The production of newly synthesized proteins is a key process of protein homeostasis that initiates the biosynthetic flux of proteins and determines the composition, stability, and functionality of the proteome. Protein synthesis is highly regulated on multiple levels to adapt the proteome to environmental and physiological challenges such as development, aging and stress conditions. However, our understanding of the regulation of ribosomal activity is limited to the control of translation initiation. In a recent publication in EMBO J,1 our lab together with our cooperation partners, Annika Scior and Elke Deuerling (University of Konstanz), provided new insight into a novel regulatory circuit of ribosomal activity in response to cytosolic protein folding conditions in the cell.
Imbalances in proteostasis are accompanied by an accumulation of misfolded and aggregated proteins that are detrimental to the cell’s function and viability. A temporary reduction of protein synthesis aids in restoring proteostasis by reducing the chaperone load as observed during the unfolded protein response (UPR) of the ER.2 A similar connection between downstream protein folding conditions and the synthesis rate of the ribosome upon heat shock in the cytosol was observed more than 30 y ago.3 However, the mechanism of the transient translational repression remained unknown. In addition, it was also not known whether there is a feedback mechanism to the ribosome in response to chronic stress conditions, such as aging, that are associated with the accumulation of misfolded and aggregated proteins or the expression of aggregation-prone proteins such as Aβ or polyglutamine proteins that escape the cell’s clearance strategies and accumulate as aggregate deposits.
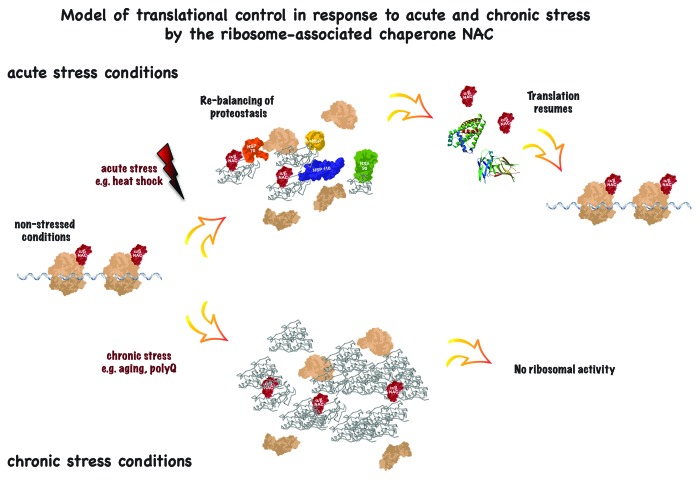
Recently it has been shown by three independent studies that the ribosome is subject to post-translational regulation in response to a variety of proteotoxic challenges.1,4,5 These findings add additional layers of regulation that enable a fine-tuned translational activity in response to imbalances of protein folding conditions. Our lab could show that acute and chronic proteotoxic stress, exemplified by heat shock, aging, and the expression of disease-causing aggregation-prone proteins is associated with a strong decline of translational activity in the nematode C. elegans. In the center of this new regulatory circuit is the ribosome-associated chaperone complex NAC (nascent polypeptide associated complex). NAC is required for ribosomal activity, assists in the folding of newly synthesized proteins, and acts as sensor for downstream protein folding conditions. NAC has the ability to dissociate from the ribosome and to re-localize toward misfolded and insoluble proteins. This functional depletion of NAC from ribosomes reduces the levels of polysomes and greatly affects the translational capacity. NAC is an important component of the proteostasis network, as it can aid in the re-solubilization of misfolded proteins in cooperation with other molecular chaperones. The re-localization, however, is reversible, as NAC can re-associate with the ribosome once misfolded and aggregated proteins are eliminated and proteostasis is re-balanced. NAC can then re-associate with the ribosome, and protein synthesis resumes. At this point, acute and chronic stress conditions differ markedly. Chronic stress conditions are characterized by a constant load of misfolded and aggregated proteins that terminally sequester chaperones, among them NAC. Indeed, we could observe that the expression of aggregation-prone proteins such as Aβ and polyQ is associated with a dramatic decline in translational activity (Fig. 1). Likewise, we could show that translational capacity starts to decline once adulthood is reached and proteostasis starts to collapse.6 These findings demonstrate that active protein synthesis is limited to only a short window of the lifespan, and that the animal is not able to compensate for the accumulation of misfolded proteins by de novo synthesis. Thus, the effect of the collapse of the proteome with age becomes even more severe than previously acknowledged.
Figure 1. Model of translational control in response to acute and chronic stress by the ribosome-associated chaperone NAC. Acute stress (upper branch) leads to a temporary translational decline due to sequestration of NAC by misfolded and aggregated proteins. Re-balancing of proteostasis by NAC and other molecular chaperones liberates NAC and allows for re-association with ribosomes and translation can resume. Chronic stress (lower branch) such as aging and polyQ expression leads to a terminal sequestration of NAC and, hence, a permanent decrease in protein synthesis.
Two additional studies analyzing the translational capacity under acute stress in cell culture propose that Hsp70 can induce ribosomal pausing in response to stress.4,5 Notably, Hsp70 can also cooperate with another ribosome-associated chaperone complex: RAC (ribosome associated complex). The pausing is induced by the sequestration of chaperones by misfolded proteins and thus follows a similar concept of a dynamic redistribution and sensor function of chaperones.
Taken together, cells and tissues are constantly monitoring and adjusting their translational capacity in response to proteotoxic imbalances to match protein production with folding capacity based on the chaperone availability. These novel chaperone-mediated regulatory mechanisms provide the cell with a dynamic, spatial, and temporal control of protein synthesis to maintain proteostasis under stressful conditions and aging.
Interestingly, the selective translational repression of the UPR, mediated by eIF2α−P, poses the question of whether there is a similar discrimination of mRNAs that escape the translational repression under acute and chronic cytosolic stress conditions. Moreover, we have only a limited understanding on how different cells and tissues within an organism orchestrate their translational capacity. Aging and especially cell-specific protein folding challenges, such as the tissue-specific expression of Aβ and polyQ, might affect different tissues within the same organism to a different extent. However, we observed that the translational capacity of the whole organism is affected.1 Future studies are required to address the question of how tissue-specific protein folding conditions are sensed and signaled to the ribosome, and whether or not the ribosome is subject to cell non-autonomous regulation.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/25703
References
- 1.Kirstein-Miles J, Scior A, Deuerling E, Morimoto RI. The nascent polypeptide-associated complex is a key regulator of proteostasis. EMBO J. 2013;32:1451–68. doi: 10.1038/emboj.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 3.Lindquist S. Translational efficiency of heat-induced messages in Drosophila melanogaster cells. J Mol Biol. 1980;137:151–8. doi: 10.1016/0022-2836(80)90322-8. [DOI] [PubMed] [Google Scholar]
- 4.Liu B, Han Y, Qian SB. Cotranslational response to proteotoxic stress by elongation pausing of ribosomes. Mol Cell. 2013;49:453–63. doi: 10.1016/j.molcel.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shalgi R, Hurt JA, Krykbaeva I, Taipale M, Lindquist S, Burge CB. Widespread regulation of translation by elongation pausing in heat shock. Mol Cell. 2013;49:439–52. doi: 10.1016/j.molcel.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Zvi A, Miller EA, Morimoto RI. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci U S A. 2009;106:14914–9. doi: 10.1073/pnas.0902882106. [DOI] [PMC free article] [PubMed] [Google Scholar]