Abstract
The basic concept for the application of vital dyes during vitreoretinal surgery is to assist in highlighting preretinal membranes and tissues which are very thin and semitransparent and thus difficult to detect. The vital dyes may be classified according to different criteria, where the most commonly applied includes chemical classification. In ophthalmic surgery, vital dyes are widely used in cataract and vitreoretinal surgery. The vital dyes, indocyanine green, infracyanine green, and brilliant blue stain the internal limiting membrane, and trypan blue and triamcinolone acetonide help to visualize epiretinal membranes and vitreous, respectively. This review exhibits the current literature regarding the properties of vital dyes, techniques of application, indications, and toxicities during vitreoretinal surgery and, also suggests that the field of chromovitrectomy represents an expanding area of research.
Keywords: Chromovitrectomy, Vital dyes, Indocyanine green, Internal limiting membrane, Vitreoretinal surgery
Introduction
The field of vitreoretinal surgery has grown over the last several decades. Scientific advances improved our understanding of disease pathology and new surgical adjuncts and techniques have decreased surgical time and increased success rate. One of the innovations in vitreoretinal surgery over the past 10 years has been the introduction of vital dyes to improve the visualization of preretinal tissues and membranes.[1], [2], [3], [4]
The term “chromovitrectomy” refers to the use of vital dyes during vitreoretinal surgery to assist in the identification of preretinal tissues and membranes.5 In 2000, the modern approach was first introduced, when the dye indocyanine green (ICG) was used to stain the thin semitransparent internal limiting membrane (ILM), and it is currently the most commonly used surgical dye in ophthalmology. However, over the past few years, controversial evidence has accumulated indicating that these dyes may have harmful effects. Following initial experience with ICG, clinical and experimental studies demonstrated signs of the retinal toxicity of ICG, which stimulated research on alternative dyes for chromovitrectomy. Some additional alternative biostains, including trypan blue (TB), or brilliant blue (BriB), and patent blue (PB), have been added to the surgical collection for chromovitrectomy.6 This review presents the latest data on chromovitrectomy in regard to the biochemical properties, indications, and clinical experience with various vital dyes available for chromovitrectomy.
Biochemical pharmacology
Vital staining refers to the coloration of living cells or tissues. Dyes are organic molecules containing chromophores. A chromophore is the part of a molecule responsible for its color.7
The staining agents may be classified according to the chemical classification. Some of the groups of dyes already used in chromovitrectomy are: (1) azo dyes; (2) arylmethane dyes; (3) cyanine dyes; (4) xanthene dyes; and (5) colored corticosteroids.
Azo dyes are a class of synthetic organic dyes with nitrogen in the azo form of –N N– in their structure. TB is an anionic hydrophilic azo dye which has the molecular formula C34H24N6Na4O14S4 and a molecular weight of 960 Da. TB is so-called because it can kill trypanosomes, the parasites that cause sleeping sickness.8 TB crosses the cell membranes of dead cells only, because of that stains dead tissues/cells blue. In ophthalmology, TB has in a preferential manner affinity for the epiretinal membrane (ERM).[9], [10], [11], [12], [13] TB may be commercially available at a concentration of 0.15% for vitreoretinal surgery, called Membrane Blue (DORC International, Zuidland, Netherlands).
Cyanine dyes are a class of dyes containing a –CH group linking two heterocyclic rings containing nitrogen. The cyanine agent has amphiphilic properties and thereby binds to both cellular and acellular elements in living tissues. ICG is a tricarbocyanine anionic vital dye with a molecular formula of C43H47N2NaO6S2 and a molecular weight of 775 Da. The sterile hydrophilic powder represents a very useful contrast agent in angiography, allowing imaging of choroidal and retinal tissues.[14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28] For ophthalmology, ICG is commercially available under the names of ICG (Pulsion Medical Systems, Munich, Germany; 25- and 50-mg vials), ICV Indocianina Verde (Ophthalmos, São Paulo, Brazil; 5-, 25-, and 50-mg vials), Diagnogreen (Daiichi Pharmaceutical, Tokyo, Japan; 25-mg vial), and IC-Green (Akorn, Buffalo Grove, USA; 25-mg vial).
Infracyanine Green (IfCG) is a green dye with a chemical formula and pharmacologic properties similar to those of ICG. However, IfCG has a clear advantage over ICG because it is synthesized without sodium iodine, as it is believed that iodine damages the cornea and retina.[29], [30], [31] On the other hand, iodide-free IfCG is not water soluble and has to be dissolved in a 5% glucose solvent IfCG.[32], [33], [34] It is commercially available under the brand name of Infracyanine (Laboratoires SERB, Paris, France; 25-mg vial).
Arylmethane dyes are stains which are formed by one carbon linked to benzene or naphthalene groups; they are commonly used in modern inks. BriB is a blue anionic arylmethane compound which has the chemical formula of C47H48N3S2O7Na and a molecular weight of 854 Da. This dye was reported to stain the ILM and to have no significant in vivo toxicity.[23], [24], [25], [26] The dye gained acceptance for intraocular use under the brand name of Brilliant Peel (Geuder, Heidelberg, Germany), and it is provided in vials containing 2 mg/ml of the vital dye. Another example is Bromophenol blue (BroB) which is an acid–base indicator that has recently been proposed as a promising alternative biostain for vitrectomy, because it has induced no damage in either in vitro or in vivo studies.[15], [16] It has a molecular weight of 670 Da and the chemical formula is C19H10Br4O5S. PB is a hydrophilic anionic triarylmethane dye with the chemical formula C27H31N2NaO6S2 and a molecular weight of 582 Da. PB has been applied as an off-label agent in vitreoretinal surgery.
