Abstract
Eph receptors, the largest family of surface-bound receptor tyrosine kinases and their ligands, the ephrins, mediate a wide variety of cellular interactions in most organ systems throughout both development and maturity. In the forming cerebral cortex, Eph family members are broadly and dynamically expressed in particular sets of cortical cells at discrete times. Here, we review the known functions of Eph-mediated intercellular signaling in the generation of progenitors, the migration of maturing cells, the differentiation of neurons, the formation of functional connections, and the choice between life and death during corticogenesis. In synthesizing these results, we posit a signaling paradigm in which cortical cells maintain a life history of Eph-mediated intercellular interactions that guides subsequent cellular decision-making.
Keywords: cerebral cortex, development, differentiation, Eph/ephrin, parcellation, proliferation
Eph Receptors and Ephrin Ligands
In the 25 years since their discovery in an erythropoietin-producing hepatocellular carcinoma cell line (Hirai et al. 1987), a wide variety of roles for signaling via the transmembrane Eph receptors have been identified and studied, particularly in the nervous system (Klein 2004). In development, adulthood and disease, this dynamic group of cell surface-bound tyrosine kinases—the largest known family of such molecules—mediates communication between neighboring cells, thus influencing intrinsic cellular processes such as proliferation, adhesion, differentiation, and migration (Pasquale 2008). This medley of cellular consequences is facilitated by a network of downstream intracellular signaling cascades, which are activated by the binding of an Eph receptor expressed by one cell to an ephrin ligand expressed by an adjacent cell (Holland et al. 1996). Originally designated as part of an A or B subtype with binding largely restricted within each group (Gale et al. 1996), promiscuity in binding between receptors and ligand subtypes is now well accepted (Kullander et al. 2003; Himanen et al. 2004; North et al. 2009).
Eph receptors are surface-bound tyrosine kinases. Ephrin ligands are also cell membrane tethered and capable of transmitting signals but are not themselves enzymatically active (Eph-Nomenclature-Committee 1997). While a few examples of soluble or substrate-based signaling exist (Nicola et al. 1996; Pascall and Brown 2004; Alford et al. 2007; Oricchio et al. 2011), a cell expressing an Eph receptor must generally come into contact with a cell expressing a ligand to trigger cell responses. These responses can be uni- or bidirectional; signaling cascades are initiated in the receptor cell, termed “forward” signaling, or in the ligand cell, called “reverse” signaling, or signaling can result in both receptor- and ligand-expressing cells. Binding of an ephrin on one cell to an Eph receptor on a neighboring cell is generally followed by autophosphorylation of the Eph receptor via its own kinase domain and then phosphorylation of other substrates (Davis et al. 1994; Holland et al. 1996). Upon engagement, ephrins also initiate cellular signaling via partner proteins (Davy et al. 1999; Jiao et al. 2008; Lim, Matsuda, et al. 2008; Lim, McLaughlin, et al. 2008). The identity and roles of downstream signaling pathways in both forward and reverse signaling are vigorously studied (Pasquale 2005; Pitulescu and Adams 2010).
In addition to their catalytic kinase domain, Eph receptors also contain a sterile alpha motif domain, which facilitates interactions with Rho and Rac guanosine triphosphate hydrolases, and a C-terminus PDZ-binding domain, which mediates Pick1, Grip, and spine-associated RapGAP interactions (Hock et al. 1998; Torres et al. 1998; Buchert et al. 1999; Richter et al. 2007). Many of these interactions ultimately exert an effect on the actin cytoskeleton, leading to changes in cell morphology or adhesion, while others influence proliferation through cyclin D1 (Genander et al. 2009) or even apoptosis (Munarini et al. 2002). A growing number of additional molecules are known to interact with Eph signaling including the atypical receptor tyrosine kinase (RYK), the chemokine receptor (CXCR4), fibroblast growth factor receptors, extracellular matrix-interacting integrins, immunoglobulin proteins, intercellular connecting molecules such as cadherins, claudins, and connexins, and neurotransmitter receptors such as the n-methy d-aspartate receptor (NMDAR) (Halford et al. 2000; Kasemeier-Kulesa et al. 2006; Arvanitis and Davy 2008; Fukai et al. 2008; Klein 2009; Akaneya et al. 2010).
Cortical Development
The mammalian neocortex, the most recent evolutionary addition to the brain, is elegantly designed with 6 layers of distinct and morphologically diverse neurons spread across functionally dedicated areas. The intricate organization of the cortex is reflected in the complex functions it subserves, such as language, sensory perception and integration, attention, memory, and even personality (Brodmann 1909; Rakic 1974; Sidman and Rakic 1982). Such complexity is achieved by the activity of many molecules, and the Eph/ephrins are invaluable in these processes. Indeed, a fundamental principle in the development, organization, and operation of the cerebral cortex is the precise compartmentalization of function, a tenet that is mirrored in the expression profiles of Eph family members.
