Abstract
Contemporary microRNA research has led to significant advances in our understanding of the process of tumorigenesis. MicroRNAs participate in different events of a cancer cell’s life, through their ability to target hundreds of putative transcripts involved in almost every cellular function, including cell cycle, apoptosis, and differentiation. The relevance of these small molecules is even more evident in light of the emerging linkage between their expression and both prognosis and clinical outcome of many types of human cancers. This identifies microRNAs as potential therapeutic modifiers of cancer phenotypes. From this perspective, we overview here the miR-10b locus and its involvement in cancer, focusing on its role in the establishment (miR-10b*) and spreading (miR-10b) of breast cancer. We conclude that targeting the locus of microRNA 10b holds great potential for cancer treatment.
Keywords: microRNA, miR-10b, miR-10b*, cancer, cell proliferation, therapeutic strategies
Introduction
microRNAs are evolutionarily conserved small non coding RNAs, 19–25 nucleotides in length, that regulate gene expression at the posttranscriptional level.1-3 This occurs through imperfect complementarity to the 3′ untranslated region (3′UTR) of target mRNAs; this partial homology recognition results in mRNAs translational inhibition and/or degradation,4,5 finally leading to a reduction in protein expression level.1 To date more than 1000 human microRNAs have been discovered (http://www.mirbase.org6). Since microRNAs are predicted to target over 50% of all human protein-coding genes,7 and each gene could be controlled by different microRNAs,8 almost every cellular function (from differentiation to cell growth, and from stress response to cell death) is putatively subjected to microRNA control.9-11 In the past decade, a great number of reports demonstrated that aberrant expression of microRNAs is linked to the insurgence of several pathologies, including cancer.12,13 From the first demonstration of the involvement of microRNAs in chronic lymphocitic leukemia (CLL),14 a very large series of studies reported that microRNAs might behave as oncogenes or tumor suppressor genes.15-17
In one of the first reports about microRNA expression profiling in breast cancer, Iorio and colleagues18 identified 29 microRNAs differentially expressed between tumoral and normal tissues. Among the 5 most deregulated microRNAs there was microRNA-10b (miR-10b), which was subsequently characterized as a pro-metastatic miR in advanced breast cancers.19,20 miR-10b is the guide strand of microRNA-10b locus, which is located on chromosome 2 within the cluster of the HOXD genes, in an intergenic region between HOXD4 and HOXD8 genes (Fig. 1A and B). During the biogenesis of microRNAs, Drosha-mediated cleavage of a long primary transcript (pri-miRNA) leads to the formation of a hairpin molecule, the pre-microRNA that is shuttled to the cytoplasm after recognition by the complex exportin-5/RAN-GTP.21,22 In the cytoplasm, the pre-miRNA terminal loop is cleaved by Dicer, to produce a ~22-nt RNA duplex, consisting of 2 distinct 5′ phosphorylated strands with 3′ overhangs.
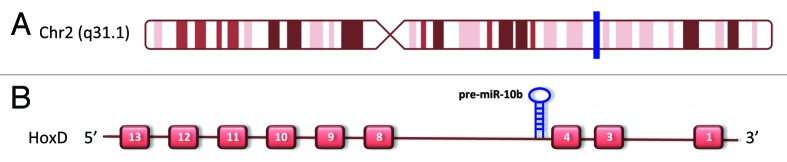
Figure 1. miR-10b locus localization (A) Chromosome 2 representation, where the blue bar indicates miR-10b position in the long arm of Chr2 (figure modified from UCSC genome browser: http://genome.ucsc.edu). (B) Overview of HOXD cluster contains miR-10b precursor.