Xanthene is a yellow organic heterocyclic compound. Its chemical formula is C13H10O. Xanthene dyes tend to be fluorescent, yellow to pink to bluish red, brilliant dyes. Fluorescein is a xanthene fluorophore with the chemical structure C20H12O5 and a molecular weight of 332 Da.
Corticosteroids are hormones produced naturally in the cortex of the adrenal gland, whose derivates may be synthetically produced to be used as drugs in the treatment of human diseases. Triamcinolone acetonide (TA) is a synthetic insoluble corticosteroid with the empirical formula C24H31FO6 and a molecular weight of 434 Da. In ocular surgery, TA has manifested a good staining of the vitreous because of the crystal composition.
Indications and toxicology
Indocyanine green
ICG facilitates ILM removal intraoperatively because it has an affinity for extracellular matrix components of the ILM, such as collagen and fibronectin.[1], [2], [3], [4], [5] ICG had been used in chromovitrectomy for macular hole treatment (Fig. 1).[1], [2], [3], [4], [5], [6], [35], [36], [37]
Figure 1.

Vitreal injection of indocyanine green (ICG) to stain Internal limiting membrane (ILM) in maculer hole surgery.
ICG has also been used to help ILM peeling in other diseases, such as diabetic macular edema (DME). Consecutive surgical experience determined that the use of ICG-assisted ILM peeling in DME induced no sign of retinal toxicity by visual acuity measurements when comparing vitrectomy with and without ILM peeling.[38], [39], [40], [41]
Although the best indication for ICG use in chromovitrectomy is for ILM staining in MH surgery, ICG has been proposed for better visualization of epiretinal membranes (ERMs) in vitrectomy for proliferative diabetic vitreoretinopathy, idiopathic ERMs, and proliferative vitreoretinopathy (PVR).[42], [43], [44] However, the green dye may stain the acellular ILM better; as for the task of ERM staining, other vital stains may be better.
Regarding the toxicity of ICG, some investigators have reported that the toxic effects of ICG are time and dose dependent.[45], [46] However, Morales et al.47 indicated that the effects of ICG are not strongly dose dependent, at least at the physiological concentrations examined. ICG has been found to be associated with the risk of damage to the photoreceptors and RPE cells,[48], [49] atrophy of the retinal pigment epithelium (RPE),50 loss of epiretinal cellular integrity,51 and cellular toxicity,[52], [53], [54], [55] among other harmful effects. In conclusion, although much evidence suggests that ICG may exert toxic effects on the retina, the staining agent in low doses should be safe for chromovitrectomy.
Infracyanine green
IfCG also binds with high affinity to the acellular ILM and facilitates its visualization and peeling similar to ICG. It is safer than ICG due to the absence of iodine in its formulation, and has reduced adverse effects.[29], [30], [31], [32], [33], [34], [56] IfCG at a concentration of 0.5 mg/ml results in adequate ILM identification and less toxic effects.57 No evidence of significant acute toxicity is associated with IfCG.47 However, IfCG can be phagocytosed by RPE cells, remaining in the interior of these cells for long periods, with a risk of inducing chronic toxicity.34
Trypan blue
TB usage recommends blue dye application mainly for ERM staining.[9], [10], [11], [12] TB exhibits outstanding affinity for ERM because of the strong presence of dead glial cells within those membranes. I personally use it for immature PVR which may minimize mechanical trauma to the retina during ERM removal and allow the recognition of the whole extent of the ERM. TB in various doses may enhance the ability to detect both the prolapsed vitreous to the anterior chamber and the posterior vitreous remaining in the vitreous cavity, but it is inferior to TA.58 Regarding the chronic toxicity of TB, it has been reported that it induces arrest of the cell cycle at G0–G1 via increased expression of p21.59 Some researchers showed that a subretinal injection of 0.05% ICG results in a more substantial retinal damage than that associated with subretinal injection of 0.15% TB.57
Brilliant blue
BriB has been introduced as a surgical adjuvant for chromovitrectomy in 2006 and, this dye was reported to stain the ILM and to have no significant in vivo toxicity.[23], [24] Cervera et al.60 showed similar outcomes with good ILM staining and clinical results and no signs of toxicity in multifocal ERG. BBG is more hydrosoluble than ICG and IfCG; it would thus penetrate less into the cells and be more easily washed away, leaving less residues after surgery.47 For this reason, BriB represents a good alternative for ICG and IfCG in chromovitrectomy due to its suitable affinity for ILM (Fig. 2).
Figure 2.

Brilliant blue is an excellent tool in optimizing internal limiting membrane peeling which enables a clear distinction between the unstained retina and the stained overlying fine ILM tissue.
