Abstract
The function of Cyclin D1 (CycD1) has been widely studied in the cell nucleus as a regulatory subunit of the cyclin-dependent kinases Cdk4/6 involved in the control of proliferation and development in mammals. CycD1 has been also localized in the cytoplasm, where its function nevertheless is poorly characterized. In this work we have observed that in normal skin as well as in primary cultures of human keratinocytes, cytoplasmic localization of CycD1 correlated with the degree of differentiation of the keratinocyte. In these conditions, CycD1 co-localized in cytoplasmic foci with exocyst components (Sec6) and regulators (RalA), and with β1 integrin, suggesting a role for CycD1 in the regulation of keratinocyte adhesion during differentiation. Consistent with this hypothesis, CycD1 overexpression increased β1 integrin recycling and drastically reduced the ability of keratinocytes to adhere to the extracellular matrix. We propose that localization of CycD1 in the cytoplasm during skin differentiation could be related to the changes in detachment ability of keratinocytes committed to differentiation.
Keywords: cell adhesion, cyclin D1, cytoplasm, keratinocytes, β1 integrin
Introduction
Cyclin D1 (CycD1) interacts and promotes the activity of cyclin-dependent kinases 4 and 6 (Cdk4/6). CycD1-Cdk4/6 complexes regulate G1-S transition in the cell cycle, a key step to determine the rate of cell proliferation. CycD1-Cdk4 acts as a transcriptional regulator inhibiting by phosphorylation the retinoblastoma protein, thus releasing the E2F transcription factor activity. E2F induces the expression of a number of genes involved in the commitment of G1-S transition (for a review, see refs. 1 and 2). CycD1 is also involved in the regulation of cell adhesion and migration. Ablation of CycD1 increases matrix-adhesion and reduces migration of macrophages and fibroblasts.3,4 These effects have been partly attributed to transcriptional mechanisms and post-transcriptional regulation.4,5 CycD1 has also been located in the cytoplasm regulating specific processes. In breast cancer cell lines for instance, CycD1 is localized in membrane ruffles and interacts with cytoskeleton proteins such as filamin A, providing a transcriptional-independent mechanism to control cell adhesion and motility.6 Furthermore, we have previously described the cytoplasmic interaction of CycD1 with components and regulators of the exocyst in both fibroblasts and epithelial cell lines.7 In particular, we found that CycD1 colocalizes with RalA and Sec6 in cytoplasmic foci, and that CycD1-Cdk4-associated activity stimulates accumulation of Ral-GTP active forms.7 Active Ral-GTP can then bind to different components of the exocyst promoting cell motility by integrin recycling or/and by targeting the exocyst to focal adhesion complexes.8,9
The epidermis exhibits a classical model of stratified epithelial differentiation, where keratinocytes move from the progenitor basal cell layer to higher layers during differentiation.10 The proliferative capacity of these progenitors is progressively lost when they leave the basal layer to become a terminally differentiated keratinocyte. It is suggested that one of the first steps in keratinocyte differentiation is the detachment of the cell from the extracellular matrix/basement membrane, which exerts an inhibitory effect on differentiation.11 β1 integrins mediate adhesion of keratinocytes to the extracellular matrix, and loss of contact of these integrins with extracellular matrix triggers terminal differentiation of cultured keratinocytes.12 In accordance with this, β1 integrin expression is mainly restricted to the basal layer and needs to be downregulated. During differentiation, β1 integrin is internalized to the cytoplasm of keratinocytes and degraded.13 Alternatively, in order to initiate their differentiation, basal epidermal cells can undergo asymmetrical divisions, but also in these conditions, interaction of keratinocytes with the extracellular matrix is needed for the regulation of the commitment to differentiation.14 Here, we report that CycD1 showed a cytoplasmic localization in suprabasal layers of normal skin. We have confirmed this observation by immunofluorescence and subcellular fractionation techniques during the in vitro differentiation of primary cultures of human keratinocytes. Importantly, we have been able to show that CycD1 co-localized with exocyst components and with β1 integrin during differentiation, and that CycD1 overexpression induced keratinocyte detachment. Overall, our data suggest that re-localization of CycD1 to the cytoplasm during keratinocyte differentiation could be an important step for the extracellular matrix detachment of the cells committed to differentiate.
Results
Cytoplasmic localization of CycD1 during keratinocyte differentiation
To study CycD1 localization, primary cultures of human keratinocytes were infected with lentivirus harboring HA-CycD1 to ensure homogeneous expression and unambiguous detection of the protein. Calcium was added to these cultures in order to induce keratinocyte differentiation.15 Successful keratinocyte differentiation was monitored by cell morphology and by the expression of the differentiation marker involucrin (Fig. 1A and B). By immunofluorescence, calcium-treated keratinocytes showed CycD1 mostly in the cytoplasm, whereas most of the untreated keratinocytes exhibited nuclear CycD1 staining (Fig. 1A and C). Note that in Figure 1A, cells exhibiting Hoescht staining but not HA-CycD1 signal are likely noninfected cells, which highlights the high specificity of the HA epitope detection. During differentiation, the levels of HA-CycD1 expressed from the heterologous promoter CMV were maintained, ensuring homogeneous staining under the different conditions assayed (Fig. 1D). These results suggest that CycD1 localization was regulated during keratinocyte differentiation. In order to test whether this also takes place in vivo, we analyzed by immunohistochemistry the localization of CycD1 in human epidermis (Fig. 2). CycD1 localization was predominantly nuclear in the proliferative basal layer of the epidermis, whereas in suprabasal layers there was a significant increase in cytoplasmic staining (Fig. 2D), even though many cells still maintain nuclear signal. No expression of CycD1 was observed in the upper skin strata of terminally differentiated keratinocytes. Hence, our results both in vitro and in vivo strongly suggest that CycD1 localization and expression could be specifically regulated during the normal differentiation of keratinocytes.