Development of the cortex begins as the neural tube folds inward. The thin epithelium of the dorsal telencephalon will ultimately become the cerebral neocortex. Rapid proliferation of neuroepithelial (NE) cells begins halfway through the gestational period (Sidman and Rakic 1982) and is well underway by embryonic day (E) 10 in the mouse (Takahashi et al. 1993, 1994, 1995; Caviness et al. 2003). NE cells divide rapidly to produce more NE cells (Gotz and Huttner 2005; Miller and Gauthier 2007; Kriegstein and Alvarez-Buylla 2009). By E11, some of these NE cells begin to display astroglial characteristics and become specialized neural progenitor cells called radial glial cells (RGCs; Alvarez-Buylla et al. 2001; Gotz and Barde 2005; Mori et al. 2005). The appearance of RGCs and the generation of the first postmitotic neuron mark the beginning of neurogenesis.
Symmetric divisions that produce mitotically competent radial glia exist initially. As time progresses, asymmetrical divisions that give rise to progeny with distinct post-division choices increase in frequency (Calegari et al. 2005). Following mitosis, postmitotic daughter cells leave the ventricular zone (VZ), migrating superficially through the intermediate zone (IZ) before settling in the cortical plate (CP). A subset of cells leaving the ventricular surface, however, are not truly postmitotic; these intermediate progenitors linger in the subventricular zone (SVZ), located between the VZ and IZ, where they undergo 1 or 2 final neurogenic divisions (Privat 1975; Sturrock and Smart 1980; Bayer et al. 1991; Tarabykin et al. 2001; Miyata et al. 2004; Noctor et al. 2004; Wu et al. 2005; Attardo et al. 2008; Javaherian and Kriegstein 2009). The proliferation of these additional progenitor cells is thought to provide an extra opportunity for the mammalian cortex to expand in size (Hevner 2006; Martinez-Cerdeno et al. 2006) and is thought to have evolutionary significance regarding the appearance of Homo sapiens (Lukaszewicz et al. 2005, 2006; Kriegstein et al. 2006; Rakic 2009). The expression of EphA3 and EphA5 in the primate SVZ hints at a role in these cells (Donoghue and Rakic 1999a, 1999b).
Maturing neurons migrate away from germinal zones, initiating cellular programs that result in morphological complexity, particularly the extension of cellular protrusions that will eventually form the axon and dendrites of the mature neuron (McConnell 1995; Banker 2003). Proper morphological differentiation is a prerequisite for proper neuronal connectivity. Fundamental to this process is the induction of a host of cytoskeletal components that scaffold the complex cellular architecture and signaling machinery that mediate neuronal responsiveness and plasticity (Hotulainen and Hoogenraad 2010). Forces that guide neuronal elaboration include signaling via soluble morphogens as well as cell–cell interactions via cellular adhesion molecules and surface-bound RYKs (Dalva et al. 2007; Yokota et al. 2010). Interestingly, molecules historically implicated in axon guidance are now also considered essential for the establishment of neuronal shape (Polleux et al. 2000; Fenstermaker et al. 2004).
A variety of Eph/ephrins are expressed in the developing cortex as it transitions into its functional adult form. Intriguingly, expression is dynamic; compartmentalized patterns of various family members change across time and space (Mackarehtschian et al. 1999; Donoghue and Rakic 1999a, 1999b; Yun et al. 2003). Indeed, shifting expression patterns of Eph receptors and ligands parallel the transition of the developing cortical cells from a predominantly germinal phase, through migration, neuronal differentiation and incorporation into neuronal circuits, and finally, to natural and disease-based apoptosis (Table 1). This review will detail Eph/ephrin signaling in the development of the cortex and consider the ways in which signaling via this family of molecules can continually influence the formation and function of this structure.
Table 1.
| Molecule | Function | References |
|---|---|---|
| EphA receptors | ||
| EphA4 | Progenitor cell division | North et al. (2009) |
| Cortical network formation | Clifford et al. (2011) | |
| Thalamocortical afferent sorting | Dufour et al. (2003) | |
| Uziel et al. (2002) | ||
| Homeostatic plasticity | Fu et al. (2011) | |
| EphA5 | Organization of the corpus callosum | Hu et al. (2003) |
| EphA7 | Corticothalamic efferent sorting | Torii and Levitt (2005) |
| Thalamocortical afferent sorting | Miller et al. (2006) | |
| Cortical cell death | Depaepe et al. (2005) | |
| EphA8 | Organization of the corpus callosum | Park et al. (1997) |
| EphB receptors | ||
| EphB | Synaptogenesis | Margolis et al. (2010) |
| EphB1 | Synaptogenesis | Kayser et al. (2008) |
| EphB2 | Inhibition of differentiation | Qiu et al. (2008) |
| Synaptogenesis | Kayser et al. (2008) | |
| Nolt et al. (2011) | ||
| Dendritic spine formation | Dalva et al. (2007) | |
| Dalva et al. (2000) | ||
| Torres et al. (1998) | ||
| EphB3 | Synaptogenesis | Kayser et al. (2008) |
| Ephrin A ligands | ||
| Ephrin-A3 | Migration of interneurons | Rudolph et al. (2010) |
| Ephrin-A5 | Thalamocortical afferent sorting | Mackarehtschian et al. (1999) |
| Miller et al. (2006) | ||
| Prakash et al. (2000) | ||
| Vanderhaeghen et al. (2000) | ||
| Organization of the corpus callosum | Hu et al. (2003) | |
| Cortical compartmentalization | Yun et al. (2003) | |
| Cortical neuron mobility and aggregation | Zimmer et al. (2007) | |
| Dendritic spine formation | Guellmar et al. (2009) | |
| Ephrin B ligands | ||
| Ephrin-B1 | Progenitor cell division | North et al. (2009) |
| Cellular adhesion | Davy et al. (1999) | |
Eph Signaling in Cell Division
Outside the nervous system, Eph/ephrin engagement modulates that cell proliferation and dysfunction of Eph signals can result in uncontrolled cell division (Pasquale 2008). A role for Eph-mediated signaling in the regulation of cell division also exists in the forming cerebral cortex. At the height of neurogenesis in the embryonic cortex, EphA4 and ephrin-B1 are selectively expressed in the initial proliferative compartment, the VZ, and their engagement influences cell division. Direct EphA4/ephrin-B1 binding in the cortex results in the stimulation of cell division within proliferative compartments (North et al. 2009; Fig. 1A). Interestingly, only receptor-containing cells were found to divide following receptor/ligand engagement, implicating forward signaling in the promotion of cell division. Investigations of potential EphA4 cosignaling molecules include the fibroblast growth factor receptor (Yokote et al. 2005; Fukai et al. 2008), an influential factor in guiding cell division in cortical development (Vaccarino et al. 1999).