Patent blue
The utility of the PB dye for applications in ophthalmology has only recently been discovered, and relatively little is known about its effects. For this reason, the present findings are of particular interest.47 It has a moderate affinity for ERM and vitreous, but a poor affinity for the ILM.17 PB affects human retinal function when applied for at least 1 min. However, no irreversible effects on the human ERG were seen even after 2 min of retinal exposure to patent blue. Thus, toxic effects on retinal function after intraoperative short-term application of patent blue 0.48% appear unlikely to occur.18 Subretinal injection of TB induced a more significant clinical and histologic damage of neurosensory retina/RPE than did PB or Balanced Salt Solution (BSS).19
Bromophenol blue
BroB has been applied in ocular surgery: the dark-blue stain may represent a novel useful adjunct for both cataract and vitreoretinal surgery. BroB stained the retinal surface and lens capsule at a low concentration (0.2%) with no signs of toxicity, this dye seems to be the most promising candidate for application in humans.15 Schuettauf et al.16 demonstrated that BroB dye tested in his study did not lead to detectable toxic effects in the rat eye, even after prolonged presence within the eye and an observation period of 7 days. Other novel dyes as well as ICG showed toxic effects, such as histologic alteration of the retina and loss of RGCs.
Sodium fluorescein
SF is highly safe for fundus angiography at concentrations of 5–25%. The intravitreal 0.20% SF dye improves the visualization of clear vitreous fibers through a green staining during chromovitrectomy. The clear vitreous can be stained markedly green by SF administered 12–16 h before surgery.61 A preoperative diagnostic fluorescein angiography in eyes with active uveitis or diabetic retinopathy may lead to a moderate accumulation of the dye in the vitreous cavity and greenish staining of the vitreous cortex at the vitreoretinal interface.[62], [63] SF 0.6% can safely be used in the vitreous cavity for easy identification of clear uncut vitreous gel for clear vitreous vitrectomy. This enhances efficient excision of this tissue.64
Triamcinolone acetonide
TA produces the best vitreous visibility in comparison to other stains.65 The crystals of the steroid adhere to the acellular tissue, thereby enabling a clear contrast between the empty vitreous cavity compared to areas with the vitreous fibers remaining.66 The surgical technique for TA application consists of a direct injection of the agent into the vitreous cavity toward the area of interest. TA-assisted removal of internal limiting membrane was used in many cases since the white specks and crystals may deposit over the ILM, thereby facilitating ILM removal (Fig. 3).[67], [68] Crystals of TA have been detected up to 40 days postsurgery with chromovitrectomy for MH surgery. For this reason, some authors suggest that postoperative residual TA could diminish the healing process necessary for MH closure.69 Injecting this steroid during vitrectomy for the management of retinal detachment may prevent fibrin reaction and PVR postoperatively.[70], [71], [72] The commonly used formulation of TA, kenalog, is not formulated for the eye, for this reason, there is a risk of pseudoendothalmitis and retina toxicity when injected intravitreally.[73], [74] There have been reports of toxicity of TA on retinal pigment epithelial cells (RPE) in vitro75 whereas ex vivo and in vivo studies have not shown any significant toxicity on the retina.76
Figure 3.
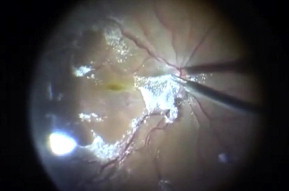
The steroid triamcinolone acetonide (TA) stains the vitreous powerfully and by that means ensures that the whole posterior vitreous cortex is removed during surgery.
Vital dyes and light sources
Interaction of light from endoillumination source and vital dye may increase or decrease the risk for toxicity. Light-induced retinal toxicity by the endoilluminator is dependent on factors such as the duration of use, type, power, and wavelength of light source.[77], [78] Vital dyes are small chemical substances that pass freely through retinal tissue and may play a role in or exacerbate retinal phototoxicity from intraoperative light exposure.79 Photosensitizing dyes could enhance phototoxicity by increasing levels of free radicals, creating a photoproduct that could be harmful to retinal cells and shifting light absorbance from one site of the retina to another.79 In this regard, the dye on the retinal surface could increase the risk for phototoxicity to the neuroretina for light greater than 450 nm, which would not occur without dyes.80 In addition, dyes in the subretinal space may exacerbate damage to the RPE after exposure to various wavelengths of light.81 From a surgery perspective, among all light sources analyzed by Costa et al.79, the greatest overlap was found with integrated laser pathway (Photon Xenon; Synergetics Photon) and halogen (Grieshaber GLS; GLS Corp.), and the least overlap was found with mercury vapor lamp (Photon 2; Synergetics). The lowest overlap values among the dyes were observed with ICG prepared in PSS, followed by IC, which showed low values for all three solvents compared with other dyes.
Dye injection techniques
There are different ways to protect the RPE cells during dye injection in the MH surgery: slow injection of the dye, placing substances over the MH such as sodium hyaluronate.82 perflourocarbons liquids (PFCL),83 autologous whole blood84, or use of VINCE (vitreoretinal internal limiting membrance color enhancer) which enables selective painting of ILM that needs to be removed without staining perifoveal and peripheral retinae.85 The most common two ways in dye injection technique are:
-
1.
The “dry method” or “air-filled technique”. This technique consists of removing the fluid in the vitreous cavity by a fluid-gas exchange before dye injection.85 While the technique has the advantage of concentrating the dye in the posterior pole and avoiding contact at the posterior capsule of the lens, it may expose the retinal surface to a higher concentration of dye to the vitreoretinal interface.5
-
2.
The “wet method” or “fluid-filled technique.” In this approach, the intravitreal fluid (usually balanced salt solution) is left inside the vitreous cavity, while the surgeon injects the dye. The amount of dye in contact with the retinal surface becomes much lower because it is immediately washed out by the fluid in the vitreous cavity.87 Czajka et al. compared the two methods in a porcine model and concluded that the air-filled technique induces a higher incidence of RPE atrophy and outer retinal degeneration than the fluid-filled technique.86 In conclusion, the wet method is more safer and faster during surgery than dry method.