The functional strand of the duplex, referred to as the guide strand, is loaded in the AGO-containing protein complex that enables target recognition.23 In the literature, the major characterization of microRNAs activities covered mostly the guide strand, which was believed to be the only one capable of controlling target mRNAs expression. The so-called passenger strand, indicated as microRNA*, has long been considered functionally irrelevant and destined to degradation. However, different groups very recently demonstrated that the biological relevance of microRNAs* may be comparable to that of the guide strand, and that deregulation of their expression could be strictly linked to cancer insurgence and development.24-26 The star strand of the miR-10b locus, miR-10b*, was first functionally characterized by our group in a recent report,27 in which we also showed that it is downregulated in primary breast tumors when compared with peritumoral non-cancerous surrounding tissues. This feature is common to all the analyzed breast cancer subtypes, suggesting that miR-10b* downregulation represents an early event in breast tumorigenesis.
In this article, we describe the role of the miR-10b locus in breast cancer establishment (miR-10b*) as well as spreading (miR-10b) and also discuss the possibility of harnessing the new information for microRNA-based therapy.
microRNA-10b
Iorio et al. (2005) first identified miR-10b as one of the most significantly downregulated microRNAs in primary breast tumors compared with normal breast samples.18 In a later study, Kim et al. reinforced the tumor suppressor role of miR-10b by demonstrating that it is also downregulated in human gastric cancer cells, where its transcriptional regulation is strictly linked to promoter hypermethylation.28 Subsequently, the Weinberg’s group reported the opposite concept that miR-10b could act as a metastasis-associated miRNA (metastamiR) in advanced breast tumors.19,20 This reveals the functional duality of miR-10b when metastatic or non-metastatic tumors are considered.
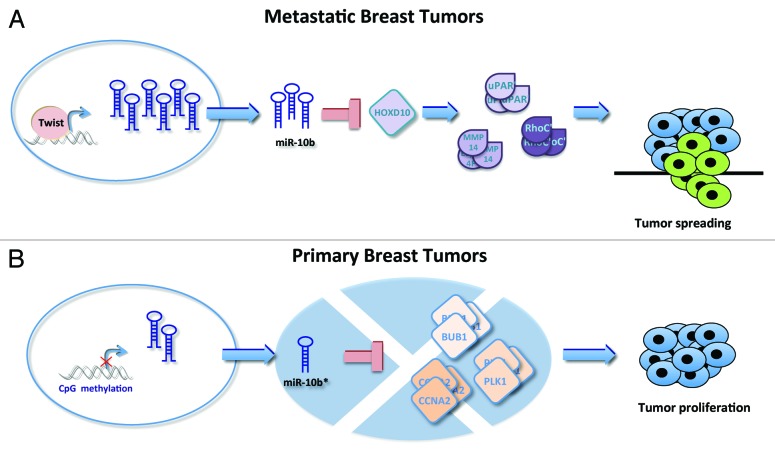
In their first paper in 2007, Ma et al. demonstrated that miR-10b is highly expressed in breast cancer metastatic cell lines, where it positively regulates cell migration and invasion processes in vitro and in vivo. This is mediated by the ability of miR-10b to target HOXD10, a repressor of several modulators of cell migration, such as RhoC19 (Fig. 2A). In glioma cells, HOXD10 targeting by miR-10b leads to the induction of extracellular matrix remodeling factors (matrix metalloproteinase-14 and urokinase-type plasminogen activator receptor), leading to cell invasion29-31 (Fig. 2A).