Figure 1.
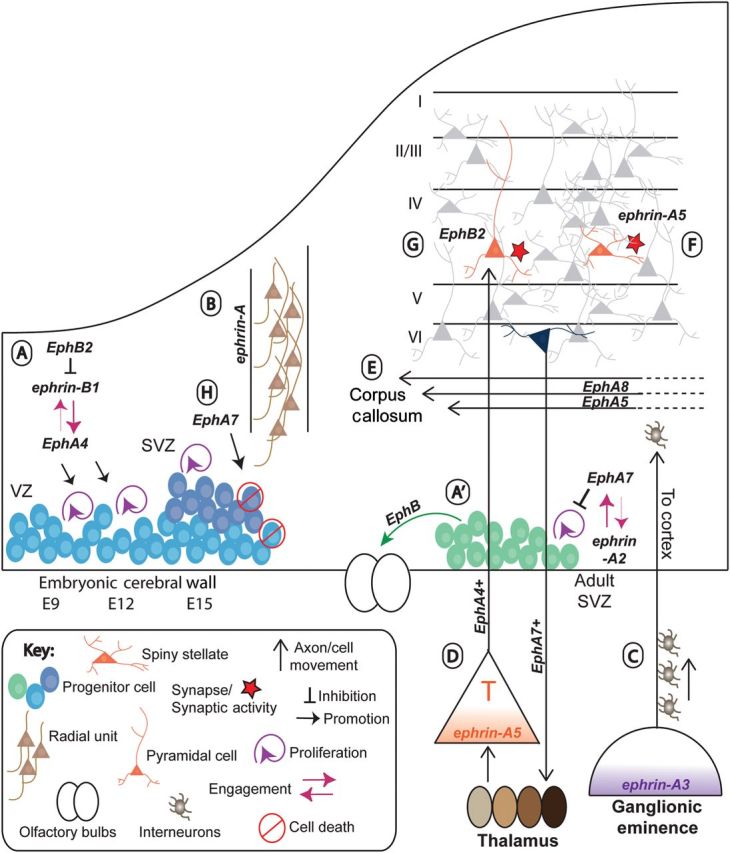
Eph receptors and ephrin ligands influence diverse cellular processes during the development of the cerebral cortex. (A). EphA4 and ephrin-B1 engage to promote the proliferation of cortical progenitor cells during expansion of the VZ (E9–E15; North et al. 2009). At the same time, EphB's engage ephrin-B1 to suppress differentiation (Qiu et al. 2008; Arvanitis et al. 2010). (A′) Ephrin-A2 and EphA7 engage to promote the proliferation of progenitor cells in the adult SVZ (Holmberg et al. 2005), which then migrate rostrally under the influence of EphB's to populate the olfactory bulbs (OB; Conover et al. 2000). (B) Ephrin-A signaling directs migration of differentiating cortical neurons within radial units (Torii et al. 2009). (C) Ephrin-A3, expressed in the ganglionic eminence, repels inhibitory neurons as they migrate (Rudolph et al. 2010), and routing them on a dorsal migratory path toward the cerebral cortex. (D) Ephrin-A5, expressed in a gradient in the subplate, repels EphA4-expressing thalamic axons, and directing them toward the cerebral cortex (Mackarehtschian et al. 1999; Uziel et al. 2002). Corticothalamic efferents, expressing EphA7, are directed toward specific nuclei within the thalamus (Torii and Levitt 2005). (E) EphA8 and EphA5 spatially segregate the fibers of the corpus callosum (Park et al. 1997; Hu et al. 2003). (F) Ephrin-A5, which is expressed in response to synaptic activity, induces spine formation (Guellmar et al. 2009). (G) EphB2 signaling influences spine formation in the CP (Nolt et al. 2011). (H) EphA7 overexpression promotes cell death within the developing cerebral wall (Depaepe et al. 2005).