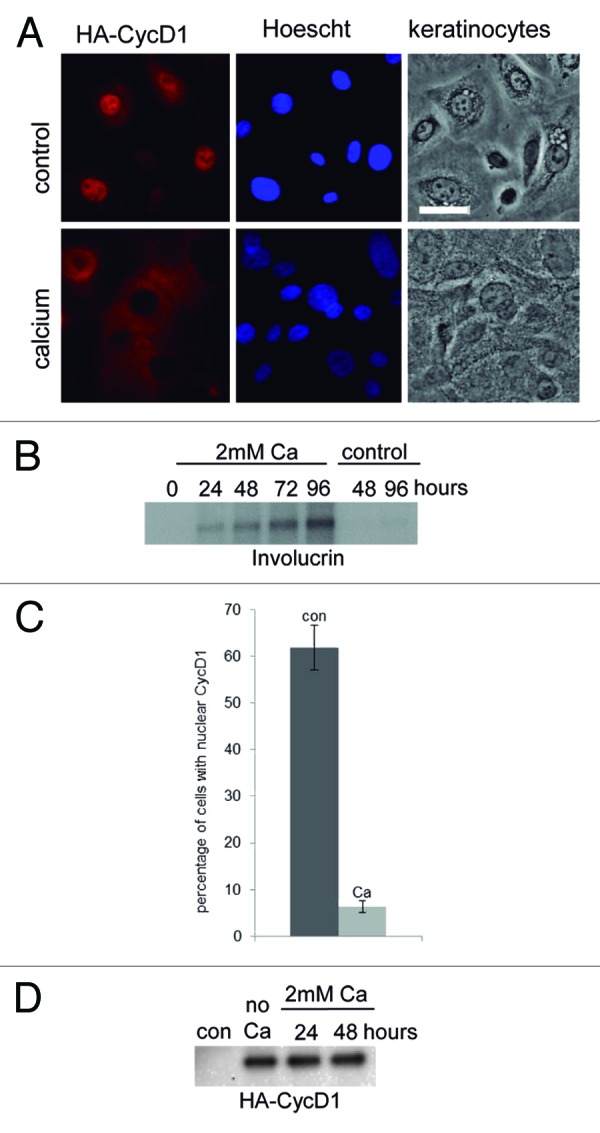
Figure 1. CycD1 accumulated in the cytoplasm during keratinocyte differentiation. (A) Primary human keratinocytes were infected with lentivirus harboring HA-CycD1. Calcium was added to induce keratinocyte differentiation. The images were taken 48 h after calcium addition. A culture without added calcium was used as a control. CycD1 was detected with a rat anti-HA 3F10 (red) antibody, and nuclei were stained with Hoescht (blue). Images were acquired using a 40× objective (20 μm bar). (B) Involucrin levels were analyzed by immunoblot with anti-involucrin antibody. Equal amounts of total protein were loaded in each well. (C) Percentage of infected cells with nuclear HA-CycD1. Data are expressed as mean ± SEM from two independent experiments (P = 0.02; n ≥ 58). (D) HA-cycD1 levels were analyzed by immunoblot with anti-HA (12CA5) antibody. Equal amounts of total protein were loaded in each well.
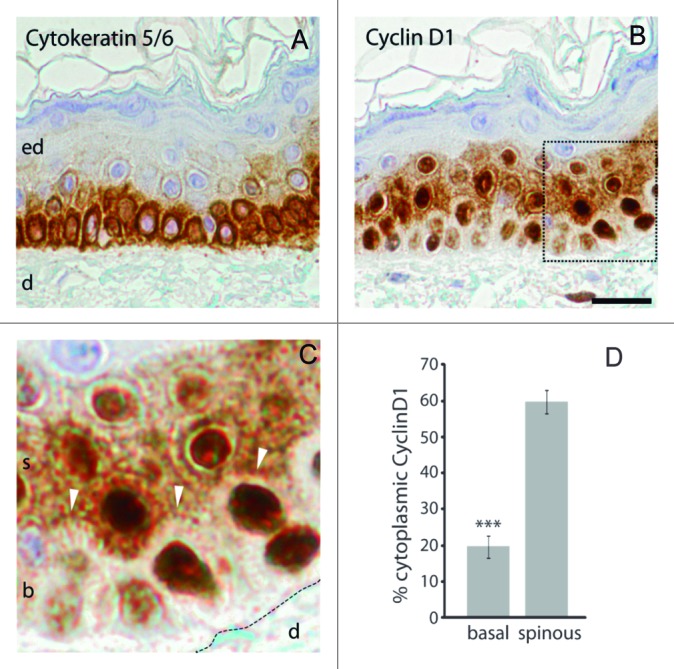
Figure 2. CycD1 accumulated in the cytoplasm of spinous layer of the skin. Consecutive sections of normal human skin were immunostained with antihuman cytokeratin5/6 (basal layer marker, (A) and CycD1 (B and C) antibodies. Sections were counterstained with hematoxylin for nuclear staining. Abbreviations: ed, epidermis; d, dermis; b, basal; s, spinous. In (C), white arrows indicate the difference of intensity in the border between basal and spinous layers. Scale bar 20 μm. (D) ImageJ software was used to quantify staining intensity. Data are expressed in mean ± sem (n ≥ 14 cells). The difference in the percentage of cytoplasmic cyclin D1 between spinous and basal layers was statistically significant (P ≤ 0.001).
To characterize further the cytoplasmic accumulation of CycD1 during keratinocyte differentiation, we performed fractionation of cells growing under proliferation and differentiation conditions. Although endogenous CycD1 was detected in both cytoplasmic and nuclear fractions of these keratinocytes, there was a clear change in CycD1 distribution during the differentiation process. In undifferentiated keratinocytes, CycD1 was both nuclear as well as associated with the vesicles-cytoskeleton fraction (Fig. 3A and B). After 48 h of calcium addition, most CycD1 was in the vesicles-cytoskeleton fraction. In the course of these experiments, the total amount of endogenous CycD1 was slightly downregulated (not shown). These results show that CycD1 was located in the cytoplasm, where it was mainly associated with cytoskeletal and membranous structures. Together our results suggest that nuclear-cytoplasmatic location is regulated during the early steps of keratinocyte differentiation.