Figure 2. miR-10b locus is involved in breast tumorigenesis. (A) In metastatic tumors, miR-10b is overexpressed by Twist transcription. This feature is closely related to the activation of invasive program through downregulation of HOXD10 and consequently overexpression of several cell migration repressor such as RhoC, uPAR, and MMP14.19 (B) In primary breast tumors miR-10b* is downregulated by CpG island hypermethylation. This feature leads to upregulation of its target genes, such as BUB1, PLK1, and CCNA2 and, in turn, to tumor proliferation.27
The close relationship between miR-10b and the metastatatic process in breast cancer cells is also based on the fact that miR-10b levels are tightly controlled by the transcription factor Twist, a well-known regulator of epithelial-to-mesenchymal transition (EMT). Twist directly binds to the E-box sequences present on the miR-10b promoter region.19 Of note, miR-10b expression level is higher in clinically advanced breast cancers and in other high-grade types of cancers, compared with metastasis-free tumors, and it correlates with clinical progression.19
Interestingly, miR-10b is expressed at higher level in the tumor vasculature compared with the vasculature of normal tissues.32 This suggests the involvement of miR-10b in the angiogenic switch that is associated with the transition to malignancy. Indeed, miR-10b is highly expressed in the vasculature of breast IDC (invasive ductal carcinoma) grade III tumors, with little or no expression in DCIS (ductal carcinoma in situ). miR-10b is upregulated in tumoral endothelial cells in response to tumor-produced growth factors, including VEGF, and administration of anti-miR-10b results in endothelial progenitor cells (EPC)-mediated impaired tumor growth in vivo.32
miR-10b overexpression, besides breast cancer, associates with progression of oral and colorectal cancer,33,34 pancreatic adenocarcinoma,35,36 and glioblastoma.31,37 In an orthotopic human glioma mouse model, inhibition of miRNA-10b diminishes the invasiveness, angiogenesis, and growth of the mesenchymal subtype-like glioma cells in the brain and significantly prolongs survival of glioma-bearing mice.38 The pleiotropic nature of miRNA-10b was due to its suppression of multiple tumor suppressors, including TP53, FOXO3, CYLD, PAX6, PTCH1, HOXD10, and NOTCH1.39 This might also suggest that miR-10b could play a critical role in many types of human cancers.
microRNA-10b*
Our group was the first to characterize miR-10b* as a tumor suppressor microRNA in primary breast cancers.27 In order to identify new molecular players in breast tumorigenesis, we performed a microRNA microarray analysis on primary breast cancers, comparing tumor and matched peritumor tissues. On the basis of multiple statistical analyses, we were able to find microRNAs that were differentially expressed (vs. peritumor) in all the 3 studied breast cancer subtypes (luminal, HER2-amplified, and triple negative) as well as microRNAs deregulated specifically in each subtype. miR-10b* emerged as a downregulated microRNA in tumor vs peritumor samples, commonly deregulated in all subtypes. We decided to further characterize miR-10b* for two main reasons: first, because many reports in the literature pointed out the importance of tumor suppressor microRNAs in the establishment and maintenance of the transformed phenotype of tumor cells,40,41 and, second, because miR-10b* is encoded by the passenger strand of the miR-10b locus, and there was no evidence in the literature about its possible role in initiation and progression of breast cancer.
Initially, we aimed at understanding how miR-10b* is downregulated in the tumor tissue. We found that there are 2 CpG islands in miR-10b/10b* regulatory regions that undergo hypermethylation, both in a breast cancer cell line and in human tumor samples, where the level of methylation in tumor tissues is significantly higher compared with matched peritumor ones.
What is the biological significance of miR-10b* downregulation? To answer this, we analyzed potential association between miR-10b* expression levels and clinical features of the analyzed lesions. Interestingly, we found that lower expression levels of miR-10b* were significantly associated with larger tumor sizes. Next, we demonstrated that ectopic miR-10b* overexpression inhibits proliferation of breast cancer cell lines, and that this regulation is exerted through the direct targeting of 3 well-known cell cycle and proliferation controllers: BUB1, PLK1, and CCNA2 (Fig. 2B). The correlation of expression of miR-10b* with its targets was also validated on human breast cancer tissues, where downregulation of miR-10b* is accompanied by BUB1, PLK1, and CCNA2 upregulation, compared with their matched peritumoral counterparts.