Conclusion
Exploration of the breakthrough chromovitrectomy supports innovative microsurgical techniques with improved patient outcomes for both anterior and posterior segment procedures. Recent recommendations for the application of dyes during vitreoretinal surgery designate that ICG, IfCG, BriB, and BroB may be the best stains for ILM, while TB and PB may be preferred for staining the glial ERM. In addition, the white steroid TA is an amazing staining agent for vitreous visualization. Regarding to the toxicity issues, no decided safety profiles for the different dyes in chromovitrectomy. In addition, some good words include avoidance of long macular exposure to endoillumination and, low amount of dye injection.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Burk S.E., Da Mata A.P., Snyder M.E., Rosa R.H., Jr, Foster R.E. Indocyanine green-assisted peeling of the retinal internal limiting membrane. Ophthalmology. 2000;107(11):2010–2014. doi: 10.1016/s0161-6420(00)00375-4. [DOI] [PubMed] [Google Scholar]
- 2.Kadonosono K., Itoh N., Uchio E., Nakamura S., Ohno S. Staining of the internal limiting membrane in macular hole surgery. Arch Ophthalmol. 2000;118(8):1116–1118. doi: 10.1001/archopht.118.8.1116. [DOI] [PubMed] [Google Scholar]
- 3.Gandorfer A., Messmer E.M., Ulbig M.W., Kampik A. Indocyanine green selectively stains the internal limiting membrane. Am J Ophthalmol. 2001;131(3):387–388. doi: 10.1016/s0002-9394(00)00924-7. [DOI] [PubMed] [Google Scholar]
- 4.Da Mata A.P., Burk S.E., Riemann C.D., et al. Indocyanine green assisted peeling of the retinal internal limiting membrane during vitrectomy surgery for macular hole repair. Ophthalmology. 2001;108(7):1187–1192. doi: 10.1016/s0161-6420(01)00593-0. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues E.B., Meyer C.H., Kroll P. Chromovitrectomy: a new field in vitreoretinal surgery. Graefes Arch Clin Exp Ophthalmol. 2005;243:291–293. doi: 10.1007/s00417-004-0992-x. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues E.B., Maia M., Meyer C.H., et al. Vital dyes for chromovitrectomy. Curr Opin Ophthalmol. 2007;18:179–187. doi: 10.1097/ICU.0b013e32811080b5. [DOI] [PubMed] [Google Scholar]
- 7.IUPAC Gold Book Chromophore.
- 8.Chapter “Detection of Caspase Activation Combined with Other Probes of Apoptosis”, Eurekah Bioscience Collection, NCBI bookshelf.
- 9.Li K., Wong D., Hiscott P., Stanga P., Groenewald C., McGalliard J. Trypan blue staining of internal limiting membrane and epiretinal membrane during vitrectomy: visual results and histopathological findings. Br J Ophthalmol. 2003;87(2):216–219. doi: 10.1136/bjo.87.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vote B.J., Russell M.K., Joondeph B.C. Trypan blue-assisted vitrectomy. Retina. 2004;24(5):736–738. doi: 10.1097/00006982-200410000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Perrier M., Sébag M. Trypan blue-assisted peeling of the internal limiting membrane during macular hole surgery. Am J Ophthalmol. 2003;135(6):903–905. doi: 10.1016/s0002-9394(02)02239-0. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues E.B., Meyer C.H., Schmidt J.C., Kroll P. Trypan blue stains the epiretinal membrane but not the internal limiting membrane. Br J Ophthalmol. 2003;87(11):1431–1432. doi: 10.1136/bjo.87.11.1431-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee K.L., Dean S., Guest S. A comparison of outcomes after indocyanine green and trypan blue assisted internal limiting membrane peeling during macular hole surgery. Br J Ophthalmol. 2005;89(4):420–424. doi: 10.1136/bjo.2004.049684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson T.L., Hillenkamp J., Knight B.C., et al. Safety testing of indocyanine green and trypan blue using retinal pigment epithelium and glial cell cultures. Invest Ophthalmol Vis Sci. 2004;45(8):2778–2785. doi: 10.1167/iovs.04-0320. [DOI] [PubMed] [Google Scholar]
- 15.Haritoglou C., Tadayoni R., May C.A., et al. Short-term in vivo evaluation of novel vital dyes for intraocular surgery. Retina. 2006;26(6):673–678. doi: 10.1097/01.iae.0000236505.42892.54. [DOI] [PubMed] [Google Scholar]
- 16.Schuettauf F., Haritoglou C., May C.A., et al. Administration of novel dyes for intraocular surgery: an in vivo toxicity animal study. Invest Ophthalmol Vis Sci. 2006;47(8):3573–3578. doi: 10.1167/iovs.06-0211. [DOI] [PubMed] [Google Scholar]
- 17.Mennel S., Meyer C.H., Tietjen A., Rodrigues E.B., Schmidt J.C. Patent blue: a novel vital dye in vitreoretinal surgery. Ophthalmologica. 2006;220(3):190–193. doi: 10.1159/000091764. [DOI] [PubMed] [Google Scholar]