At the same time that EphA4 and ephrin-B1 are coexpressed in the VZ-promoting cell division, another receptor, EphB2, is present in the more superficial differentiated compartment, the CP (Fig. 1A). Results from both gain- and loss-of-function experiments support a model in which activated ephrin-B1 acts to prevent the differentiation of progenitor cells, keeping them in the proliferative niche (Qiu et al. 2008). Thus, Eph signaling appears to play 2 discrete roles during cortical proliferation: Promoting the division and proliferation of progenitor cells while also, separately preventing differentiation of post-mitotic cells.
Roles for Eph signaling have also been described in postnatal proliferative neural compartments, the adult SVZ of the olfactory system (Conover et al. 2000; Holmberg et al. 2005; Fig. 1A′), and the hippocampal subgranular zone (Khodosevich et al. 2011). Overall, particular Eph receptors are expressed by progenitor cells of the postnatal cortex, and changes in Eph function lead to shifts in proliferation and differentiation of those progenitor cells. Additional data support a broad role for Eph signaling in the suppression of cell division in these neurogenic proliferative niches (Jiao et al. 2008).
Eph Signaling in Migration
As cortical cells adjacent to the lateral ventricle cease cell division, a program of neuronal differentiation is initiated; newly post-mitotic neurons migrate superficially to seed differentiated zones, eventually producing all of the excitatory neurons in the mature cortex (Sidman and Rakic 1973; Rakic 1985, 1990). In coordination with this radial movement, inhibitory neurons derived from the basal ganglia tangentially migrate into the cerebral cortex, thus supplying interneurons to differentiated cortical domains (Anderson et al. 1997, 1999; Butt et al. 2005). Eph signaling also impacts cell movement in non-neocortical regions; in the hippocampal archeocortex, both radial migration of excitatory neurons and tangential migration of inhibitory neuron are impacted (Catchpole and Henkemeyer 2011).
In the cerebral cortex, the proliferative and subsequent migratory states are actively maintained by Eph/ephrin signaling (Arvanitis and Davy 2008; Qiu et al. 2008). VZ cells initiate differentiation, and the expression of pro-proliferative ephrin-B1 is actively suppressed via a microRNA mechanism (Arvanitis et al. 2010). As these neurons move radially toward superficial cortical zones, they tend to remain in register with the point of their origin (Walsh and Cepko 1988, 1992, 1993; Kornack and Rakic 1995). Eph signaling appears to regulate this proximity while allowing some lateral dispersion; elimination of 3 ephrins resulted in less diffuse radial units (Torii et al. 2009), supporting the idea that Eph engagement maintains the spatial register of radially migrating neurons (Fig. 1B). In addition, ephrin-Bs participate in the well established Reelin pathway to limit radial movement during corticogenesis; it was recently demonstrated that ephrin-B binds the secreted glycoprotein Reelin, thereby anchoring it to the cell surface and facilitating its induction of its intracellular signaling pathway beginning with Dab-1 (Senturk et al. 2011).
Interneuron migration is also affected by Eph signaling: Ventrally expressed ephrin-A3 acts as a repulsive cue for migrating interneurons, corralling cells to move dorsally (Rudolph et al. 2010; Fig. 1C).
Eph Signaling in Mature Cortical Neurons
Eph Signaling in Axon Guidance
Eph signaling was first identified in the nervous system when a role in axon guidance in the retinotectal system was defined (Cheng et al. 1995; Drescher et al. 1995). Indeed, the molecules are canonically recognized in the central nervous system as indicators of topographical information and were first identified as being positional cues (Drescher et al. 1995; Frisen et al. 1998; O'Leary and Wilkinson 1999; Knoll and Drescher 2002). Consistent with this original role, subsequent studies demonstrated that Eph engagement influences afferent, efferent, and intracortical axon pathfinding in the forming cortex. Thalamocortical (TC) axons traverse subcortical regions during embryogenesis, arriving in the thalamorecipient layer, layer IV, just as recipient cells complete their maturation (Miller et al. 1993). A gradient of ephrin-A5 expression in the posterior subplate was hypothesized to be important for early sorting of TC axons prior to the formation of layer IV (Mackarehtschian et al. 1999) and opposing gradients of Eph receptor and ephrin ligands in regions of the cortex and their corresponding thalamic nuclei predict patterns of innervation (Mackarehtschian et al. 1999; Vanderhaeghen et al. 2000; Dufour et al. 2003; Yun et al. 2003; Torii and Levitt 2005; Fig. 1D). Consistent with in vitro results demonstrating the decreased axonal outgrowth of limbic axons on ephrin-A5 substrates (Gao et al. 1998), in vivo analysis revealed that EphA4-positive axons emerging from the thalamus were repulsed from ephrin-A5 domains since they were misrouted to the somatosensory cortex in ephrin-A5−/− and ephrin-A5−/−; EphA4−/− animals (Uziel et al. 2002; Dufour et al. 2003; Fig. 1D). Finally, the ephrin family also affects efferent pathfinding of corticothalamic axons: EphA7-mediated signaling influences the topography of projections to thalamic nuclei (Torii and Levitt 2005; Fig. 1D).
