Abstract
Enterovesical fistulae in Crohn's disease are relatively rare. We present the first report of a child presenting with an enterovesical fistula as the initial presentation of Crohn's disease. Management comprises of timely diagnosis, and treatment involving surgical resection with adjunctive medical management including immunomodulators. This case highlights the need to be aware of the rare but important occurrence of Crohn's enterovesical fistula as a cause for urinary symptoms in a child with inadequate weight gain.
Background
Crohn's disease is one of the most important chronic diseases that affect children and adolescents. While abdominal pain, diarrhoea and weight loss remain the most frequent presenting features of Crohn's disease,1 signs related to the involvement of extraintestinal sites, such as the urinary tract, musculoskeletal system and liver, are well documented. However, it is rare for these to occur in the absence of significant intestinal manifestations. We report the first case of Crohn's disease in a child presenting with urinary tract fistulisation in the absence of significant gastrointestinal (GI) tract manifestations. The case highlights the need to be aware of this differential diagnosis in a child with urinary symptoms and suboptimal growth.
Case presentation
A 13-year-old boy was referred to his local paediatric service with three episodes of urinary tract infection over 6 months. His blood tests were unremarkable. A urine microscopy revealed a raised leukocyte count (white cell count; WCC 2800) although subsequent culture yielded no microbial growth. He was subsequently referred to the regional paediatric urology service following a urinary tract ultrasound scan (USS) which showed a trabeculated and thickened bladder.
Investigations
A cystoscopy revealed a frond-like, pale lesion with the appearance of a coral reef over the entire right wall of the bladder. The bladder appeared distorted in general and only a single ureteric orifice was identified, to the left of the midline. Multiple small biopsies taken from the distorted area were suggestive of a benign papillary lesion with features of papillary urothelial hyperplasia.
An MRI scan showed irregular thickening of the posterior wall of the bladder extending to the dome and frond-like lesions on the bladder and several small gas bubbles were visible within the bladder possibly representing recent instrumentation or a connection with the bowel. The rectum appeared grossly normal but there was some focal wall thickening anteriorly with apparent tethering up towards the thickened bladder wall. Reactive lymph nodes were present along the vascular chains. A thickened small bowel was closely associated with a thickened posterior bladder wall. Overall, the MRI scan suggested an aggressive, inflammatory process, with a distribution suggestive of Crohn's disease with thickening of the small bowel, proliferation of the small bowel mesentery, thickening of the bladder with possible fistulation and lesser involvement of the anterior aspect of the rectum.
Gastroenterology evaluation revealed further history of seeing brown flecks in the urine occasionally and intermittent lower urinary tract symptoms in keeping with recurrent cystitis. The bowel habits were regular, however, he was on the <0.4th centile for weight. He had no other symptoms or family history of inflammatory bowel disease. Perianal and digital rectal examination were unremarkable. Blood tests showed a hypochromic, microcytic picture (haemoglobin 11.4 g/dl, mean cell volume 67.6 fl; mean corpuscular haemoglobin 67.6%). The patient had normal renal and liver function tests and raised inflammatory markers (C reactive protein 50.4, erythrocyte sedimentation rate 41 mm/hr, albumin 38 g/l, WCC 9.7×106/ml, plat 517×106/ml).
A barium meal and follow-through confirmed a fairly long segment of thickened distal ileum with barium seen to trickle into rectum and bladder in keeping with fistulae (figure 1). Mild gastritis only was identified on an upper endoscopy, while the colonoscopy limited by preparation up to splenic flexure, showed a normal distal colon. Multiple biopsies taken in both endoscopies were normal.
Figure 1.