- 18.Lüke C., Lüke M., Sickel W., Schneider T. Effects of patent blue on human retinal function. Graefes Arch Clin Exp Ophthalmol. 2006;244(9):1188–1190. doi: 10.1007/s00417-005-0239-5. [DOI] [PubMed] [Google Scholar]
- 19.Maia M., Penha F., Rodrigues E.B., et al. Effects of subretinal injection of patent blue and trypan blue in rabbits. Curr Eye Res. 2007;32(4):309–317. doi: 10.1080/02713680701199377. [DOI] [PubMed] [Google Scholar]
- 20.Laughton C. Quantification of attached cells in microtiter plates based on Coomassie brilliant blue G-250 staining of total cellular protein. Anal Biochem. 1984;140(2):417–423. doi: 10.1016/0003-2697(84)90187-8. [DOI] [PubMed] [Google Scholar]
- 21.Said-Fernández S., González-Garza M.T., Mata-Cárdenas B.D., Navarro-Marmolejo L. A multipurpose solid-phase method for protein determination with Coomassie brilliant blue G-250 (published correction in Anal Biochem 1991;197(1):276) Anal Biochem. 1990;191(1):119–126. doi: 10.1016/0003-2697(90)90397-r. [DOI] [PubMed] [Google Scholar]
- 22.Westermeier R. Sensitive, quantitative, and fast modifications for Coomassie blue staining of polyacrylamide gels. Proteomics. 2006;6(Suppl. 2):61–64. doi: 10.1002/pmic.200690121. [DOI] [PubMed] [Google Scholar]
- 23.Enaida H., Hisatomi T., Goto Y., et al. Preclinical investigation of internal limiting membrane staining and peeling using intravitreal brilliant blue G. Retina. 2006;26(6):623–630. doi: 10.1097/01.iae.0000236470.71443.7c. [DOI] [PubMed] [Google Scholar]
- 24.Enaida H., Hisatomi T., Hata Y., et al. Brilliant blue G selectively stains the internal limiting membrane/brilliant blue G-assisted membrane peeling. Retina. 2006;26(6):631–636. doi: 10.1097/01.iae.0000236469.71443.aa. [DOI] [PubMed] [Google Scholar]
- 25.Hisatomi T., Enaida H., Matsumoto H., et al. Staining ability and biocompatibility of brilliant blue G: preclinical study of brilliant blue G as an adjunct for capsular staining. Arch Ophthalmol. 2006;124(4):514–519. doi: 10.1001/archopht.124.4.514. [DOI] [PubMed] [Google Scholar]
- 26.Ueno A., Hisatomi T., Enaida H., et al. Biocompatibility of brilliant blue G in a rat model of subretinal injection. Retina. 2007;27(4):499–504. doi: 10.1097/IAE.0b013e318030a129. [DOI] [PubMed] [Google Scholar]
- 27.Dunn K.C., Aotaki-Keen A.E., Putkey F.R., Hjelmeland L.M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62(2):155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 28.Peters S., Altvater A., Bopp S., et al. Systematic evaluation of ICG and trypan blue related effects on ARPE-19 cells in vitro. Exp Eye Res. 2007;85(6):880–889. doi: 10.1016/j.exer.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 29.Ullern M., Roman S., Dhalluin J.F., et al. Contribution of intravitreal infracyanine green to macular hole and epimacular membrane surgery: preliminary study. J Fr Ophthalmol. 2002;25(9):915–920. [PubMed] [Google Scholar]
- 30.Stalmans P., Van Aken E.H., Veckeneer M., Feron E.J., Stalmans I. Toxic effect of indocyanine green on retinal pigment epithelium related to osmotic effects of the solvent. Am J Ophthalmol. 2002;134(2):282–285. doi: 10.1016/s0002-9394(02)01468-x. [DOI] [PubMed] [Google Scholar]
- 31.Rivett K., Kruger L., Radloff S. Infracyanine-assisted internal limiting membrane peeling in macular hole repair: does it make a difference? Graefes Arch Clin Exp Ophthalmol. 2004;242(5):393–396. doi: 10.1007/s00417-003-0857-8. [DOI] [PubMed] [Google Scholar]
- 32.Haritoglou C., Gandorfer A., Gass C.A., Kampik A. Histology of the vitreoretinal interface after staining of the internal limiting membrane using glucose 5% diluted indocyanine and infracyanine green. Am J Ophthalmol. 2004;137(2):345–348. doi: 10.1016/S0002-9394(03)00845-6. [DOI] [PubMed] [Google Scholar]
- 33.Jackson T.L., Vote B., Knight B.C., El-Amir A., Stanford M.R., Marshall J. Safety testing of infracyanine green using retinal pigment epithelium and glial cell cultures. Invest Ophthalmol Vis Sci. 2004;45(10):3697–3703. doi: 10.1167/iovs.04-0387. [DOI] [PubMed] [Google Scholar]
- 34.Kodjikian L., Richter T., Halberstadt M., et al. Toxic effects of indocyanine green, infracyanine green, and trypan blue on the human retinal pigmented epithelium. Graefes Arch Clin Exp Ophthalmol. 2005;243(9):917–925. doi: 10.1007/s00417-004-1121-6. [DOI] [PubMed] [Google Scholar]
- 35.Haritoglou C., Gass C.A., Schaumberger M., et al. Long-term follow-up after macular hole surgery with internal limiting membrane peeling. Am J Ophthalmol. 2002;134:661–666. doi: 10.1016/s0002-9394(02)01751-8. [DOI] [PubMed] [Google Scholar]
