in layers 2-3 of monkey dorsolateral prefrontal cortex. J Neurophysiol. 2005;94:3009–3022. doi: 10.1152/jn.00156.2005. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Mateo C, Avermann M, Gentet LJ, Zhang F, Deisseroth K, Petersen CC. In vivo optogenetic stimulation of neocortical excitatory neurons drives brain-state-dependent inhibition. Curr Biol. 2011;21:1593–1602. doi: 10.1016/j.cub.2011.08.028. [DOI] [PubMed] [Google Scholar]
- Meyer HS, Schwarz D, Wimmer VC, Schmitt AC, Kerr JN, Sakmann B, Helmstaedter M. Inhibitory interneurons in a cortical column form hot zones of inhibition in layers 2 and 5A. Proc Natl Acad Sci U S A. 2011;108:16807–16812. doi: 10.1073/pnas.1113648108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB. An organizing principle of cerebral function: the unit module and the distributed system. In: Schmitt HO, editor. The Mindful Brain. MIT Press; Cambridge, Mass: 1982. pp. 1–50. [Google Scholar]
- Oswald AM, Doiron B, Rinzel J, Reyes AD. Spatial profile and differential recruitment of GABAB modulate oscillatory activity in auditory cortex. J Neurosci. 2009;29:10321–10334. doi: 10.1523/JNEUROSCI.1703-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka T, Kawaguchi Y. Cortical inhibitory cell types differentially form intralaminar and interlaminar subnetworks with excitatory neurons. J Neurosci. 2009;29:10533–10540. doi: 10.1523/JNEUROSCI.2219-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci. 2011;31:13260–13271. doi: 10.1523/JNEUROSCI.3131-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petilla Interneuron Nomenclature G, Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvarday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Munoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo-Rodriguez M, Wang Y, West DC, Yuste R. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povysheva NV, Zaitsev AV, Rotaru DC, Gonzalez-Burgos G, Lewis DA, Krimer LS. Parvalbumin-positive basket interneurons in monkey and rat prefrontal cortex. J Neurophysiol. 2008;100:2348–2360. doi: 10.1152/jn.90396.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid CA, Bekkers JM, Clements JD. Presynaptic Ca2+ channels: a functional patchwork. Trends Neurosci. 2003;26:683–687. doi: 10.1016/j.tins.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Ren JQ, Aika Y, Heizmann CW, Kosaka T. Quantitative analysis of neurons and glial cells in the rat somatosensory cortex, with special reference to GABAergic neurons and parvalbumin-containing neurons. Exp Brain Res. 1992;92:1–14. doi: 10.1007/BF00230378. [DOI] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Rozov A, Burnashev N, Sakmann B, Neher E. Transmitter release modulation by intracellular Ca2+ buffers in facilitating and depressing nerve terminals of pyramidal cells in layer 2/3 of the rat neocortex indicates a target cell-specific difference in presynaptic calcium dynamics. J Physiol. 2001;531:807–826. doi: 10.1111/j.1469-7793.2001.0807h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Sun QQ, Huguenard JR, Prince DA. Barrel cortex microcircuits: thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J Neurosci. 2006;26:1219–1230. doi: 10.1523/JNEUROSCI.4727-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Somogyi P. Fast IPSPs elicited via multiple synaptic release sites by different types of GABAergic neurone in the cat visual cortex. J Physiol. 1997;500(Pt 3):715–738. doi: 10.1113/jphysiol.1997.sp022054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM. Molecular frequency filters at central synapses. Prog Neurobiol. 2000;62:159–196. doi: 10.1016/s0301-0082(00)00008-3. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Lamy C. Functional maps of neocortical local circuitry. Front Neurosci. 2007;1:19–42. doi: 10.3389/neuro.01.1.1.002.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, West DC, Hahn J, Deuchars J. Single axon IPSPs elicited in pyramidal cells by three classes of interneurones in slices of rat neocortex. J Physiol. 1996;496(Pt 1):81–102. doi: 10.1113/jphysiol.1996.sp021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, West DC, Wang Y, Bannister AP. Synaptic connections and small circuits involving excitatory and inhibitory neurons in layers 2-5 of adult rat and cat neocortex: triple intracellular recordings and biocytin labelling in vitro. Cereb Cortex. 2002;12:936–953. doi: 10.1093/cercor/12.9.936. [DOI] [PubMed] [Google Scholar]
- Uematsu M, Hirai Y, Karube F, Ebihara S, Kato M, Abe K, Obata K, Yoshida S, Hirabayashi M, Yanagawa Y, Kawaguchi Y. Quantitative chemical composition of cortical GABAergic neurons revealed in transgenic venus-expressing rats. Cereb Cortex. 2008;18:315–330. doi: 10.1093/cercor/bhm056. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gupta A, Toledo-Rodriguez M, Wu CZ, Markram H. Anatomical, physiological, molecular and circuit properties of nest basket cells in the developing somatosensory cortex. Cereb Cortex. 2002;12:395–410. doi: 10.1093/cercor/12.4.395. [DOI] [PubMed] [Google Scholar]
- Wang Y, Markram H, Goodman PH, Berger TK, Ma J, Goldman-Rakic PS. Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nat Neurosci. 2006;9:534–542. doi: 10.1038/nn1670. [DOI] [PubMed] [Google Scholar]
- Woodruff A, Xu Q, Anderson SA, Yuste R. Depolarizing effect of neocortical chandelier neurons. Front Neural Circuits. 2009;3:15. doi: 10.3389/neuro.04.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y, Callaway EM. Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nat Neurosci. 2005;8:1552–1559. doi: 10.1038/nn1565. [DOI] [PubMed] [Google Scholar]
- Yuan K, Shih JY, Winer JA, Schreiner CE. Functional networks of parvalbumin-immunoreactive neurons in cat auditory cortex. J Neurosci. 2011;31:13333–13342. doi: 10.1523/JNEUROSCI.1000-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev AV, Povysheva NV, Gonzalez-Burgos G, Rotaru D, Fish KN, Krimer LS, Lewis DA. Interneuron diversity in layers 2-3 of monkey prefrontal cortex. Cereb Cortex. 2009;19:1597–1615. doi: 10.1093/cercor/bhn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev AV, Povysheva NV, Lewis DA, Krimer LS. P/Q-type, but not N-type, calcium channels mediate GABA release from fast-spiking interneurons to pyramidal cells in rat prefrontal cortex. J Neurophysiol. 2007;97:3567–3573. doi: 10.1152/jn.01293.2006. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]