Dysregulated Expression of miR-10b* and of its Targets BUB1, PLK1, and CCNA2 is Associated with Poor Survival of Breast Cancer Patients
Recently a wealth of breast cancer data has become available, in the form of mRNA42 and miR expression data43 measured for more than 1000 primary tumors of patients with available clinical follow-up. The METABRIC data set contains 1286 primary breast cancer tumors, for which both mRNA42 and miR43 expression data were measured. The patients are from the 5 subtypes of breast cancer: Her2+ (127 patients), Basal-like (209), Luminal A (479), Luminal B (312), and normal-like (151). We applied the CoSMic algorithm44 , that improves miR target prediction by combining joint mRNA and miR expression profiling with sequence-based analysis, to the METABRIC data set. Using miRanda predictions (http://www.ebi.ac.uk/enright-srv/microcosm/targets/v5/) (the only one of the available sequence-based predictors that predicts targets for miR-10b*), we found 15 target genes for miR-10b* (data not shown). BUB1, PLK1, CCNA2 appear among these, together with other genes connected to cell cycle. This might suggest that miR-10b* downregulation releases aberrant expression of diverse cell cycle-related proteins through which miR-10b* plays a major role in the insurgence of breast tumors. In accordance with its proposed role as tumor suppressor, Kaplan-Meier analysis found significant association between low expression levels of miR-10b* and poor disease-specific survival (Fig. 3A). Correspondingly, high expression levels of the 15 target genes were also significantly associated with poor survival (Fig. 3B–D and data not shown). These findings strongly reinforce our previous observations27 and highlight miR-10b* as a potential predictor of breast cancer patient survival.
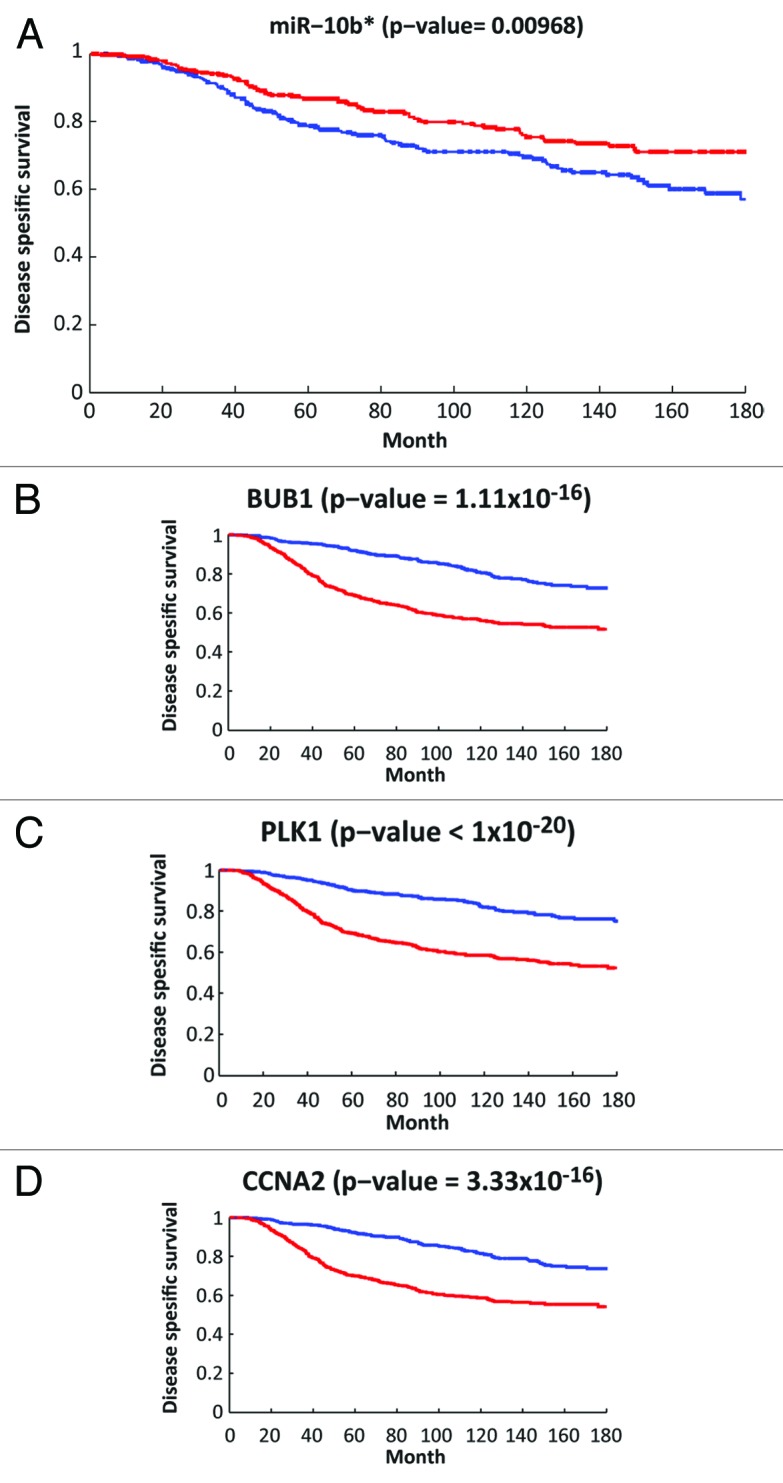
Figure 3. Association between disease-specific survival and the expression levels of miR-10b* and 3 of its target genes (BUB1, PLK1, and CCNA2). (A) Kaplan-Meier analysis based on the METABRIC miR expression data.43 Survival of the 370 patients with the highest (top third) expression levels of miR-10b* (red line) was compared with the 370 patients with the lowest expression levels (blue line). The plot was truncated at 15 y, P value is indicated in the title. (B–D) Kaplan-Meier analysis based on the METABRIC mRNA expression data.42 The 2 compared groups are 565 patients with the highest (top third) expression levels of each target gene (red line) vs. 565 patients with the lowest (bottom third) expression levels (blue line). The plot was truncated at 15 y, P values are indicated in the titles. Only patients with available clinical follow-up were included in this analysis.