- 36.Sheidow T.G., Blinder K.J., Holekamp N., et al. Outcome results in macular hole surgery: an evaluation of internal limiting membrane peeling with and without indocyanine green. Ophthalmology. 2003;110(9):1697–1701. doi: 10.1016/S0161-6420(03)00562-1. [DOI] [PubMed] [Google Scholar]
- 37.Wolf S., Reichel M.B., Wiedemann P., Schnurrbusch U.E. Clinical findings in macular hole surgery with indocyanine green-assisted peeling of the internal limiting membrane. Graefes Arch Clin Exp Ophthalmol. 2003;241(7):589–592. doi: 10.1007/s00417-003-0673-1. [DOI] [PubMed] [Google Scholar]
- 38.Yanyali A., Horozoglu F., Celik E., Nohutcu A.F. Long-term outcomes of pars plana vitrectomy with internal limiting membrane removal in diabetic macular edema. Retina. 2007;27(5):557–566. doi: 10.1097/01.iae.0000249390.61854.d5. [DOI] [PubMed] [Google Scholar]
- 39.Ando F., Yasui O., Hirose H., Ohba N. Optic nerve atrophy after vitrectomy with indocyanine green-assisted internal limiting membrane peeling in diffuse diabetic macular edema. Adverse effect of ICG-assisted ILM peeling. Graefes Arch Clin Exp Ophthalmol. 2004;242(12):995–999. doi: 10.1007/s00417-004-0864-4. [DOI] [PubMed] [Google Scholar]
- 40.Hartley K.L., Smiddy W.E., Flynn H.W., Jr, Murray T.G. Pars plana vitrectomy with internal limiting membrane peeling for diabetic macular edema. Retina. 2008;28(3):410–419. doi: 10.1097/IAE.0b013e31816102f2. [DOI] [PubMed] [Google Scholar]
- 41.Dehghan M.H., Salehipour M., Naghib J., Babaeian M., Karimi S., Yaseri M.J. Pars plana vitrectomy with internal limiting membrane peeling for refractory diffuse diabetic macular edema. Ophthalmic Vis Res. 2010;5(3):162–167. [PMC free article] [PubMed] [Google Scholar]
- 42.Hillenkamp J., Saikia P., Gora F., et al. Macular function and morphology after peeling of idiopathic epiretinal membrane with and without the assistance of indocyanine green. Br J Ophthalmol. 2005;89(4):437–443. doi: 10.1136/bjo.2004.051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwok A.Kh., Lai T.Y., Yuen K.S. Epiretinal membrane surgery with or without internal limiting membrane peeling. Clin Experiment Ophthalmol. 2005;33(4):379–385. doi: 10.1111/j.1442-9071.2005.01015.x. [DOI] [PubMed] [Google Scholar]
- 44.Lee J.W., Kim I.T. Outcomes of idiopathic macular epiretinal membrane removal with and without internal limiting membrane peeling: a comparative study. Jpn J Ophthalmol. 2010;54(2):129–134. doi: 10.1007/s10384-009-0778-0. [DOI] [PubMed] [Google Scholar]
- 45.Hsu S.L., Kao Y.H., Wu W.C. Effect of indocyanine green on the growth and viability of cultured human retinal pigment epithelial cells. J Ocul Pharmacol Ther. 2004;20(4):353–362. doi: 10.1089/1080768041725362. [DOI] [PubMed] [Google Scholar]
- 46.Yuen D., Gonder J., Proulx A., Liu H., Hutnik C. Comparison of the in vitro safety of intraocular dyes using two retinal cell lines: a focus on brilliant blue G and indocyanine green. Am J Ophthalmol. 2009;147(2):251–259. doi: 10.1016/j.ajo.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 47.Morales M.C., Freire V., Asumendi A., et al. Comparative effects of six intraocular vital dyes on retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2011;51(11):6018–6029. doi: 10.1167/iovs.09-4916. [DOI] [PubMed] [Google Scholar]
- 48.Engelbrecht N.E., Freeman J., Sternberg P., Jr, et al. Retinal pigment epithelial changes after macular hole surgery with indocyanine green-assisted internal limiting membrane peeling. Am J Ophthalmol. 2002;133(1):89–94. doi: 10.1016/s0002-9394(01)01293-4. [DOI] [PubMed] [Google Scholar]
- 49.Maia M., Kellner L., de Juan E., Jr Effects of indocyanine green injection on the retinal surface and into the subretinal space in rabbits. Retina. 2004;24(1):80–91. doi: 10.1097/00006982-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Hirata A., Inomata Y., Kawaji T., Tanihara H. Persistent subretinal indocyanine green induces retinal pigment epithelium atrophy. Am J Ophthalmol. 2003;136(2):353–355. doi: 10.1016/s0002-9394(03)00121-1. [DOI] [PubMed] [Google Scholar]
- 51.Rezai K.A., Farrokh-Siar L., Ernest J.T., van Seventer G.A. Indocyanine green induces apoptosis in human retinal pigment epithelial cells. Am J Ophthalmol. 2004;137(5):931–933. doi: 10.1016/j.ajo.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 52.Ho J.D., Tsai R.J., Chen S.N., Chen H.C. Toxic effect of indocyanine green on retinal pigment epithelium related to osmotic effects of the solvent. Am J Ophthalmol. 2003;135(2):258. doi: 10.1016/s0002-9394(02)01976-1. [DOI] [PubMed] [Google Scholar]
