Abstract
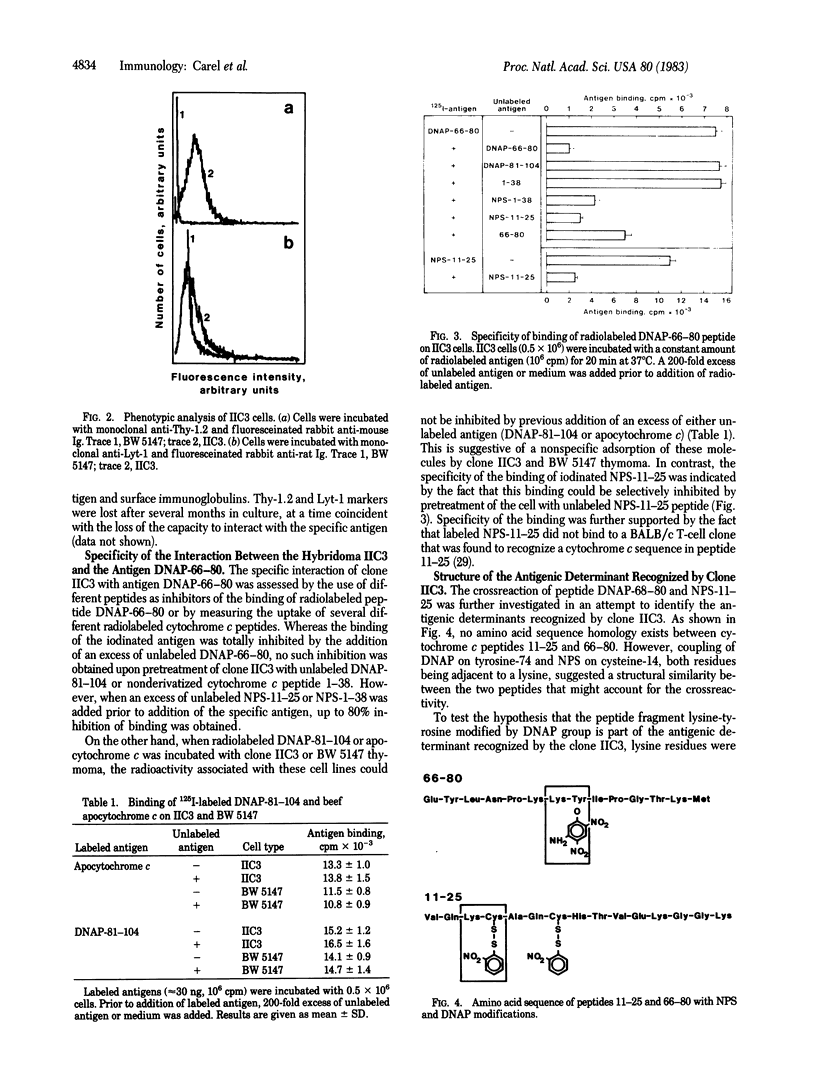
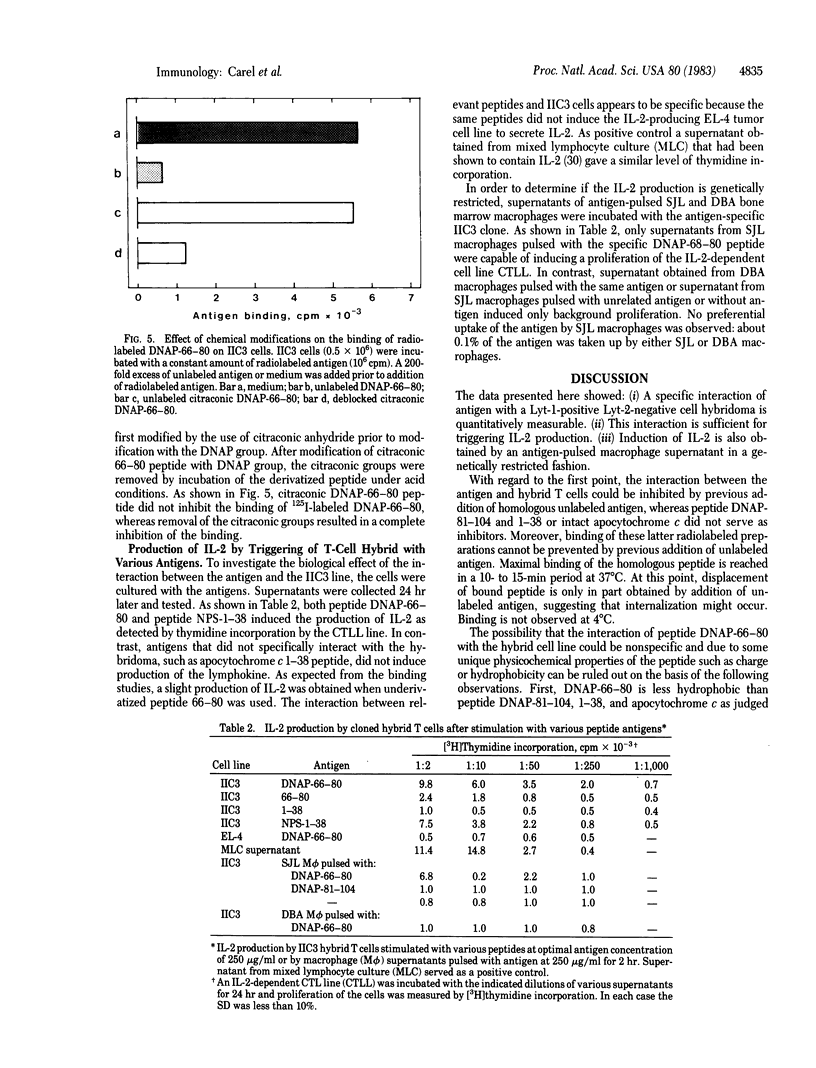
T-cell hybridomas were obtained after fusion of BW 5147 thymoma and long-term cultured T cells specific for cytochrome c peptide 66-80 derivatized with a 2,4-dinitroaminophenyl (DNAP) group. The resulting hybridomas were selected for their capacity to specifically bind to soluble radiolabeled peptide antigen. One T-cell hybrid was positive for antigen binding. This hybrid T cell exhibits surface phenotypic markers of the parent antigen-specific T cells. The binding could be inhibited either by an excess of unlabeled homologous antigen or by cytochrome c peptide 11-25 derivatized with a 2-nitrophenylsulfenyl group. Several other peptide antigens tested failed to inhibit binding of the radioactive peptide. This suggests that a specific amino acid sequence, modified by a DNAP group, is the antigenic structure recognized by the putative T-cell receptor. In addition, direct interaction of DNAP-66-80 peptide with the hybridoma cell line induced production of the T-cell growth factor interleukin 2. Furthermore, supernatants derived from syngeneic macrophages pulsed with the relevant peptide also induced the antigen-specific hybridoma to produce interleukin 2.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binz H., Wigzell H. Antigen-binding, idiotypic T-lymphocyte receptors. Contemp Top Immunobiol. 1977;7:113–177. doi: 10.1007/978-1-4684-3054-7_4. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carel S., Bron C., Corradin G. Cytochrome c specific T cell hybrid. Curr Top Microbiol Immunol. 1982;100:111–116. doi: 10.1007/978-3-642-68586-6_13. [DOI] [PubMed] [Google Scholar]
- Corradin G., Etlinger H. M., Chiller J. M. Lymphocyte specificity to protein antigens. I. Characterization of the antigen-induced in vitro T cell-dependent proliferative response with lymph node cells from primed mice. J Immunol. 1977 Sep;119(3):1048–1053. [PubMed] [Google Scholar]
