Abstract
Invasive pulmonary aspergillosis is a rare and fatal complication in patients with cystic fibrosis (CF) who lack concomitant risk factors. The few documented cases in children have all resulted in deaths during hospitalisation. We present the case of a 12-year-old boy with CF who was admitted for an exacerbation which was unresponsive to antibiotic therapy. The findings on imaging raised concerns about a possible fungal infection. As a result, voriconazole therapy was started prior to his respiratory deterioration. He was later found to be β-D glucan and Aspergillus Ag galactomannan positive confirming the suspicion for invasive pulmonary aspergillosis. Three months after diagnosis, he was discharged home under stable condition. Voriconazole was continued beyond discharge and resulted in improvement of respiratory symptoms. This underscores the importance of early treatment of pulmonary aspergillosis in patients with CF. Unfortunately, the patient died 6 months after diagnosis from a CF exacerbation.
Background
Invasive pulmonary aspergillosis (IPA) can occur in patients with immunodeficiencies or corticosteroid-induced immunosuppression.1 Despite the fact that Aspergillus can be found in up to 60% of cystic fibrosis (CF) patients’ airways, invasive aspergillosis is a very rare and fatal complication.2 Invasive aspergillosis can be diagnosed with serology like the galactomannan (GM) test, which is negative in patients with CF colonised with Aspergillus.3 In this case report, we describe a child admitted with a CF exacerbation with no risk factors for invasive aspergillosis who failed to improve with standard antibiotic therapy. Chest radiographs and CT scan findings raised concern of a possible fungal infection, and suspicions were confirmed when he was found to be β-D glucan (BG) and GM positive. He was then started on voriconazole for Aspergillus coverage.
Case presentation
We present the case of a 12-year-old boy with CF δ 508 mutation with pancreatic insufficiency associated with lung and sinus diseases. He presented at 3 months of age with persistent vomiting, diarrhoea and respiratory distress. A sweat chloride test was positive and CF DNA analysis confirmed his diagnosis. His regular medications included inhaled tobramycin alternating with aztreonam, dornase alfa, acetylcysteine, hypertonic saline, azithromycin, digestive enzymes, sertraline and vitamin D and K supplements. He had been chronically infected with methicillin-sensitive Staphylococcus aureus (MSSA) and Pseudomonas. His most recent admission was 6 months ago for a CF exacerbation.
He presented with a 2-week history of worsening cough, intermittent low-grade fever and sputum production managed with oral levofloxacin as an outpatient with no response. The patient was admitted based on worsening symptoms and decreased pulmonary function from his baseline. He was initiated on intravenous antibiotic therapy (tobramycin, meropenem and nafcillin) due to his history of positive cultures for MSSA and Pseudomonas. A chest radiograph at admission revealed left lower lobe alveolar space disease. Respiratory cultures, acid-fast bacilli and fungal cultures were obtained at admission.
On day 4, sputum culture was reported with MSSA and yeast which raised concern of possible allergic bronchopulmonary aspergillosis (ABPA). Oral itraconazole was started. The patient continued with low grade fevers and infectious diseases recommended serologies for Chlamydiae, Mycoplasma, Aspergillus and total IgE level. On day 12, total IgE was mildly elevated decreasing concerns for ABPA, and results for Aspergillus fumigatus IgG returned mildly elevated (77, normal range <46). Chlamydiae and Mycoplasma serologies were negative.
After 2 weeks, the patient did not show significant clinical improvement; a chest radiograph demonstrated a new right lower lobe patchy opacity; blood and sputum cultures were sent. Ciprofloxacin was added for extended Pseudomonas coverage. The lack of response and new radiographic findings prompted an evaluation by chest CT at 2 weeks after admission. The CT demonstrated ‘tree in bud’ opacities and scattered alveolar densities bilaterally, left lower lobe collapse, extensive tubular bronchiectasis, ground glass opacities and mediastinal and hilar lymphadenopathy (figure 1). These findings raised concern for Mycobacterium avium complex (MAC) and various fungal infections. Infectious diseases recommended gastric aspirates and sputum for MAC as well as BG and GM enzyme assays for aspergillosis.
Figure 1.