Studies have also examined the role of EphA/ephrinA signaling on cortico-cortical circuitry (Fig. 1E). An early constitutive deletion of EphA8 eliminated the corpus callosum (Park et al. 1997), while another study involving gene replacement introduced a kinase-dead mutant of EphA5 and generated defective callosal projections from deep layers, but not from upper layers where EphA5 expression is lower. Interestingly, the authors posit that ephrin-A5 is attractive to some neurons and repulsive to others (Hu et al. 2003).
Additional studies implicate Eph signaling in establishing connectivity within a particular cortical area. For example, axons from the ventroposterior nucleus of the thalamus of ephrin-A5−/− and/or EphA7−/− mice still innervate somatosensory cortex but either the representations of individual whiskers or the size of the entire barrel field were altered, respectively (Prakash et al. 2000; Vanderhaeghen et al. 2000; Miller et al. 2006).
Cortical Compartmentalization
The hundreds of distinct functional areas that exist in the mature cerebral cortex are established during development (Rakic 1988). Cortical areas arise via a combination of intrinsic specification (Rakic 1988; Miyashita-Lin et al. 1999; Nakagawa et al. 1999; Bishop et al. 2000; Hamasaki et al. 2004) and environmental cues (O'Leary 1989; Roe et al. 1990; Schlaggar and O'Leary 1991; Sharma et al. 2000; von Melchner et al. 2000). Eph receptors and ephrin ligands are uniquely expressed as functional areas emerge and impact compartmentalization. Studies in monkey demonstrated that Eph signaling molecules are broadly and dynamically expressed in the developing cortex, marking emerging cortical areas (Donoghue and Rakic 1999a, 1999b). Indeed, Eph receptor expression delineates visual domains prior to significant afferent innervation and the emergence of clear cytoarchitectural distinctions (Sestan et al. 2001). In mice, most of EphA receptor patterning is maintained in the absence of TC connections (Miyashita-Lin et al. 1999; Yun et al. 2003), suggesting that some patterned Eph expression is independent of innervation. In contrast, ephrin-A5 expression is altered when afferent input is eliminated, suggesting that activity may play a role in the induction or maintenance of the ligand's expression (Yun et al. 2003; Fig. 1F). Thus, Eph signaling appears to be both independent of and responsive to cortical areal formation.
Neuronal Differentiation and Synapse Formation
Consistent with roles for Eph-mediated signaling in the morphological elaboration of retinal and hippocampal neurons (Grunwald et al. 2004; Marler et al. 2008), a similar role for Eph signaling in the cerebral cortex is emerging. Indeed, cortical neurons in vitro are more motile and aggregate more in the presence of ephrin-A5, an effect that requires signaling through Src family kinases (Zimmer et al. 2007; Fig. 1F). Moreover, spiny stellate neurons from layer IV have increased dendritic branching and filopodia, but fewer spines in ephrin-A5−/− (Guellmar et al. 2009). EphB-mediated signaling also plays a role: Cultured cortical neurons or cortical slices from EphB1−/−; EphB2−/−; EphB3−/− mice had increased filopodia with decreased motility and decreased synaptic density, respectively (Kayser et al. 2008). EphB2 appears to be the essential element since downregulation resulted in decreases in both spine number and extent of filopodia, while overexpression increased spine density (Kayser et al. 2008; Nolt et al. 2011; Fig. 1G).
Postnatal Cortical Synapses and Plasticity
Postnatal distribution and localization of Eph family members in the cerebral cortex is unclear. General cataloging of gene expression demonstrates considerably lower levels for most Eph family members postnatally than in development (www.brain-map.org). Still, one receptor, EphA4, is clearly present, localized to spines and axons of the adult rat (Martone et al. 1997), as well as to synaptosomes, post-synaptic densities, and clathrin coated and synaptic vesicles (Bouvier et al. 2008).
In parallel to a widely recognized role for Eph receptors and ephrin ligands in the modulation of synaptic characteristics in the hippocampus (Murai et al. 2003; Hruska and Dalva 2012), Eph signaling also impacts neuronal excitability in the cortex. For example, EphA4 overexpression in a subset of neurons in cortical cultures increased spontaneous bursting activity in cortical neurons in vitro (Clifford et al. 2011). In addition, Eph signaling affects shifts in neuronal responsivity, termed plasticity, within the neocortex. Indeed, the ability of neocortical neurons to change electrophysiological properties, due to the characteristics and/or the strength of synaptic partners or based upon the overall degree of cell excitability (Turrigiano 2008), is supported, at least in part, by Eph/ephrin signaling. In vitro analyses demonstrated that a reduction in GABA-mediated inhibition diminished the amplitude of mEPSC's via a decrease in AMPA receptors, but that this effect was lost when EphA4 was reduced. In keeping with a role for Eph signaling in neocortical plasticity, an experimentally induced increase in neuronal activity increased the phosphorylated, and thus active, form of EphA4 whereas EphA4-null neurons had decreased GluR1 expression (Fu et al. 2011). While these results are tantalizing and support a role for Eph signaling in neocortical synaptic function, in vivo experiments must be performed to fully understand Eph-mediated synaptic changes in the neocortex.