Conclusions
The emerging role of microRNAs as oncogenes or tumor suppressors opens new scenarios for cancer treatment. It has in fact been proposed that microRNA-based therapy might become a realistic option, by demonstrating that specific microRNAs can act as anticancer drugs in a model system.45,46 The manipulation of microRNA expression includes either silencing the overexpression of an oncogenic miRNA or restoring the presence of a tumor suppressor miRNA. Why should such approaches be effective? The answer relates to one of the most appealing properties of microRNAs, namely their capability to target multiple genes. The complexity of the cellular context is represented by networks of genes and proteins that, during carcinogenesis, are modified and rewired. microRNAs could be seen as the hubs of such rewiring. Perturbing the hubs properly could restore the normal connectivity map. An example of how this could work is presented by the miR-10b locus, for which in vivo approaches have been exemplified, either miR-10b silencing or miR-10b* restoration.
Based on the correlation between miR-10b expression and metastasis control, Weinberg’s group in 2010 tested the capability of miR-10b antagomiR treatment in vivo to target metastatic breast cancers.20 Using a mouse metastasis mammary model, their study elegantly demonstrated that silencing miR-10b might be an effective therapeutic strategy.
In the case of miR-10b*, we attempted restoring expression in an established breast tumor model in vivo.27 Injection of miR-10b* mimic into breast cancer xenografts induced a strong inhibitory effect on tumor growth, which decreased proliferative markers and miR-10b* targets in the treated tumors relative to control tumors. Thus, although more studies are needed to firmly elucidate the efficacy and safety of such therapeutic approaches and their translation to clinical practice, microRNAs application could represent a turning point in the battle against cancer.
Acknowledgment
Work in Blandino’s lab is supported by AIRC grant N (08/30R/89). Work of E Domany and N Bossel Ben-Moshe was supported by the Leir Charitable Foundation.