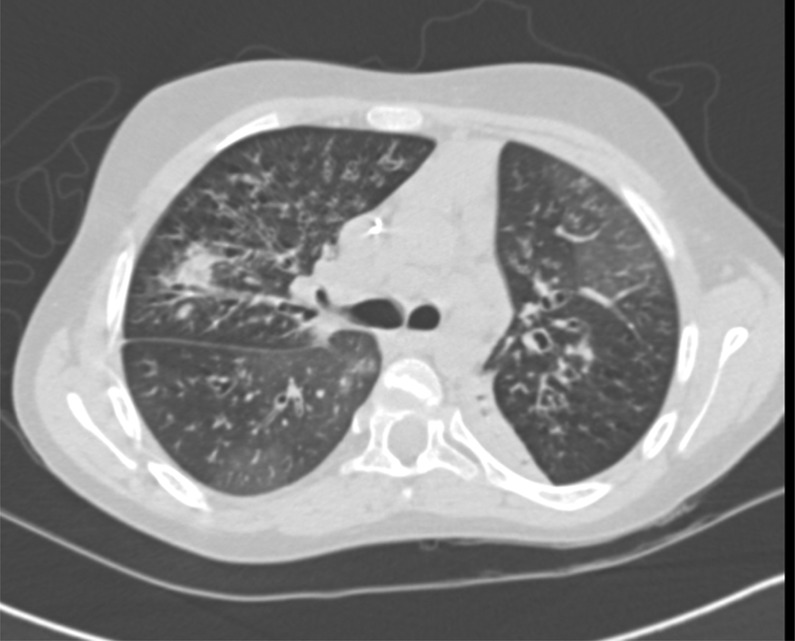
Chest CT scan: ‘tree in bud’ opacities, extensive bronchiectasis and ground glass opacities in a 12-year-old patient with cystic fibrosis with invasive pulmonary aspergillosis.
The patient continued with low-grade fevers and reported with a ‘tight feeling’ in his chest. Blood cultures remained with no growth. On day 23, gastric aspirates were reported negative. On day 25 of hospitalisation, serum BG (147 pg/mL) and GM (0.58) returned positive increasing suspicion of invasive aspergillosis. The patient was started on intravenous voriconazole (8 mg/kg/day) and itraconazole was discontinued.
As the patient continued to have poor clinical progression, it was decided to perform a bronchoscopy on day 33 of hospitalisation. The bronchoalveolar lavage (BAL) sample was sent for Gram staining, cytopathology, the GM assay and aerobic, fungal and mycobacterial cultures. One day after the procedure, the patient reported with chest pain and developed increased work of breathing and desaturations (80 s) requiring O2 via facemask. Gram stain for BAL demonstrated many white blood cells, moderate budding yeast and pseudohyphae. A chest radiograph on day 34 revealed increased alveolar space disease in the right middle and right lower lobes (figure 2).
Figure 2.
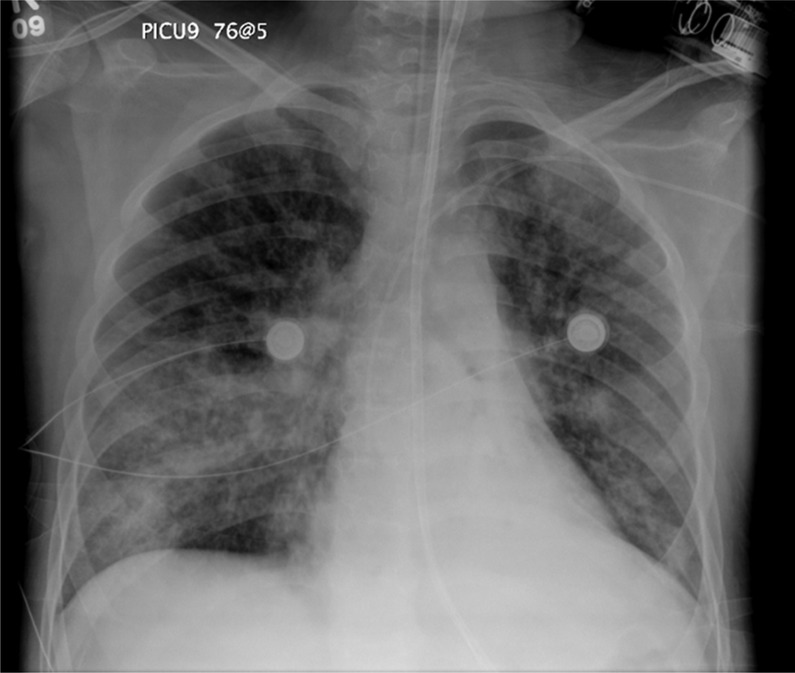
Chest radiograph: alveolar space disease in the right middle and right lower lobes.