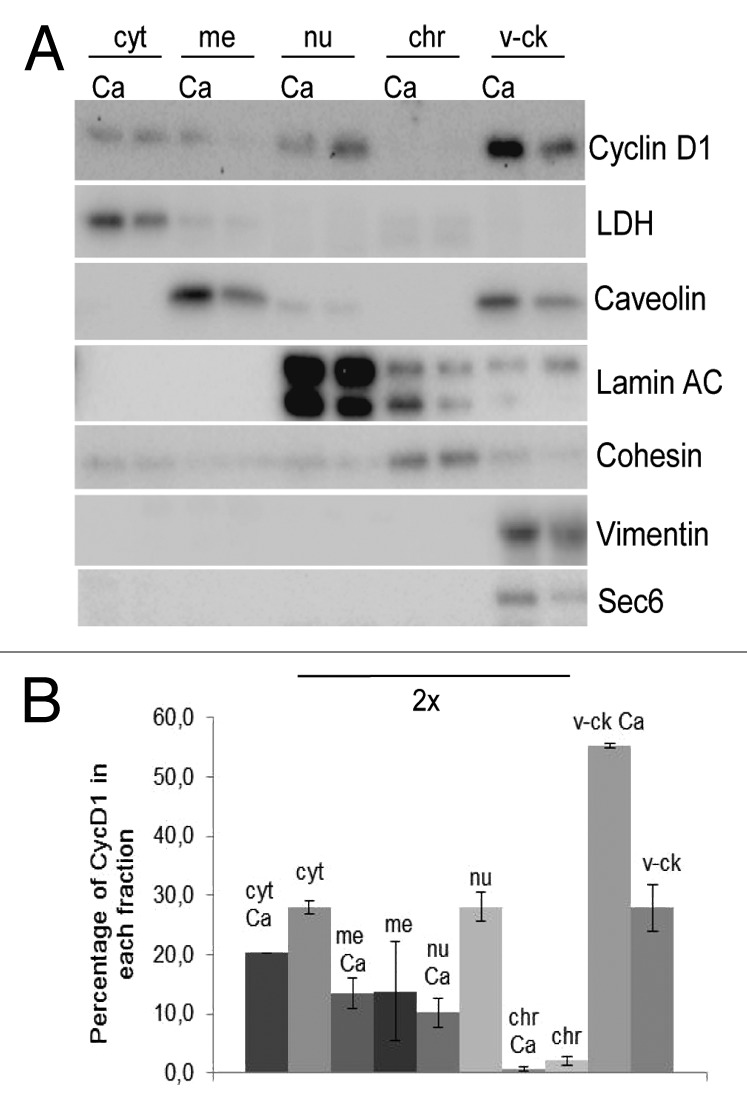
Figure 3. CycD1 localization by cell fractionation during keratinocyte differentiation. (A) Calcium was added to a semi-confluent culture of primary human keratinocytes; cells were recovered 48 h later and submitted to subcellular fractionation (see “Materials and Methods”). As a control, we used keratinocytes growing under the same conditions, but without calcium addition. Fractions were then analyzed by immunoblotting to detect CycD1, LDH as a cytosol marker, caveolin as a membrane marker, lamin A/C as a nucleoplasm marker, cohesin subunit Scc1 as a chromatin marker, exocyst subunit Sec6 as a vesicle marker, and vimentin as a cytoskeleton marker. Nucleoplasm, chromatin and vesicle-cytoskeleton fractions are 2-fold concentrated (2×) (B) Quantification of CycD1 levels in different subcellular fractions. The values are the mean ± SEM of two independent experiments. Statistically significant differences (P ≤ 0.05) were observed between −Ca and +Ca in the cytosolic, nucleoplasm and vesicles-cytoskeleton fractions.
CycD1 co-localized with exocyst components and β1 integrin
In a previous work, we have observed that CycD1 interacts in cytoplasmic foci with exocyst components (Sec6) and regulators (RalA) and also regulates cell-matrix adhesion in fibroblasts.7 We hypothesized that these interactions could also be relevant for keratinocytes in regulating cell-matrix adhesion during differentiation. By using a low permeabilization protocol for immunofluorescence to detect membranous cell structures adequately16 (E Montanez and S Wickström personal communication), we have analyzed the co-localization of CycD1 with RalA and Sec6 in primary human keratinocytes a short time after being seeded on collagen plates. Under these low-confluent conditions, cells showed co-localization of CycD1 with RalA and Sec6 in cytoplasmic foci (Fig. 4) as in fibroblasts,7 suggesting that CycD1 may be involved in the regulation of keratinocyte adhesion to the extracellular cell matrix.
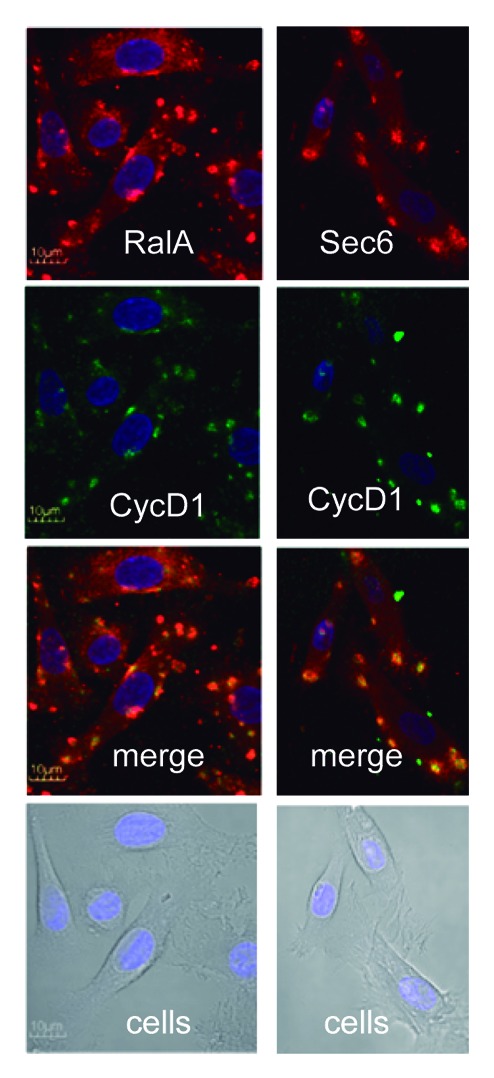
Figure 4. Co-localization of CycD1 with exocyst in human keratinocytes. After trypsinization, keratinocytes were seeded on collagen plates and incubated 4 h before fixation. After fixation, we used 0.02% tween20 to permeabilize. Co-localization of CycD1 with exocyst regulator RalA and exocyst component Sec6 was analyzed by immunofluorescence. Images were acquired by confocal microscopy using a 60× objective (10 μm bar).