- 53.Maia M., Margalit E., Lakhanpal R., et al. Effects of intravitreal indocyanine green injection in rabbits. Retina. 2004;24(1):69–79. doi: 10.1097/00006982-200402000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Iriyama A., Uchida S., Yanagi Y., et al. Effects of indocyanine green on retinal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45(3):943–947. doi: 10.1167/iovs.03-1026. [DOI] [PubMed] [Google Scholar]
- 55.Murata M., Shimizu S., Horiuchi S., Sato S. The effect of indocyanine green on cultured retinal glial cells. Retina. 2005;25(1):75–80. doi: 10.1097/00006982-200501000-00011. [DOI] [PubMed] [Google Scholar]
- 56.Farah M.E., Maia M., Rodrigues E.B. Dyes in ocular surgery: principles for use in chromovitrectomy. Am J Ophthalmol. 2009;148(3):332–340. doi: 10.1016/j.ajo.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Penha F.M., Maia M., Farah M.E., et al. Morphologic and clinical effects of subretinal injection of indocyanine green and infracyanine green in rabbits. J Ocul Pharmacol Ther. 2008;24(1):52–61. doi: 10.1089/jop.2007.0047. [DOI] [PubMed] [Google Scholar]
- 58.Guo S., Tutela A.C., Wagner R., et al. A comparison of the effectiveness of four biostains in enhancing visualization of the vitreous. J Pediatr Ophthalmol Strabismus. 2006;43:281–284. doi: 10.3928/01913913-20060901-02. [DOI] [PubMed] [Google Scholar]
- 59.Kwok A.K., Yeung C.K., Lai T.Y., Chan K.P., Pang C.P. Effects of trypan blue on cell viability and gene expression in human retinal pigment epithelial cells. Br J Ophthalmol. 2004;88(12):1590–1594. doi: 10.1136/bjo.2004.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cervera E., Diaz-Llopis M., Salom D., et al. Internal limiting membrane staining using intravitreal brilliant blue G: good help for vitreo-retinal surgeon in training. Arch Soc Esp Oftalmol. 2007;82:71–72. doi: 10.4321/s0365-66912007000200003. [DOI] [PubMed] [Google Scholar]
- 61.Yao Y., Wang Z.J., Wei S.H., Huang Y.F., Zhang M.N. Oral sodium fluorescein to improve visualization of clear vitreous during vitrectomy for proliferative diabetic retinopathy. Clin Experiment Ophthalmol. 2007;35(9):824–827. doi: 10.1111/j.1442-9071.2007.01610.x. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt J.C., Chofflet J., Hörle S., Mennel S., Meyer C.H. Three simple approaches to visualize the transparent vitreous cortex during vitreoretinal surgery. Dev Ophthalmol. 2008;42:35–42. doi: 10.1159/000138923. [DOI] [PubMed] [Google Scholar]
- 63.Yokoi T., Yokoi T., Hiraoka M., Nishina S., Azuma N. Fluorescein staining of the vitreous during vitrectomy for retinopathy of prematurity. Kobayashi. Retina. 2011;31(8):1717–1719. doi: 10.1097/IAE.0b013e318227a9d9. [DOI] [PubMed] [Google Scholar]
- 64.Das T., Vedantham V. Intravitreal sodium fluorescein enhances visualization of clear vitreous during vitreous surgery for macular hole: a safety and efficacy study. Clin Experiment Ophthalmol. 2004;32(1):55–57. doi: 10.1046/j.1442-9071.2003.00758.x. [DOI] [PubMed] [Google Scholar]
- 65.Kaynak S., Celik L., Kocak N., Oner F.H., Kaynak T., Cingil G. Staining of vitreous with triamcinolone acetonide in retained lens surgery with phacofragmentation. J Cataract Refract Surg. 2006;32(1):56–59. doi: 10.1016/j.jcrs.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 66.Yamakoshi T., Kachi S., Sugita J., Asami T., Ishikawa K., Ito Y., et al. Triamcinolone-assisted removal of internal limiting membrane enhances the effect of vitrectomy for diabetic macular edema. Ophthalmic Res. 2009;41(4):203–209. doi: 10.1159/000217724. [DOI] [PubMed] [Google Scholar]
- 67.Koto T., Inoue M., Shinoda K., et al. Residual crystals of triamcinolone acetonide in macular hole may prevent complete closure. Acta Ophthalmol Scand. 2007;85:913–914. doi: 10.1111/j.1600-0420.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 68.Kumar A., Gogia V., Shah V.M., Nag T.C. Comparative evaluation of anatomical and functional outcomes using brilliant blue G versus triamcinolone assisted ILM peeling in macular hole surgery in Indian population. Graefes Arch Clin Exp Ophthalmol. 2011;249(7):987–995. doi: 10.1007/s00417-010-1609-1. [DOI] [PubMed] [Google Scholar]
- 69.Takasu I., Shiraga F., Otsuki H. Triamcinolone acetonide-assisted internal limiting membrane peeling in macular hole surgery. Retina. 2004;24:620–622. doi: 10.1097/00006982-200408000-00021. [DOI] [PubMed] [Google Scholar]