The patient continued to deteriorate and developed respiratory failure. He was transferred to the paediatric intensive care unit (ICU) where he was intubated on day 35 of hospitalisation and continued to have low-grade fevers. On day 37, BAL Aspergillus Ag GM from right lower lobe returned positive (0.669) and negative from the left lower lobe (0.194) which confirmed the diagnosis of IPA. The voriconazole dose was increased to 14 mg/kg/day with close monitoring of kidney and liver function as well as the serum drug level. The patient was extubated to bilevel positive airway pressure (BiPAP) on day 56 and his voriconazole dose was increased to 30 mg/kg/day to maintain optimal levels.
During the remainder of his hospital stay, the patient experienced episodes of respiratory exacerbation accompanied by low-grade fevers. Sputum cultures returned positive for MSSA and methicillin-resistant S aureus, and antibiotic therapy was given according to sensitivities. The patient gradually began to improve. GM remained undetectable since day 39, and BG was slowly trending down (137 pg/mL, day 115). He continued to improve clinically and was discharged on day 122 of hospitalisation. Intravenous voriconazole was continued at home to complete a total of 6 months, and he required O2 by nasal cannula during the day and BiPAP at night. He was followed regularly by pulmonology and infectious diseases with the plan to transfer him to a lung transplant centre given his forced expiratory volume in 1 s persisted in 30%.
Outcome and follow-up
Three months after discharge, the patient was admitted to a different hospital for a CF exacerbation. During this hospitalisation, he developed respiratory failure and died.
Discussion
A fumigatus is a saprophytic mould found throughout the environment in potting soil, crawl spaces and decaying vegetation.4 5 Airborne spores can be inhaled into the lower respiratory airways and cause six types of lung diseases based on the host's immune status. These diseases include a hypersensitivity pneumonitis, ABPA, Aspergillus-sensitive asthma, aspergilloma, chronic necrotising aspergillosis and IPA.4 The latter is often life threatening and occurs in immunosuppressed patients with an overall mortality rate of 50–80%.6 Although A fumigatus is isolated from the respiratory secretions of 9–57% of patients with CF, only up to 15% will actually mount an allergic response to the filamentous mould.7 Disseminated aspergillosis in the paediatric CF population with the absence of immunosuppression secondary to lung transplantation rarely occurs, and the outcome is almost always fatal.8 We present the case of a 12-year-old boy with CF who developed IPA and recovered after receiving early empiric therapy with voriconazole.
To the best of our knowledge, there are four known cases in the literature which describe the development of IPA in the CF paediatric and young adult population. All presented with at least one predisposing factor for developing IPA. One patient received intravenous methylprednisolone during his hospitalisation for a presumed CF exacerbation. Two patients were previously colonised with A fumigatus and showed a history of inhaled/oral corticosteroid use. The fourth patient had a history of viral-induced lymphocytopenia.6 8–10 In all these cases, the outcome was fatal. Our patient's case is different in that not only did he not have any identifiable factors leading to the development of IPA, but also he survived for 6 months after diagnosis. We assumed that this was due to early empiric antifungal therapy and diagnosis.