Exocyst regulates β1 integrin recycling, a crucial process for keratinocyte detachment from the basal membrane during differentiation.12,13 We analyzed the co-localization of CycD1 with β1 integrin in cultured keratinocytes a short time (16 h) after calcium addition (akin to skin epithelia in the initial steps of differentiation). Under these conditions, a significant number of keratinocytes showed β1 integrin, forming a punctuate pattern in their cytoplasm, whereas cells incubated without calcium did not exhibit these foci13 (Fig. 5A and B). Moreover, we observed co-localization of CycD1 with β1 integrin and Sec6 in those cytoplasmic foci (Fig. 5C). These results are consistent with the idea that cytoplasmic CycD1 could collaborate with exocyst in β1 integrin recycling during keratinocyte differentiation. In order to address this question, we have analyzed whether high levels of cyclin D1 enhanced the loss of β1 integrin signal in the membrane during keratinocyte differentiation. We transfected keratinocytes either with wild-type or k112E CycD1 alleles fused to GFP, and GFP alone as a control. Calcium was added 24 h later to induce differentiation. After a short time of incubation with calcium, which is not sufficient to trigger full keratinocyte differentiation, cells were fixed and processed for IF to detect β1 integrin, and the percentage of cells that had completely lost β1 integrin signal in the membrane was counted. We have observed that the number of cells that had lost β1 integrin signal in the membrane was higher for cells overexpressing cyclin D1, suggesting that this cyclin is involved in the efficient recycling of β1 integrin (Fig. 5D). On the contrary, the overexpression of k112 allele was unable to raise the number of cells that had lost β1 integrin signal in the membrane, indicat ing that cyclin D1-associated kinase activity is required for cyclin D1 function in β1 integrin recycling (Fig. 5D).
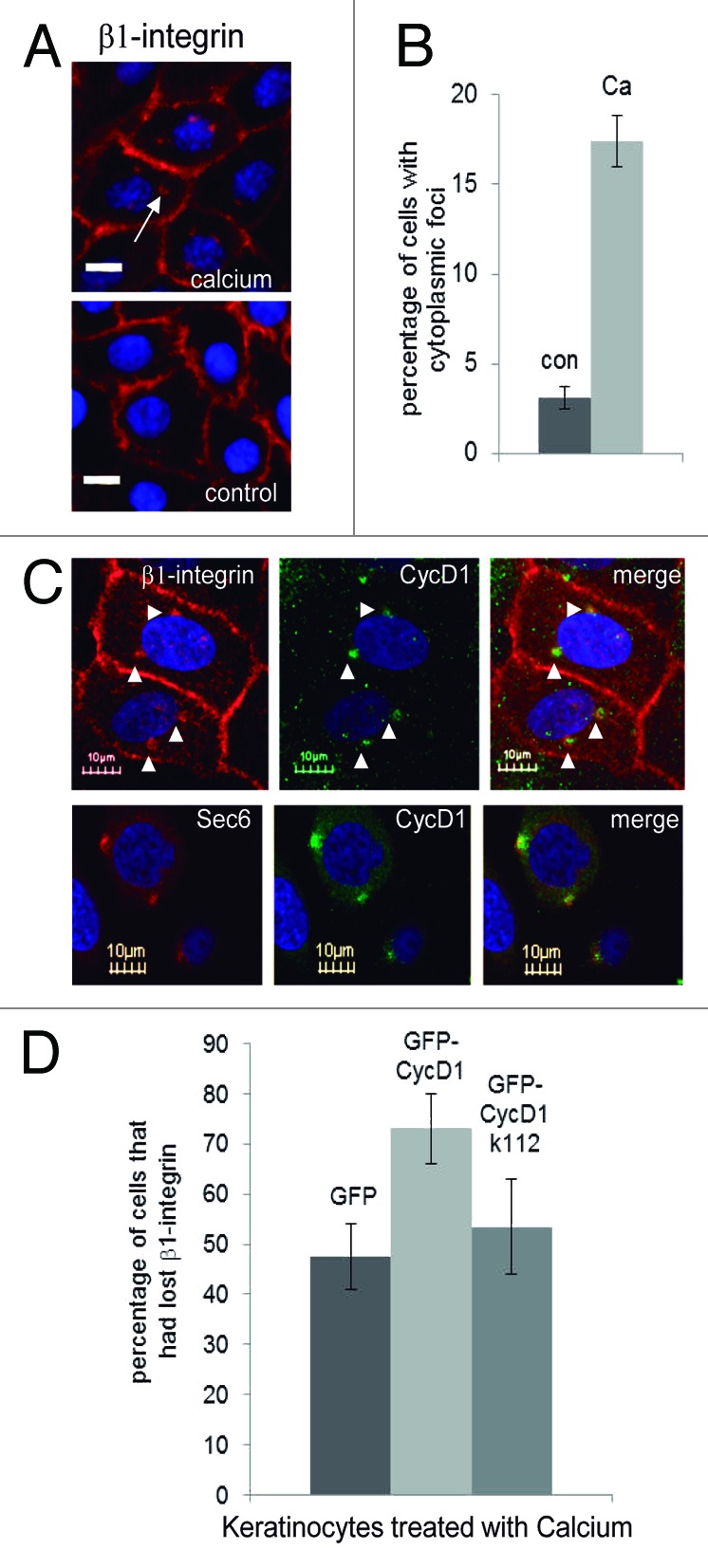
Figure 5. Co-localization of CycD1 with β1 integrin during keratinocyte differentiation. Before fixation, keratinocytes were incubated for only 16 h in the presence of 2 mM calcium to look at cells at the initial steps of differentiation. (A) β1 integrin was analyzed by immunofluorescence. Images were acquired by using a 40× objective in an Olympus IX71 inverted microscope (20 µm bar) (B) Quantification of cells with β1 integrin in cytoplasmic foci. Data are expressed as mean ± SEM from two independent experiments (P = 0.045; n ≥ 122). (C) Co-localization of CycD1 with β1 integrin and Sec6 was analyzed by confocal microscopy using a 60× objective (10 μm bar). Nuclei were stained with Hoescht. (D) Keratinocytes were transfected with either GFP-CycD1, GFP-CycD1k112, or GFP and after a short time of incubation with calcium, cells were fixed and processed for IF to detect β1 integrin, and the percentage of cells that had completely lost β1 integrin signal in the membrane was counted. Percentage of cells and confidence limits (α = 0.05; n ≥ 110) are plotted.