EphBs have also been a focus of synaptic studies because of their ability to bind NMDARs and other post-synaptic partners and modulate synaptic signaling in other brain regions (Torres et al. 1998; Dalva et al. 2000, 2007). In cultures of mature cortical neurons, EphB2 overexpression causes an increase in the co-localization of GluR1 and VGlut1 on spines, indicating an increase in mature dendritic spines. Indeed, these neurons had an increase in mEPSC amplitude largely due to an increased NMDA component. While brain slices from triple EphB receptor mutant mice (EphB1−/−; EphB2−/−; EphB3−/−) had an unexpected increase in surface NR2B expression, less NR2B was localized to synaptosomes (Nolt et al. 2011).
EphB activation can affect synapse density through its interaction with ephexin5. Without ligand present, EphB activates ephexin5, a guanine exchange factor for RhoA, which, when activated, decreases excitatory synapses. Ligand binding leads to the phosphorylation of ephexin5, which allows ubiquitination by Ube3A, targeting ephexin5 for degradation by the proteosome. This ultimately promotes the formation of excitatory synapses (Fig. 1G). Most of the functional effects of this interaction were demonstrated in the hippocampus, while the biochemistry and binding studies were performed on cortical neurons (Margolis et al. 2010).
Eph Signaling in Cell Death
During brain development, Eph signaling may also modulate cell survival. In a gain-of-function study, exogenous expression of EphA7 resulted in increased cell death in the forming cortex, whereas the complimentary loss-of-function elimination of EphA7 resulted in a subset of mutant embryonic brains becoming too large (Depaepe et al. 2005; Fig. 1H). A connection between Eph signaling in maturity is equally intriguing: Supplementation with EphB2 improved cognition in a mouse model of Alzheimer's disease and investigation of gamma-secretase targets led researchers to identify EphA4 as a neuronal target in neural degeneration (Inoue et al. 2009; Cisse et al. 2011). While it remains unclear how these results inform our understanding of cortical development and function, the possibility of a role for Eph signaling in determining survival is interesting.
Synthesis and Speculation
Eph signaling is a fundamental part of intercellular communication in the cerebral cortex from its formation through maturity, impacting proliferation, differentiation, migration, cellular elaboration, synaptic target selection, and synaptic function. Based upon the stereotyped, dynamic spatial and temporal expression of Eph family members during corticogenesis as well as the myriad roles of Eph signaling that have been shown to play a role in the life of a cortical neuron, we hypothesize that balanced and sequential signaling underlies the normal progression of cortical neurons from birth through death. An imbalance in Eph/ephrin interaction at a given stage of a cortical neuron's life may change its course: Different levels of Eph stimulation can produce “opposite” cellular consequences (Hansen et al. 2004) and relative amounts of signaling between cells, as opposed to absolute amounts, appears to impact cellular fates (Brown et al. 2000). It is also possible that signaling through related family members, acting discretely but via overlapping downstream mediators occurs in a well-coordinated manner as development proceeds. Given the importance of Eph signaling in most milestones in the life of a cortical neuron, the Eph signaling experienced by any given cortical cell at any point during development may impact that cell's future behavior. According to this hypothesis, cortical cells maintain a sense, either actively through potentiation of downstream signalers or passively by the genes expressed by a new cellular state, of previous interactions via specific Eph receptors and ligands. This potential “memory” may influence subsequent cellular responsiveness. It remains unclear whether this kind of cellular memory would be accomplished by accumulation of signaling events, or if the “slate” of signaling events is wiped clean with each new stage of existence for a given cell. We look forward to additional studies of Eph/ephrin function in cortical development and to understanding whether this family of molecules is so widely used during the formation of this complex structure simply because of its diversity and flexibility, or if there is a concerted “Ephfort” to direct cells from cradle to grave.
Funding
This work was supported by NSF IOS-0923642 to MJD and NIH F31NS062550 to HAN.
Notes
Conflict of Interest: None declared.