Several studies on the use of biomarkers, BG and GM, along with BAL cultures have been shown to accurately diagnose IPA. GM is a polysaccharide with a mannose backbone and galactose side groups that compose a majority of the cell wall of many Aspergillus species. During an infection with IPA, GM is released allowing for antigen detection in the serum.11 Although biomarker studies have dealt primarily with the detection of IPA in the adult immunocompromised, neutropenic population, serum GM antigen testing showed a specificity of 95% in the paediatric haematology population.12 Another study investigating the diagnostic utility of BAL fluid GM in the paediatric immunocompromised population showed sensitivity, specificity, positive and negative predictive values of 78%, 100%, 58% and 96%, respectively.11 After a diagnosis of IPA has been made, GM is useful to track the progression of the disease.13 Serial assessment of GM antigenemia until discharge is suggested, but additional data in various patient settings are necessary to refine the optimal testing window, that is, in non-neutropenic patients and those receiving prophylactic antifungal therapy.11 13
BG is a group of glucose polysaccharides found in the cell wall of pathogenic fungi like Aspergillus, Candida and Fusarium as well as in the bacteria Pneumocystis jirovecii. Adult data on BG testing have consistently shown high specificity and sensitivity when using the recommended cut-offs for a positive value of >80 pg/mL, an indeterminate range of 60–79 pg/mL and a negative result of <60 pg/mL.11 Currently there are no recommendations on the use of BG testing in the paediatric population at risk for IPA as there are very limited data on the subject. Preliminary studies of children without risk for IPA suggested that they can have dramatically elevated levels of BG; however, the combined use of BG and GM assays can be used as adjuncts to clinical and radiographic results in order to guide clinicians in the diagnosis of IPA and subsequent treatment with antifungal medications.11
In this case report, the patient's sputum culture grew yeast on day 4 of his hospital stay prompting suspicions of ABPA which resulted in the rapid initiation of oral itraconazole. Although orally administered itraconazole is considered the first line for ABPA, it can also be effective for salvage therapy of IPA.13 When further testing revealed positive GM and BG antigenemia, the patient was switched to intravenous voriconazole 8 mg/kg/day and continued beyond discharge. This occurred early on day 25 and before the patient deteriorated. We believe that it was the high index of suspicion for an invasive fungal infection and subsequent treatment that led to our patient's recovery. Voriconazole and amphotericin B are the only two licensed medications for the use of IPA.13 However, because of better survival and improved responses of initial therapy with voriconazole, primary therapy with amphotericin B is not recommended.13 In addition, the management of IPA has become increasingly difficult due to the development of acquired azole resistance; however, the Infectious Diseases Society of America continues to recommend voriconazole as the first-choice therapy for invasive infections caused by invasive aspergillosis.13 14
A fumigatus is the most common mould reported from the sputa of patients with CF.15 For years, the patient had positive sputum cultures for A fumigatus without any clinical manifestations. Although up to 60% of patients with CF can be colonised with the mould, it is unusual for Aspergillus to become invasive in CF without any predisposing risk factors.8 16 Risk factors for invasive disease include prolonged steroid use (intravenous or inhaled), systemic treatment with immunosuppressants, malnutrition, ICU stay >21 days, neutropenia and haematological malignancy.17 Our patient had a relatively good pulmonary function with no history of CF-related diabetes or neutropenia. He was well nourished and had no history of prolonged steroid use, either inhaled or intravenous. The use of steroids during hospitalisation has been cited as a possible source of invasive infection; however, the threshold dose or duration has not been determined to identify those at risk.8
Investigations linking Pseudomonas and Aspergillus spp. colonisation and infection have yielded conflicting results; however, there is evidence that sensitisation to A fumigatus in patients with chronic pseudomonal colonisation can occur.15 This is due to protease production of the Pseudomonas aeruginosa bacterium which works to directly damage respiratory epithelium and change the airway milieu.15 In addition, the aggressive approach to the treatment via nebulised antipseudomonal antibiotics, oral ciprofloxacin and oral macrolides in the population with CF is another possible reason for the increasing prevalence of Aspergillus lung disease.15 18 Owing to his complicated medical history, he was receiving chronic pseudomonal treatment. At this point of time, it is unclear whether other risk factors, which are not well described, such as prolonged use of intravenous antibiotics or chronic use of macrolides could be playing a role in the development of IPA.
Learning points.
Physicians should include invasive pulmonary aspergillosis (IPA) on their differential when presented with a patient with cystic fibrosis who has failed a trial of antibiotics.
A patient without any obvious predisposing factors may develop IPA.
There may be other risk factors for IPA like chronic use of antibiotics.