CycD1 expression induced cell detachment in primary keratinocytes
In order to test the functional relevance of CycD1 in the control of keratinocyte-matrix attachment, we assayed the effect of CycD1 overexpression in the ability of keratinocytes to spread and adhere on collagen and fibronectin plates, respectively. We observed a significant reduction in the number of spread cells after 48 h overexpressing CycD1 (Fig. 6A). Keratinocytes overexpressing CycD1 showed a retracted morphology, indicative of alterations in spreading (Fig. 6B). We have also observed delocalization of E-cadherin in keratinocytes overexpressing CycD1 (Fig. 6B). This suggests that overexpression of CycD1 also modified cell-to-cell adhesion, even though this effect could be indirect due to cell-matrix detachment. We have also tested whether the alteration in the efficiency of keratinocytes adhesion was dependent on Cdk activity. For this, we transfected a construct harboring GFP fused to cyclin D1-k112 allele, which is unable to produce active kinase complexes. In accordance with previous data obtained in other cell types, the overexpression of this allele did not promote either morphological alterations (not shown) or detachment of keratinocytes, indicating that these phenotypes are dependent on Cdk activity (Fig. 6A). Finally, in order to evaluate the adhesion capacity, transfected keratinocytes were seeded on fibronectin plates and incubated for 90 min before washing and fixing to count the number of attached cells. Keratinocytes overexpressing CycD1 showed a highly reduced adhesion capacity (Fig. 6C). Taken together our results demonstrate that high levels of CycD1 promoted cell-matrix detachment of primary keratinocytes.
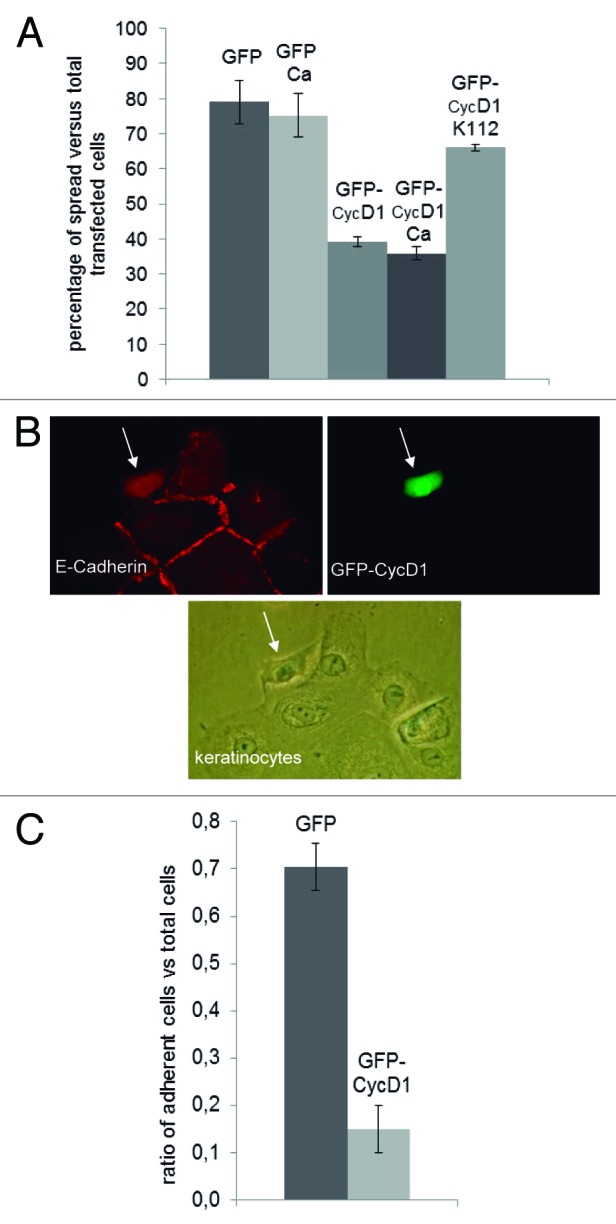
Figure 6. Expression of CycD1 reduced adhesion and spreading efficiency of keratinocytes. (A) Cells were transfected with GFP-CycD1, GFP-CycD1k112, or GFP alone. Forty-eight hours after transfection, cells were fixed, and the proportion of spread green cells was determined. Percentages of spread cells are plotted. The mean ± SEM is shown from 2 or 4 independent experiments depending on the samples. For sample comparisons, GFP vs. GFP-CycD1 and GFP-CycD1 vs. GFP-CycD1k112 were both significantly different with P ≤ 0.001; GFP Ca vs. GFP-CycD1 Ca were also significantly different with P = 0.013. (B) Representative image of the morphological changes of keratinocytes overexpressing GFP-CycD1 as in (A). Cells were also stained to detect E-cadherin (red). (C) Keratinocytes transfected with either GFP-CycD1 or GFP were seeded in serum-free medium in 35 mm plates coated with 10 μg/ml fibronectin. After 90 min, plates were washed twice with temperate PBS, fixed, and green cells counted. Relative adhesion values and confidence limits (α = 0.05; n ≥ 219) are plotted.