- Corradin G., Harbury H. A. Cleavage of cytochrome c with cyanogen bromide. Biochim Biophys Acta. 1970 Dec 22;221(3):489–496. doi: 10.1016/0005-2795(70)90219-9. [DOI] [PubMed] [Google Scholar]
- Eichmann K., Ben-Neriah Y., Hetzelberger D., Polke C., Givol D., Lonai P. Correlated expression of VH framework and VH idiotypic determinants on T helper cells and on functionally undefined T cells binding group A streptococcal carbohydrate. Eur J Immunol. 1980 Feb;10(2):105–112. doi: 10.1002/eji.1830100207. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Vanderkooi J. M., Wilson D. F. Cytochrome c interactions with membranes. A photoaffinity-labeled cytochrome c. Arch Biochem Biophys. 1975 Nov;171(1):108–116. doi: 10.1016/0003-9861(75)90013-2. [DOI] [PubMed] [Google Scholar]
- Eshhar Z., Apte R. N., Löwy I., Ben-Neriah Y., Givol D., Mozes E. T-cell hybridoma bearing heavy chain variable region determinants producing (T,G)-A--L-specific helper factor. Nature. 1980 Jul 17;286(5770):270–272. doi: 10.1038/286270a0. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Harwell L., Skidmore B., Marrack P., Kappler J. Concanavalin A-inducible, interleukin-2-producing T cell hybridoma. J Exp Med. 1980 Oct 1;152(4):893–904. doi: 10.1084/jem.152.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensenius J. C., Williams A. F. The T lymphocyte antigen receptor--paradigm lost. Nature. 1982 Dec 16;300(5893):583–588. doi: 10.1038/300583a0. [DOI] [PubMed] [Google Scholar]
- Lonai P., Puri J., Hämmerling G. H-2-restricted antigen binding by a hybridoma clone that produces antigen-specific helper factor. Proc Natl Acad Sci U S A. 1981 Jan;78(1):549–553. doi: 10.1073/pnas.78.1.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. A one-receptor view of T-cell behaviour. Nature. 1981 Aug 6;292(5823):497–501. doi: 10.1038/292497a0. [DOI] [PubMed] [Google Scholar]