Early empiric therapy with voriconazole may improve outcomes in patients with IPA.
Acknowledgments
The authors would like to thank Cheryl Samuels, Paediatrics Nurse Practitioner who helped with editing the draft.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev 2009;2013:447–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens DA, Moss RB, Kurup VP, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis-state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis 2003;2013(Suppl 3):S225–64 [DOI] [PubMed] [Google Scholar]
- 3.Warren TA, Yau Y, Ratjen F, et al. Serum galactomannan in cystic fibrosis patients colonized with Aspergillus species. Med Mycol 2012;2013:658–60 [DOI] [PubMed] [Google Scholar]
- 4.Bains SN, Judson MA. Allergic bronchopulmonary aspergillosis. Clin Chest Med 2012;2013:265–81 [DOI] [PubMed] [Google Scholar]
- 5.Knutsen AP, Slavin RG. Allergic bronchopulmonary aspergillosis in asthma and cystic fibrosis. Clin Dev Immunol 2011;2013:843763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown K, Rosenthal M, Bush A. Fatal invasive aspergillosis in an adolescent with cystic fibrosis. Pediatr Pulmonol 1999;2013:130–3 [DOI] [PubMed] [Google Scholar]
- 7.Chadhary N, Datta K, Askin FB, et al. Cystic fibrosis transmembrane conductance regulator regulates epithelial cell response to Aspergillus and resultant pulmonary inflammation. Am J Respir Crit Care Med 2012;2013:301–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massam J, Bitnun A, Solomon M, et al. Invasive aspergillosis in cystic fibrosis: a fatal case in an adolescent and review of the literature. Pediatr Infect Dis J 2011;2013:178–80 [DOI] [PubMed] [Google Scholar]
- 9.Guidotti TL, Luetzeler J, di Sant’ Agnese PA, et al. Fatal disseminated aspergillosis in a previously well young adult with cystic fibrosis. Am J Med Sci 1982;2013:157–60 [DOI] [PubMed] [Google Scholar]
- 10.Chung Y, Kraut JR, Stone AM, et al. Disseminated aspergillosis in a patient with cystic fibrosis and allergic bronchopulmonary aspergillosis. Pediatr Pulmonol 1994;2013:131–4 [DOI] [PubMed] [Google Scholar]
- 11.Fisher BT. The role of biomarkers for diagnosis of and therapeutic decisions related to invasive aspergillosis in children. Curr Fungal Infect Rep 2013;2013:7–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher BT, Zaoutis TE, Park JR, et al. Galactomannan antigen testing for diagnosis of invasive aspergillosis in pediatric hematology patients. J Pediatr Infect Dis Soc 2012;2013:103–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2008;2013:327–60 [DOI] [PubMed] [Google Scholar]
- 14.Seyedmousavi S, Melchers WJ, Mouton JW, et al. Pharmacodynamics and dose-response relationships of liposomal amphotericin B against different azole-resistant Aspergillus fumigatus isolates in a murine model of disseminated aspergillosis. Antimicrob Agents Chemother 2013;2013:1866–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu JC, Modha DE, Gaillard EA. What is the clinical significance of filamentous fungi positive sputum cultures in patients with cystic fibrosis? J Cyst Fibros 2013;2013:187–93 [DOI] [PubMed] [Google Scholar]
- 16.Chow L, Brown NE, Kunimoto D. An unusual case of pulmonary invasive aspergillosis and aspergilloma cured with voriconazole in a patient with cystic fibrosis. Clin Infect Dis 2002;2013:e106–10 [DOI] [PubMed] [Google Scholar]
- 17.Garnacho-Montero J, Olaechea P, Alvarez-Lerma F, et al. Epidemiology, diagnosis and treatment of fungal respiratory infections in the critically ill patient. Rev Esp Quimioter 2013;2013:173–88 [PubMed] [Google Scholar]
- 18.Jubin V, Ranque S, Stremler N, et al. Risk factors for Aspergillus colonization and allergic bronchopulmonary aspergillosis in children with cystic fibrosis. Pediatr Pulmonol 2010;2013:764–71 [DOI] [PubMed] [Google Scholar]