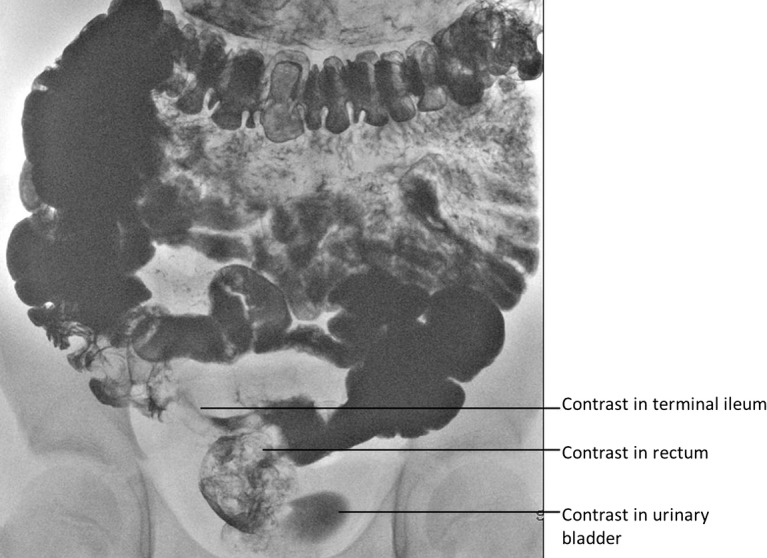
Contrast-meal and follow-through demonstrating early rectal filling with contrast (prior to contrast reaching the colon), followed by contrast appearing in the urinary bladder.
Treatment
Inpatient management with total parenteral nutrition and intravenous antibiotics resulted in weight gain before planned surgical intervention. Laparotomy confirmed the MRI findings of the enterovesical fistula entering the right posterior wall of the bladder, and the enterorectal fistula, closely related to an inflammatory mass, with the caecum ‘stuck’ in the pelvis attached to the bladder (figure 2). The right ureter was encased in inflammatory tissue and was therefore protected during surgery by passage of a feeding tube, which was left in situ for a week postoperatively. Fistula excision was performed with a cuff of normal bladder along with right hemicolectomy, temporary ileostomy and formation of mucus fistula. A histopathology examination showed multifocal ulceration, patchy mixed inflammation of the lamina propria, foci of cryptitis and crypt abscesses. Transmural inflammation was noted with fissuring ulcers and a few compact epithelioid cell granulomata and prominent lymphoid follicular hyperplasia. The presence of the two fistulae was confirmed.
Figure 2.
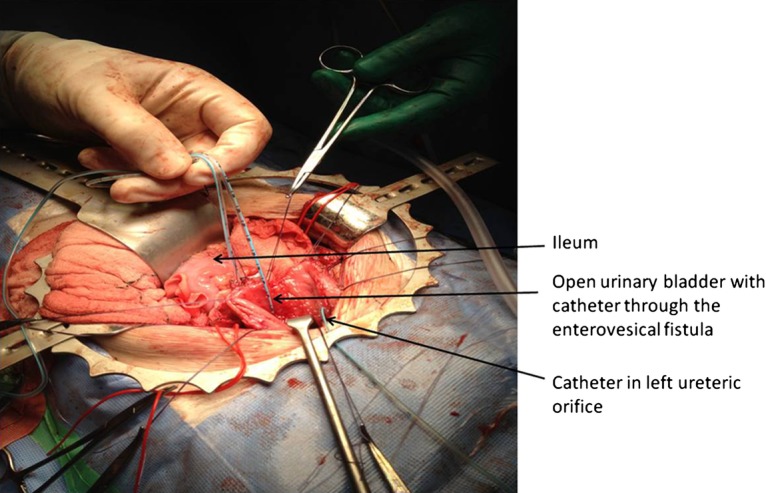
Operative image showing one catheter through the enterovesical fistula and the second catheter tube in the right ureter.
Postoperatively, he was managed with a course of antibiotics, parenteral nutrition and a suprapubic catheter (SPC) for 2 weeks. Micturating cystourethrogram 2 weeks postprocedure revealed no leak of urine and so SPC was removed. USS performed 8 weeks later showed normal kidneys and bladder. He was discharged on azathioprine, oxybutynin and prophylactic trimethoprim.