- Mauel J., Defendi V. Infection and transformation of mouse peritoneal macrophages by simian virus 40. J Exp Med. 1971 Aug 1;134(2):335–350. doi: 10.1084/jem.134.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerpohl H. G., Lohmann-Matthes M. L., Fischer H. Studies on the activation of mouse bone marrow-derived macrophages by the macrophage cytotoxicity factor (MCF). Eur J Immunol. 1976 Mar;6(3):213–217. doi: 10.1002/eji.1830060313. [DOI] [PubMed] [Google Scholar]
- Mitchell W. M., Harrington W. F. Purification and properties of clostridiopeptidase B (Clostripain). J Biol Chem. 1968 Sep 25;243(18):4683–4692. [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Paul W. E., Benacerraf B. Functional specificity of thymus- dependent lymphocytes. Science. 1977 Mar 25;195(4284):1293–1300. doi: 10.1126/science.320663. [DOI] [PubMed] [Google Scholar]
- Rajewsky K., Eichmann K. Antigen receptors of T helper cells. Contemp Top Immunobiol. 1977;7:69–112. doi: 10.1007/978-1-4684-3054-7_3. [DOI] [PubMed] [Google Scholar]
- Ruddle N. H., Beezley B., Lewis G. K., Goodman J. W. Antigen specific T cell hybrids--II. T cell hybrids which bind azobenzenearsonate. Mol Immunol. 1980 Jul;17(7):925–931. doi: 10.1016/0161-5890(80)90041-3. [DOI] [PubMed] [Google Scholar]
- Ryser J. E., Cerottini J. C., Brunner K. T. Generation of cytolytic T lymphocytes in vitro. IX. induction of secondary CTL responses in primary long-term MLC by supernatants from secondary MLC. J Immunol. 1978 Feb;120(2):370–377. [PubMed] [Google Scholar]
- Schrier R. D., Skidmore B. J., Kurnick J. T., Goldstine S. N., Chiller J. M. Propagation of antigen-specific T cell helper function in vitro. J Immunol. 1979 Dec;123(6):2525–2531. [PubMed] [Google Scholar]
- Williamson A. R. Three-receptor, clonal expansion model for selection of self-recognition in the thymus. Nature. 1980 Feb 7;283(5747):527–532. doi: 10.1038/283527a0. [DOI] [PubMed] [Google Scholar]