Glossary
Abbreviations:
- miRNA
microRNA
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/25380
References
- 1.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Pasquinelli AE, Hunter S, Bracht J. MicroRNAs: a developing story. Curr Opin Genet Dev. 2005;15:200–5. doi: 10.1016/j.gde.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Carleton M, Cleary MA, Linsley PS. MicroRNAs and cell cycle regulation. Cell Cycle. 2007;6:2127–32. doi: 10.4161/cc.6.17.4641. [DOI] [PubMed] [Google Scholar]
- 4.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–40. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doench JG, Petersen CP, Sharp PA. siRNAs can function as miRNAs. Genes Dev. 2003;17:438–42. doi: 10.1101/gad.1064703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39(Database issue):D152–7. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 8.Rajewsky N. microRNA target predictions in animals. Nat Genet. 2006;38(Suppl):S8–13. doi: 10.1038/ng1798. [DOI] [PubMed] [Google Scholar]
- 9.Miska EA. How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev. 2005;15:563–8. doi: 10.1016/j.gde.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Zamore PD, Haley B. Ribo-gnome: the big world of small RNAs. Science. 2005;309:1519–24. doi: 10.1126/science.1111444. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Taniguchi T. MicroRNAs and DNA damage response: implications for cancer therapy. Cell Cycle. 2013;12:32–42. doi: 10.4161/cc.23051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calin GA, Liu CG, Sevignani C, Ferracin M, Felli N, Dumitru CD, et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc Natl Acad Sci U S A. 2004;101:11755–60. doi: 10.1073/pnas.0404432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng S, Calin GA, Croce CM, Coukos G, Zhang L. Mechanisms of microRNA deregulation in human cancer. Cell Cycle. 2008;7:2643–6. doi: 10.4161/cc.7.17.6597. [DOI] [PubMed] [Google Scholar]
- 14.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99:15524–9. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–47. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Voorhoeve PM, le Sage C, Schrier M, Gillis AJ, Stoop H, Nagel R, et al. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell. 2006;124:1169–81. doi: 10.1016/j.cell.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 17.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102:13944–9. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–70. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 19.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–8. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 20.Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol. 2010;28:341–7. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–6. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303:95–8. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 23.Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell. 2004;15:185–97. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Kuchenbauer F, Mah SM, Heuser M, McPherson A, Rüschmann J, Rouhi A, et al. Comprehensive analysis of mammalian miRNA* species and their role in myeloid cells. Blood. 2011;118:3350–8. doi: 10.1182/blood-2010-10-312454. [DOI] [PubMed] [Google Scholar]
- 25.Eichner LJ, Perry MC, Dufour CR, Bertos N, Park M, St-Pierre J, et al. miR-378(∗) mediates metabolic shift in breast cancer cells via the PGC-1β/ERRγ transcriptional pathway. Cell Metab. 2010;12:352–61. doi: 10.1016/j.cmet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Bhayani MK, Calin GA, Lai SY. Functional relevance of miRNA sequences in human disease. Mutat Res. 2012;731:14–9. doi: 10.1016/j.mrfmmm.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biagioni F, Bossel Ben-Moshe N, Fontemaggi G, Canu V, Mori F, Antoniani B, et al. miR-10b*, a master inhibitor of the cell cycle, is down-regulated in human breast tumours. EMBO Mol Med. 2012;4:1214–29. doi: 10.1002/emmm.201201483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim K, Lee HC, Park JL, Kim M, Kim SY, Noh SM, et al. Epigenetic regulation of microRNA-10b and targeting of oncogenic MAPRE1 in gastric cancer. Epigenetics. 2011;6:740–51. doi: 10.4161/epi.6.6.15874. [DOI] [PubMed] [Google Scholar]