Outcome and follow-up
The ileostomy was closed after 8 months uneventfully. The gastroenterology team performed follow-up upper GI endoscopy and colonoscopy which were unremarkable. At 1-year follow-up, the child was gaining weight on normal enteral feed supplemented with high-calorie drinks. He has no further urinary or bowel symptoms.
Discussion
Genitourinary tract involvement is well-described in Crohn's disease in both adults and children,2 3 ranging from fistulisation, renal calculi, hydronephrosis secondary to compression, nephrolithiasis, amyloidosis and even scrotal abscesses. However, this is the first report in literature of Crohn's disease in a child with an initial presentation with enterovesical (EV) fistula. EV fistula itself has been reported to occur in 2–5% of patients with Crohn's disease.3 These are usually caused by transmural fissures from the diseased segment of the bowel to the bladder.4 EV fistulae have been most commonly reported to be from the ileum or sigmoid colon.5 Complex fistulae can also happen with coexisting ileo ileal, ileo colonic or enterocutaneous fistulae. Pneumaturia and dysuria are the most frequent clinical manifestations, occurring in two thirds of patients, while recurrent urinary tract infections and fecaluria have been reported in about one third of the patients.
Most previous reports of EV fistula in patients with Crohn's disease have been of 2.5–4 years following the diagnosis of Crohn's. Cystoscopy enables visualisation of a fistula, alongside the typical ‘bullous oedema’ surrounded by a localised patch of inflammation associated with inflammatory polyps. Adjacent or adherent inflammatory bowel and air within the bladder can be seen on CT or MRI. Although the most common site for fistulation into the urinary tract is the urinary bladder itself, fistulae have been previously reported into the urethra and the ureter.
Medical management of Crohn's EV fistula includes immunosuppressive therapy alongside antibiotics and optimisation of nutrition.3 Most reported cases have needed definitive surgical resection of the fistula for resolution of symptoms. The recommended procedure is fistulectomy with ‘pinching off’ of the involved dome of the bladder followed by repair of the bladder defect.3 Recent literature suggests a role for infliximab, the chimeric mouse/human monoclonal antibody directed against tumour necrosis factor. Although up to 40% complete remission rates have been reported following infliximab and non-surgical management for Crohn's EV fistulae in adults, the success rate has been low for non-operative treatment in children.3 Complications of untreated EV fistula include pyelonephritis and symptomatic bacteraemia. Postoperative management includes adequate bladder drainage using a suprapubic or urethral catheter for 2 weeks or until radiological evidence of watertight bladder repair. Continuing assessment and supportive and nutritional therapy for Crohn's disease is essential to minimise recurrence.
Learning points.
Consider Crohn’s enterovesical fistula as a rare but important cause of urinary symptoms in a child with suboptimal growth.
Effective treatment is achieved by a combination of surgical resection and supportive medical therapy.
Footnotes
Contributors: PC and DM wrote the initial drafts of the report. HC and CB revised the manuscript. All authors approved the final draft of the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sawczenko A, Sandhu BK. Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch Dis Child 2003;2013:995–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simoneaux SF, Patrick LE. Genitourinary complications of Crohn's disease in pediatric patients. AJR Am J Roentgenol 1997;2013:197–9 [DOI] [PubMed] [Google Scholar]
- 3.Teitelbaum JE, Saeed S, Triantafyllopoulou M, et al. Infliximab in pediatric Crohn disease patients with enterovesicular fistulas. J Pediatr Gastroenterol Nutr 2007;2013:279–82 [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto T, Keighley MR. Enterovesical fistulas complicating Crohn's disease: clinicopathological features and management. Int J Colorectal Dis 2000;2013:211–15; discussion 6–7 [DOI] [PubMed] [Google Scholar]
- 5.McNamara MJ, Fazio VW, Lavery IC, et al. Surgical treatment of enterovesical fistulas in Crohn's disease. Dis Colon Rectum 1990;2013:271–6 [DOI] [PubMed] [Google Scholar]