Discussion
Here we report that CycD1 localization was largely reduced in the cell nucleus and accumulated in the cytoplasm of keratinocytes under differentiation conditions in vitro and in vivo. The question that immediately arises from our results is which would be the function of the change in CycD1 localization regarding skin homeostasis? We know that basal keratinocytes progressively reduce their adhesiveness to the basement membrane during skin differentiation, and that the levels of β1 integrin attachment to the extracellular matrix regulate the balance between skin proliferation and differentiation.12,17 β1 integrin is internalized during differentiation and can be visualized forming punctuate foci in the cytoplasm of keratinocytes.13 Moreover, we and others have shown that CycD1 exerts cytoplasmic functions collaborating with Ral and Rho GTPases to control cell-matrix adhesion and motility.4,7 RalGTPases, in turn, control exocyst formation and activity, which increases β1 integrin recycling.9 Based on these facts, we hypothesize that the increment of cytoplasmic CycD1 that we observed in keratinocytes may positively regulate the recycling of β1 integrin through exocyst activation and therefore stimulate detachment from the basal membrane and differentiation. In support of this idea, we have observed: (1) co-localization of CycD1 with RalA and Sec6 in keratinocytes, (2) an increment in the co-localization of CycD1 and β1 integrin in cytoplasmic foci during keratinocyte differentiation, and (3) that overexpression of CycD1 in keratinocytes induced β1 integrin recycling and loss of cell-matrix adhesion. Overall, our results strongly suggest that CycD1 exerts cytoplasmic functions involved in the regulation of keratinocyte adherence during differentiation.
We cannot exclude that the increment of cyclin D1 in the cytoplasm (and perhaps total increment) observed in suprabasal layer may be associate with other cellular processes. For instance, senescent keratinocytes appear in basal and suprabasal layers of the human epidermis and are more abundant in aged skin.18 According to this, it is well known that aging induces accumulation of cellular alterations in skin. Interestingly, accumulation of cyclin D1 has been observed in senescent cells promoting cellular hypertrophy.19 Then, the strong signal of cytoplasmic cyclin D1 (perhaps also the nuclear signal) observed in suprabasal layers of human epidermis could also indicate the presence of senescent keratinocytes. Further analyses with more human skin samples grouped by age have to be done in order to test whether the cytoplasmic accumulation of cyclin D1 would be related to senescence and aged skin. Also it should be tested whether cyclin D1 is accumulated in the cytoplasm of senescent cells.
CycD1-knockout mice are viable and do not show obvious alterations in their skin,20-22 which might be explained by the redundant function of other D-type cyclins.23,24 In the reverse situation, by means of its overexpression, we have shown the relevance of CycD1 in the control of keratinocyte attachment to the cell-matrix. Because loss of keratinocyte adherence to the basal lamina is required for differentiation, high levels of CycD1 may have been expected to promote keratinocyte differentiation. However, overexpression of CycD1 actually blocks keratinocyte differentiation in vitro because of the role of CycD1 in promoting cell proliferation.25 To explain this, we can assume that the fine regulation of cyclin D1 localization may be important in differentiation and developmental processes. However, we have observed that under overexpression conditions GFP-cyclin D1 is located both in the nucleus and cytoplasm (Fig. 6B), probably because the high amount of the protein is able to detour the machinery that controls CycD1 localization. In this situation, even though high levels of cytoplasmic cyclin D1-Cdk4 activity efficiently promote cell detachment, nuclear cyclin D1-associated activity may be dominant by phosphorylating several transcriptional regulators to trigger cell proliferation and to block differentiation.
The predominant subcellular localization of CycD1 has been largely controversial. For a long time, it was believed that CycD1 was exclusively nuclear, with the cytoplasm as the site of its degradation (for a review, see ref. 26). More recently, though, CycD1 has been shown to accumulate in cytoplasmic fractions of cell lines and differentiated neurons.27,28 By using soft permeabilization conditions, here we have detected CycD1 distributed in cytoplasmic foci. Also, by using biochemical methods, we have found CycD1 in both nuclear and cytoplasmic fractions. Importantly, we have shown that CycD1 is present in the cytosol, in particular, associated with vesicles and the cytoskeleton, which agrees with previous findings showing that CycD1 interacts with the cytoskeleton and membrane-associated proteins, such as filamin A, translokin, and Sec6.6,7,29 Our study places CycD1 both in the nucleus and in the cytoplasm of primary human keratinocytes, and strongly suggests that the prevalence of a specific subcellular localization likely depends on the function to be performed in a given context, either proliferation or differentiation.
Since abnormal alterations of CycD1 localization could be critical under pathological conditions, this may be an interesting feature to use diagnostically. The control of cell motility by CycD1 might be relevant for the ability of CycD1 to promote invasiveness and metastases.30 Amplification and overexpression of Cyclin D1 is frequently found in nonmelanocitic skin cancers.31,32 Based on the heterogeneity of cells within tumors, it is conceivable that an abnormal amount of cytoplasmic CycD1 in one part of the population of tumor cells may promote their migration and enhance invasiveness of the surrounding tissue. Consistent with this, high levels of cytoplasmic CycD1 seem to correlate with augmented invasion and metastasis in several cases of cutaneous squamous cell carcinoma.33 Further work will be required to address this question.
Materials and Methods
Cell culture and expression vectors
Primary human keratinocytes were obtained from Invitrogen-Gibco (Cat. No. 12332-011). Cells were maintained at 37 °C in 5% CO2 environment and grown in defined keratinocyte serum-free medium (Cat. No 10744-019) with 10 μg/ml gentamycin. For “in vitro” differentiation, 2 mM calcium chloride was added to semi-confluent keratinocyte cultures that were incubated as above, except that the medium only contained one-third of the growth supplement. Human CycD1 (a gift from N. Agell) was used to make pCMV-EGFP-CycD1 in pcDNA3 (Invitrogen), or pUBI-3xHA-CycD1 in a lentiviral vector derived from pDSL (Invitrogen). CycD1-K112E allele was constructed by site directed mutagenesis. pEGFP-N1 was obtained from Clontech. Details of all constructs are available upon request. Transient transfection of vectors was performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
Immunohistochemistry
Normal skin samples were obtained from Hospital Arnau de Vilanova Histopathology archive (with explicit informed consent for research use obtained from each patient, according to the regulations approved by the hospital ethics committee). Blocks were sectioned at a thickness of 3 μm and dried for 1 h at 65 °C before being dewaxed in xylene and rehydrated through a graded ethanol series, then washed with PBS. Antigen retrieval was performed by heat treatment in a pressure cooker for 2 min in EDTA (pH 8.9). Before staining the sections, endogenous peroxidase was blocked. The antibodies used were: cytokeratin 5 (rabbit monoclonal ERP1600Y, Epitomics) and CycD1 (rabbit monoclonal SP4, Dako). After incubation, the reaction was visualized with the EnVision Detection Kit (Dako), using diaminobenzidine chromogen as a substrate. Sections were counterstained with hematoxylin. ImageJ software was used to quantify staining intensity. We have converted the color images to 8-bit gray values. Using the selection tools the nuclear area and total cell area were drawn. We defined a threshold (same for all images), and pixel intensity was measured. To determine the percentage of cytoplasmic cyclin D1 (C), nuclear intensity (N) was subtracted from total cell intensity (T) and calculated as %C = (T-N)*100/T.