- 29.Sun L, Yan W, Wang Y, Sun G, Luo H, Zhang J, et al. MicroRNA-10b induces glioma cell invasion by modulating MMP-14 and uPAR expression via HOXD10. Brain Res. 2011;1389:9–18. doi: 10.1016/j.brainres.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Myers C, Charboneau A, Cheung I, Hanks D, Boudreau N. Sustained expression of homeobox D10 inhibits angiogenesis. Am J Pathol. 2002;161:2099–109. doi: 10.1016/S0002-9440(10)64488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciafrè SA, Galardi S, Mangiola A, Ferracin M, Liu CG, Sabatino G, et al. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun. 2005;334:1351–8. doi: 10.1016/j.bbrc.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 32.Plummer PN, Freeman R, Taft RJ, Vider J, Sax M, Umer BA, et al. MicroRNAs regulate tumor angiogenesis modulated by endothelial progenitor cells. Cancer Res. 2013;73:341–52. doi: 10.1158/0008-5472.CAN-12-0271. [DOI] [PubMed] [Google Scholar]
- 33.Lu YC, Chen YJ, Wang HM, Tsai CY, Chen WH, Huang YC, et al. Oncogenic function and early detection potential of miRNA-10b in oral cancer as identified by microRNA profiling. Cancer Prev Res (Phila) 2012;5:665–74. doi: 10.1158/1940-6207.CAPR-11-0358. [DOI] [PubMed] [Google Scholar]
- 34.Nishida N, Yamashita S, Mimori K, Sudo T, Tanaka F, Shibata K, et al. MicroRNA-10b is a prognostic indicator in colorectal cancer and confers resistance to the chemotherapeutic agent 5-fluorouracil in colorectal cancer cells. Ann Surg Oncol. 2012;19:3065–71. doi: 10.1245/s10434-012-2246-1. [DOI] [PubMed] [Google Scholar]
- 35.Nakata K, Ohuchida K, Mizumoto K, Kayashima T, Ikenaga N, Sakai H, et al. MicroRNA-10b is overexpressed in pancreatic cancer, promotes its invasiveness, and correlates with a poor prognosis. Surgery. 2011;150:916–22. doi: 10.1016/j.surg.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–8. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 37.Huse JT, Brennan C, Hambardzumyan D, Wee B, Pena J, Rouhanifard SH, et al. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev. 2009;23:1327–37. doi: 10.1101/gad.1777409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabriely G, Yi M, Narayan RS, Niers JM, Wurdinger T, Imitola J, et al. Human glioma growth is controlled by microRNA-10b. Cancer Res. 2011;71:3563–72. doi: 10.1158/0008-5472.CAN-10-3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin J, Teo S, Lam DH, Jeyaseelan K, Wang S. MicroRNA-10b pleiotropically regulates invasion, angiogenicity and apoptosis of tumor cells resembling mesenchymal subtype of glioblastoma multiforme. Cell Death Dis. 2012;3:e398. doi: 10.1038/cddis.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39:673–7. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 41.Ozen M, Creighton CJ, Ozdemir M, Ittmann M. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene. 2008;27:1788–93. doi: 10.1038/sj.onc.1210809. [DOI] [PubMed] [Google Scholar]
- 42.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. METABRIC Group The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–52. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dvinge H, Git A, Gräf S, Salmon-Divon M, Curtis C, Sottoriva A, et al. The shaping and functional consequences of the microRNA landscape in breast cancer. Nature. 2013;497:378–82. doi: 10.1038/nature12108. [DOI] [PubMed] [Google Scholar]
- 44.Bossel Ben-Moshe N, Avraham R, Kedmi M, Zeisel A, Yitzhaky A, Yarden Y, et al. Context-specific microRNA analysis: identification of functional microRNAs and their mRNA targets. Nucleic Acids Res. 2012;40:10614–27. doi: 10.1093/nar/gks841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nana-Sinkam SP, Croce CM. Clinical applications for microRNAs in cancer. Clin Pharmacol Ther. 2013;93:98–104. doi: 10.1038/clpt.2012.192. [DOI] [PubMed] [Google Scholar]
- 46.Bisso A, Faleschini M, Zampa F, Capaci V, De Santa J, Santarpia L, et al. Oncogenic miR-181a/b affect the DNA damage response in aggressive breast cancer. Cell Cycle. 2013;12:1679–87. doi: 10.4161/cc.24757. [DOI] [PMC free article] [PubMed] [Google Scholar]