References
- Akaneya YK, Sohya A, Kitamura F, Kimura C, Washburn R, Zhou I, Ninan T, Tsumoto T, Ziff EB. Ephrin-A5 and EphA5 interaction induces synaptogenesis during early hippocampal development. PLoS One. 2010;5:e12486. doi: 10.1371/journal.pone.0012486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford SC, Bazowski J, Lorimer H, Elowe S, Howard PL. Tissue transglutaminase clusters soluble A-type ephrins into functionally active high molecular weight oligomers. Exp Cell Res. 2007;313:4170–4179. doi: 10.1016/j.yexcr.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Anderson S, Mione M, Yun K, Rubenstein JL. Differential origins of neocortical projection and local circuit neurons: role of Dlx genes in neocortical interneuronogenesis. Cereb Cortex. 1999;9:646–654. doi: 10.1093/cercor/9.6.646. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Arvanitis D, Davy A. Eph/ephrin signaling: networks. Genes Dev. 2008;22:416–429. doi: 10.1101/gad.1630408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitis DN, Jungas T, Behar A, Davy A. Ephrin-B1 reverse signaling controls a posttranscriptional feedback mechanism via miR-124. Mol Cell Biol. 2010;30:2508–2517. doi: 10.1128/MCB.01620-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardo A, Calegari F, Haubensak W, Wilsch-Brauninger M, Huttner WB. Live imaging at the onset of cortical neurogenesis reveals differential appearance of the neuronal phenotype in apical versus basal progenitor progeny. PLoS One. 2008;3:e2388. doi: 10.1371/journal.pone.0002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker G. Pars, PI 3-kinase, and the establishment of neuronal polarity. Cell. 2003;112:4–5. doi: 10.1016/s0092-8674(02)01280-1. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Dai XF, Humphreys L. Planar differences in nuclear area and orientation in the subventricular and intermediate zones of the rat embryonic neocortex. J Comp Neurol. 1991;307:487–498. doi: 10.1002/cne.903070311. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Goudreau G, O'Leary DDM. Regulation of area identity in the mammalian neocortex by emx-2 and pax-6. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- Bouvier D, Corera AT, Tremblay ME, Riad M, Chagnon M, Murai KK, Pasquale EB, Fon EA, Doucet G. Pre-synaptic and post-synaptic localization of EphA4 and EphB2 in adult mouse forebrain. J Neurochem. 2008;106:682–695. doi: 10.1111/j.1471-4159.2008.05416.x. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Localisationsationslehre der Grosshir-hinde. Leipzig: Barth; 1909. [Google Scholar]
- Brown A, Yates PA, Burrola P, Ortuno D, Vaidya A, Jessell TM, Pfaff SL, O'Leary DD, Lemke G. Topographic mapping from the retina to the midbrain is controlled by relative but not absolute levels of EphA receptor signaling. Cell. 2000;102:77–88. doi: 10.1016/s0092-8674(00)00012-x. [DOI] [PubMed] [Google Scholar]
- Buchert M, Schneider S, Meskenaite V, Adams MT, Canaani E, Baechi T, Moelling K, Hovens CM. The junction-associated protein AF-6 interacts and clusters with specific Eph receptor tyrosine kinases at specialized sites of cell-cell contact in the brain. J Cell Biol. 1999;144:361–371. doi: 10.1083/jcb.144.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Calegari FW, Haubensak C, Haffner C, Huttner WB. Selective lengthening of the cell cycle in the neurogenic subpopulation of neural progenitor cells during mouse brain development. J Neurosci. 2005;25:6533–6538. doi: 10.1523/JNEUROSCI.0778-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catchpole T, Henkemeyer M. EphB2 tyrosine kinase-dependent forward signaling in migration of neuronal progenitors that populate and form a distinct region of the dentate niche. J Neurosci. 2011;31:11472–11483. doi: 10.1523/JNEUROSCI.6349-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS, Jr, Goto T, Tarui T, Takahashi T, Bhide PG, Nowakowski RS. Cell output, cell cycle duration and neuronal specification: a model of integrated mechanisms of the neocortical proliferative process. Cereb Cortex. 2003;13:592–598. doi: 10.1093/cercor/13.6.592. [DOI] [PubMed] [Google Scholar]
- Cheng HJ, Nakamoto M, Bergemann AD, Flanagan JG. Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell. 1995;82:371–381. doi: 10.1016/0092-8674(95)90426-3. [DOI] [PubMed] [Google Scholar]
- Cisse M, Halabisky B, Harris J, Devidze N, Dubal DB, Sun B, Orr A, Lotz G, Kim DH, Hamto P, et al. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature. 2011;469:47–52. doi: 10.1038/nature09635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford MA, Kanwal JK, Dzakpasu R, Donoghue MJ. EphA4 expression promotes network activity and spine maturation in cortical neuronal cultures. Neural Dev. 2011;6:21. doi: 10.1186/1749-8104-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, Goldfarb M, Yancopoulos GD. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–819. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- Davy A, Gale NW, Murray EW, Klinghoffer RA, Soriano P, Feuerstein C, Robbins SM. Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev. 1999;13:3125–3135. doi: 10.1101/gad.13.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depaepe V, Suarez-Gonzalez N, Dufour A, Passante L, Gorski JA, Jones KR, Ledent C, Vanderhaeghen P. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature. 2005;435:1244–1250. doi: 10.1038/nature03651. [DOI] [PubMed] [Google Scholar]