Protein fractionation and immunoblot
Protein fractionation was performed with the Subcellular Protein Fractionation kit for cultured cells (Thermo Scientific-Pierce; 78840) following supplier’s instructions. Protein samples were resolved by SDS–PAGE, transferred to PVDF membranes (Millipore), and analyzed by immunoblotting. Appropriate peroxidase-linked secondary antibodies (GE Healthcare UK Ltd) were detected using the chemiluminescent HRP substrate Immobilon Western (Millipore). Chemiluminescence originated from western blots was recorded with the aid of a CCD-based camera (Lumimager, Roche). Intensity of CycD1 bands was quantified with the Image Lab software (Biorad). Primary antibodies were LDH (goat polyclonal), Caveolin (mouse monoclonal 2297, BD), Lamin A/C (mouse monoclonal 346, SC), Scc1 (rabbit polyclonal, kindly provided by N. Colomina), Vimentin (mouse monoclonal RV202, BD), CycD1 (monoclonal DCS-6, BD PharMingen), and Sec6 (mouse monoclonal 9H5, Abcam).
Total extracts to detect HA-CycD1 and involucrin were obtained by scraping the plate surface in lysis buffer (2% SDS in 0.125M TRIS-HCl at pH6.8). Primary antibodies were HA (monoclonal 12CA5, our own stocks) and Involucrin (mouse monoclonal SY5, Sigma).
Immunofluorescence
Keratinocytes were quickly washed in PBS and fixed in 4% paraformaldehyde for 15 min at room temperature and were permeabilized with either 0.02% (low) or 0.1% (medium) tween20 for 2 min at room temperature. Cells were blocked with 3% BSA for 30 min. Primary antibodies were detected with adequate Alexa488 and/or Alexa594-labeled secondary antibodies (Molecular Probes) in PBS with 0.3% BSA. Nuclei were stained with Hoechst (Sigma). Images were acquired using a 40× objective in an Olympus IX71 inverted microscope. Confocal images were acquired in an Olympus FV1000 confocal system using a 60× objective. Antibodies used were HA (rat monoclonal 3F10, Roche), Sec6 (monoclonal 9H5, Abcam), RalA (monoclonal 610221, BD Trans. Lab), CycD1 (polyclonal 06-137, Upstate), E-cadherin (mouse monoclonal HECD-1, Calbiochem), GFP (rabbit polyclonal 488-conjugate, Invitrogen), and β1 integrin (monoclonal P5D2, Abcam).
Cell spreading and adhesion assay
Keratinocytes were seeded in collagen plates and transfected with GFP or with GFP-CycD1 expression vectors. Cells were incubated in normal conditions and 48 h later fixed. Images were taken, and spread green cells were counted. Round and bright cells were considered to be unspread. For cell adhesion analysis, keratinocytes were transfected with expression vectors harboring GFP or GFP-CycD1. Forty-eight hours later, cells were trypsinized, and a total of 20 000 cells were seeded in serum-free medium in a 35 mm plate coated with 10 μg/ml fibronectin (Sigma). After 90 min, plates were washed twice with temperate PBS and fixed.
Statistical analyses
Data are expressed as mean ± SEM. Comparisons among groups were made using an equal variance t test under one-tailed hypothesis. In the figures, error bars indicate SEM P ≤ 0.05 was considered significant. In Figures 5D and 6C, bars indicate confidence limits for a proportion.
Acknowledgments
We thank Eloi Montanez and Sara Wickström for technical advice; Neus Agell for providing human cyclin D1; Neus Colomina for the Scc1 antibody; Maria Santacana and Xavier Matias-Guiu for immunohistochemistry; Sònia Rius for technical assistance; and the members of the CYC and MDN labs for helpful discussions. This work was funded by the Spanish Ministry of Education and Science (BFU2010-20293/BMC), by the Catalan Government (SGR-559), and by Consolider-Ingenio 2010 (CSD2007-00015). Joaquim Egea is a researcher of the Ramon y Cajal program (RYC-2008-03342). R Fernández-Hernández, M Rafel, and NP. Fusté were supported by predoctoral fellowships from the Spanish Ministry of Education and Science, and from the University of Lleida.