- 70.Peyman G.A., Cheema R., Conway M.D., et al. Triamcinolone acetonide as an aid to visualization of the vitreous and the posterior hyaloid during pars plana vitrectomy. Retina. 2000;20:554–555. doi: 10.1097/00006982-200005000-00024. [DOI] [PubMed] [Google Scholar]
- 71.Hiebl W., Günther B., Meinert H. Substances for staining biological tissues: use of dyes in ophthalmology. Klin Monatsbl Augenheilkd. 2005;222:309–311. doi: 10.1055/s-2005-858030. [DOI] [PubMed] [Google Scholar]
- 72.Cheema R.A., Peyman G.A., Fang T., Jones A., Lukaris A.D., Lim K. Triamcinolone acetonide as an adjuvant in the surgical treatment of retinal detachment with proliferative vitreoretinopathy. Ophthalmic Surg Lasers Imaging. 2007;38:365–370. doi: 10.3928/15428877-20070901-02. [DOI] [PubMed] [Google Scholar]
- 73.Moshfeghi A.A., Scott I.U., Flynn H.W., Jr, Puliafito C.A. Pseudohypopyon after intravitreal triamcinolone acetonide injection for cystoid macular edema. Am J Ophthalmol. 2004;138(3):489–492. doi: 10.1016/j.ajo.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 74.Nelson M.L., Tennant M.T., Sivalingam A., Regillo C.D., Belmont J.B., Martidis A. Infectious and presumed noninfectious endophthalmitis after intravitreal triamcinolone acetonide injection. Retina. 2003;23(5):686–691. doi: 10.1097/00006982-200310000-00014. [DOI] [PubMed] [Google Scholar]
- 75.Yeung C.K., Chan K.P., Chan C.K., Pang C.P., Lam D.S. Cytotoxicity of triamcinolone on cultured human retinal pigment epithelial cells: comparison with dexamethasone and hydrocortisone. Jpn J Ophthalmol. 2004;48(3):236–242. doi: 10.1007/s10384-003-0053-8. [DOI] [PubMed] [Google Scholar]
- 76.Kivilcim M., Peyman G.A., El-Dessouky E.S., Kazi A.A., Cheema R., Hegazy H. Retinal toxicity of triamcinolone acetonide in silicone-filled eyes. Ophthalmic Surg Lasers. 2000;31(6):474–478. [PubMed] [Google Scholar]
- 77.Narayanan R., Mungcal J.K., Kenney M.C., Seigel G.M., Kuppermann B.D. Toxicity of triamcinolone acetonide on retinal neurosensory and pigment epithelial cells. Invest Ophthalmol Vis Sci. 2006;47(2):722–728. doi: 10.1167/iovs.05-0772. [DOI] [PubMed] [Google Scholar]
- 78.Ham W.T., Jr, Ruffolo J.J., Jr, Mueller H.A., Guerry D., 3rd. The nature of retinal radiation damage: dependence on wavelength, power level and exposure time. Vision Res. 1980;20:1105–1111. doi: 10.1016/0042-6989(80)90047-4. [DOI] [PubMed] [Google Scholar]
- 79.Costa Ede P., Rodrigues E.B., Farah M.E., Dib E., Penha F., Magalhães O., Jr, et al. Vital dyes and light sources for chromovitrectomy: comparative assessment of osmolarity, pH, and spectrophotometry. Invest Ophthalmol Vis Sci. 2009;50(1):385–391. doi: 10.1167/iovs.08-2285. [DOI] [PubMed] [Google Scholar]
- 80.Gorgels T.G., van Norren D. Ultraviolet and green light cause different types of damage in rat retina. Invest Ophthalmol Vis Sci. 1995;36:851–863. [PubMed] [Google Scholar]
- 81.Yanagi Y., Inoue Y., Jang W.D., Kadonosono K. A2e mediated phototoxic effects of endoilluminators. Br J Ophthalmol. 2006;90:229–232. doi: 10.1136/bjo.2005.076711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu S.Y., Damico F.M., Viola F., D’Amico D.J., Young L.H. Retinal toxicity of intravitreal triamcinolone acetonide: a morphological study. Retina. 2006;26(5):531–536. doi: 10.1097/00006982-200605000-00006. [DOI] [PubMed] [Google Scholar]
- 83.Rodrigues E.B., Meyer C.H., Mennel S., Farah M.E. Protecting the retinal pigment epithelium during macular hole surgery. Retina. 2007;27(7):958–970. doi: 10.1097/01.iae.0000253051.01194.ab. [DOI] [PubMed] [Google Scholar]
- 84.Chuang L.H., Wang N.K., Yeung L., Chen Y.P., Hwang Y.S., Wu W.C., et al. Use of autologous whole blood during internal limiting membrane peeling and macular hole surgery is protective for indocyanine green toxicity. Cutan Ocul Toxicol. 2010;29(2):98–104. doi: 10.3109/15569521003627867. [DOI] [PubMed] [Google Scholar]
- 85.Meyer C.H., Rodrigues E.B. A novel applicator for the selective painting of the pre-retinal structure during vitreoretinal surgery. Graefes Arch Clin Exp Ophthalmol. 2005;243:487–489. doi: 10.1007/s00417-004-1054-0. [DOI] [PubMed] [Google Scholar]
- 86.Czajka M.P., McCuen B.W., Cummings T.J., et al. Effects of indocyanine green on the retina and retinal pigment epithelium in a porcine model of retinal hole. Retina. 2004;24:275–282. doi: 10.1097/00006982-200404000-00014. [DOI] [PubMed] [Google Scholar]
- 87.Sorcinelli R. Surgical management of epiretinal membrane with indocyanine-green-assisted peeling. Ophthalmologica. 2003;217:107–110. doi: 10.1159/000068556. [DOI] [PubMed] [Google Scholar]