Glossary
Abbreviations:
- CycD1
Cyclin D1
- LDH
lactate dehydrogenase
- HA
haemagglutinin
- CMV
cytomegalovirus
- PBS
phosphate buffer saline
- cyt
cytosol
- me
membrane
- nu
nucleoplasm
- chr
chromatin
- v-ck
vesicles-cytoskeleton
- con
control
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/25590
References
- 1.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 2.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 3.Neumeister P, Pixley FJ, Xiong Y, Xie H, Wu K, Ashton A, et al. Cyclin D1 governs adhesion and motility of macrophages. Mol Biol Cell. 2003;14:2005–15. doi: 10.1091/mbc.02-07-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Wang C, Jiao X, Lu Y, Fu M, Quong AA, et al. Cyclin D1 regulates cellular migration through the inhibition of thrombospondin 1 and ROCK signaling. Mol Cell Biol. 2006;26:4240–56. doi: 10.1128/MCB.02124-05. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Jiao X, Wang C, Ju X, Lu Y, Yuan L, et al. Cyclin D1 induction of cellular migration requires p27(KIP1) Cancer Res. 2006;66:9986–94. doi: 10.1128/MCB.02124-05. b. [DOI] [PubMed] [Google Scholar]
- 6.Zhong Z, Yeow WS, Zou C, Wassell R, Wang C, Pestell RG, et al. Cyclin D1/cyclin-dependent kinase 4 interacts with filamin A and affects the migration and invasion potential of breast cancer cells. Cancer Res. 2010;70:2105–14. doi: 10.1158/0008-5472.CAN-08-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández RMH, Ruiz-Miró M, Dolcet X, Aldea M, Garí E. Cyclin D1 interacts and collaborates with Ral GTPases enhancing cell detachment and motility. Oncogene. 2011;30:1936–46. doi: 10.1038/onc.2010.577. [DOI] [PubMed] [Google Scholar]
- 8.Bodemann BO, White MA. Ral GTPases and cancer: linchpin support of the tumorigenic platform. Nat Rev Cancer. 2008;8:133–40. doi: 10.1038/nrc2296. [DOI] [PubMed] [Google Scholar]
- 9.Spiczka KS, Yeaman C. Ral-regulated interaction between Sec5 and paxillin targets Exocyst to focal complexes during cell migration. J Cell Sci. 2008;121:2880–91. doi: 10.1242/jcs.031641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs E. Skin stem cells: rising to the surface. J Cell Biol. 2008;180:273–84. doi: 10.1083/jcb.200708185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watt FM, Fujiwara H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb Perspect Biol. 2011;3:a005124. doi: 10.1101/cshperspect.a005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watt FM. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 2002;21:3919–26. doi: 10.1093/emboj/cdf399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lotti R, Marconi A, Truzzi F, Dallaglio K, Gemelli C, Borroni RG, et al. A previously unreported function of β(1)B integrin isoform in caspase-8-dependent integrin-mediated keratinocyte death. J Invest Dermatol. 2010;130:2569–77. doi: 10.1038/jid.2010.195. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs E, Horsley V. More than one way to skin . . . Genes Dev. 2008;22:976–85. doi: 10.1101/gad.1645908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245–54. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- 16.Montanez E, Piwko-Czuchra A, Bauer M, Li S, Yurchenco P, Fässler R. Analysis of integrin functions in peri-implantation embryos, hematopoietic system, and skin. Methods Enzymol. 2007;426:239–89. doi: 10.1016/S0076-6879(07)26012-4. [DOI] [PubMed] [Google Scholar]
- 17.Levy L, Broad S, Diekmann D, Evans RD, Watt FM. β1 integrins regulate keratinocyte adhesion and differentiation by distinct mechanisms. Mol Biol Cell. 2000;11:453–66. doi: 10.1091/mbc.11.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leontieva OV, Lenzo F, Demidenko ZN, Blagosklonny MV. Hyper-mitogenic drive coexists with mitotic incompetence in senescent cells. Cell Cycle. 2012;11:4642–9. doi: 10.4161/cc.22937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H, et al. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–30. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 21.Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364–72. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- 22.Robles AI, Rodriguez-Puebla ML, Glick AB, Trempus C, Hansen L, Sicinski P, et al. Reduced skin tumor development in cyclin D1-deficient mice highlights the oncogenic ras pathway in vivo. Genes Dev. 1998;12:2469–74. doi: 10.1101/gad.12.16.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozar K, Ciemerych MA, Rebel VI, Shigematsu H, Zagozdzon A, Sicinska E, et al. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118:477–91. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Sistrunk C, Miliani de Marval PL, Kim Y, Rodriguez-Puebla ML. Combined effect of cyclin D3 expression and abrogation of cyclin D1 prevent mouse skin tumor development. Cell Cycle. 2012;11:335–42. doi: 10.4161/cc.11.2.18774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto H, Ochiya T, Takeshita F, Toriyama-Baba H, Hirai K, Sasaki H, et al. Enhanced skin carcinogenesis in cyclin D1-conditional transgenic mice: cyclin D1 alters keratinocyte response to calcium-induced terminal differentiation. Cancer Res. 2002;62:1641–7. [PubMed] [Google Scholar]
- 26.Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alao JP, Gamble SC, Stavropoulou AV, Pomeranz KM, Lam EW, Coombes RC, et al. The cyclin D1 proto-oncogene is sequestered in the cytoplasm of mammalian cancer cell lines. Mol Cancer. 2006;5:7. doi: 10.1186/1476-4598-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sumrejkanchanakij P, Tamamori-Adachi M, Matsunaga Y, Eto K, Ikeda MA. Role of cyclin D1 cytoplasmic sequestration in the survival of postmitotic neurons. Oncogene. 2003;22:8723–30. doi: 10.1038/sj.onc.1206870. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz-Miró M, Colomina N, Fernández RM, Garí E, Gallego C, Aldea M. Translokin (Cep57) interacts with cyclin D1 and prevents its nuclear accumulation in quiescent fibroblasts. Traffic. 2011;12:549–62. doi: 10.1111/j.1600-0854.2011.01176.x. [DOI] [PubMed] [Google Scholar]
- 30.Velasco-Velázquez MA, Li Z, Casimiro M, Loro E, Homsi N, Pestell RG. Examining the role of cyclin D1 in breast cancer. Future Oncol. 2011;7:753–65. doi: 10.2217/fon.11.56. [DOI] [PubMed] [Google Scholar]
- 31.Liang SB, Furihata M, Takeuchi T, Iwata J, Chen BK, Sonobe H, et al. Overexpression of cyclin D1 in nonmelanocytic skin cancer. Virchows Arch. 2000;436:370–6. doi: 10.1007/s004280050461. [DOI] [PubMed] [Google Scholar]
- 32.Staibano S, Lo Muzio L, Pannone G, Mezza E, Argenziano G, Vetrani A, et al. Association of Directors of Anatomic and Surgical Pathology DNA ploidy and cyclin D1 expression in basal cell carcinoma of the head and neck. Am J Clin Pathol. 2001;115:805–13. doi: 10.1309/GGE7-WL7J-VRWD-R4VG. [DOI] [PubMed] [Google Scholar]
- 33.Huang K, Huang C, Shan K, Chen J, Li H. Significance of PC cell-derived growth factor and cyclin D1 expression in cutaneous squamous cell carcinoma. Clin Exp Dermatol. 2012;37:411–7. doi: 10.1111/j.1365-2230.2011.04275.x. [DOI] [PubMed] [Google Scholar]