- Donoghue MJ, Rakic P. Molecular evidence for the early specification of presumptive functional domains in the embryonic primate cerebral cortex. J Neurosci. 1999a;19:5967–5979. doi: 10.1523/JNEUROSCI.19-14-05967.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue MJ, Rakic P. Molecular gradients and compartments in the embryonic primate cerebral cortex. Cereb Cortex. 1999b;9:586–600. doi: 10.1093/cercor/9.6.586. [DOI] [PubMed] [Google Scholar]
- Drescher U, Kremoser C, Handwerker C, Loschinger J, Noda M, Bonhoeffer F. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- Dufour A, Seibt J, Passante L, Depaepe V, Ciossek Y, Frisen J, Kullander K, Flanagan JG, Polleux F, Vanderhaeghen P. Area specificity and topography of thalamocortical projections are controlled by ephrin/Eph genes. Neuron. 2003;39:453–465. doi: 10.1016/s0896-6273(03)00440-9. [DOI] [PubMed] [Google Scholar]
- Eph-Nomenclature-Committee. Unified nomenclature for Eph family receptors and their ligands, the ephrins. Eph nomenclature committee [letter] Cell. 1997;90:403–404. doi: 10.1016/s0092-8674(00)80500-0. [DOI] [PubMed] [Google Scholar]
- Fenstermaker V, Chen Y, Ghosh A, Yuste R. Regulation of dendritic length and branching by semaphorin 3A. J Neurobiol. 2004;58:403–412. doi: 10.1002/neu.10304. [DOI] [PubMed] [Google Scholar]
- Frisen J, Yates PA, McLaughlin T, Friedman GC, O'Leary DD, Barbacid M. Ephrin-A5 (AL-1/RAGS) is essential for proper retinal axon guidance and topographic mapping in the mammalian visual system. Neuron. 1998;20:235–243. doi: 10.1016/s0896-6273(00)80452-3. [DOI] [PubMed] [Google Scholar]
- Fu AK, Hung KW, Fu WY, Shen C, Chen Y, Xia J, Lai KO, Ip NY. APC(Cdh1) mediates EphA4-dependent downregulation of AMPA receptors in homeostatic plasticity. Nat Neurosci. 2011;14:181–189. doi: 10.1038/nn.2715. [DOI] [PubMed] [Google Scholar]
- Fukai J, Yokote H, Yamanaka R, Arao T, Nishio K, Itakura T. EphA4 promotes cell proliferation and migration through a novel EphA4-FGFR1 signaling pathway in the human glioma U251 cell line. Mol Cancer Ther. 2008;7:2768–2778. doi: 10.1158/1535-7163.MCT-07-2263. [DOI] [PubMed] [Google Scholar]
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- Gao PP, Yue T, Zhang JH, Cerretti DP, Levitt P, Zhou R. Regulation of thalamic neurite outgrowth by the Eph ligand ephrin-A5: implications in the development of thalamocortical projections. Proc Natl Acad Sci USA. 1998;95:5329–5334. doi: 10.1073/pnas.95.9.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genander M, Halford MM, Xu NJ, Eriksson M, Yu Z, Qiu Z, Martling A, Greicius G, Thakar S, Catchpole T, et al. Dissociation of EphB2 signaling pathways mediating progenitor cell proliferation and tumor suppression. Cell. 2009;139:679–692. doi: 10.1016/j.cell.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Barde YA. Radial glial cells defined and major intermediates between embryonic stem cells and CNS neurons. Neuron. 2005;46:369–372. doi: 10.1016/j.neuron.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Grunwald IC, Korte M, Adelmann G, Plueck A, Kullander K, Adams RH, Frotscher M, Bonhoeffer T, Klein R. Hippocampal plasticity requires postsynaptic ephrinBs. Nat Neurosci. 2004;7:33–40. doi: 10.1038/nn1164. [DOI] [PubMed] [Google Scholar]
- Guellmar A, Rudolph J, Bolz J. Structural alterations of spiny stellate cells in the somatosensory cortex in ephrin-A5-deficient mice. J Comp Neurol. 2009;517:645–654. doi: 10.1002/cne.22198. [DOI] [PubMed] [Google Scholar]
- Halford MM, Armes J, Buchert M, Meskenaite V, Grail D, Hibbs ML, Wilks AF, Farlie PG, Newgreen DF, Hovens CM, et al. Ryk-deficient mice exhibit craniofacial defects associated with perturbed Eph receptor crosstalk. Nat Genet. 2000;25:414–418. doi: 10.1038/78099. [DOI] [PubMed] [Google Scholar]
- Hamasaki T, Leingartner A, Ringstedt T, O'Leary DD. EMX2 regulates sizes and positioning of the primary sensory and motor areas in neocortex by direct specification of cortical progenitors. Neuron. 2004;43:359–372. doi: 10.1016/j.neuron.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Hansen MJ, Dallal GE, Flanagan JG. Retinal axon response to ephrin–as shows a graded, concentration-dependent transition from growth promotion to inhibition. Neuron. 2004;42:717–730. doi: 10.1016/j.neuron.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Hevner RF. From radial glia to pyramidal-projection neuron: transcription factor cascades in cerebral cortex development. Mol Neurobiol. 2006;33:33–50. doi: 10.1385/MN:33:1:033. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, et al. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987;238:1717–1720. doi: 10.1126/science.2825356. [DOI] [PubMed] [Google Scholar]
- Hock B, Bohme B, Karn T, Yamamoto T, Kaibuchi K, Holtrich U, Holland S, Pawson T, Rubsamen-Waigmann H, Strebhardt K. PDZ-domain-mediated interaction of the Eph-related receptor tyrosine kinase EphB3 and the ras-binding protein AF6 depends on the kinase activity of the receptor. Proc Natl Acad Sci USA. 1998;95:9779–9784. doi: 10.1073/pnas.95.17.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland SJ, Gale NW, Mbamalu G, Yancopoulos GD, Henkemeyer M, Pawson T. Bidirectional signalling through the Eph-family receptor Nuk and its transmembrane ligands. Nature. 1996;383:722. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- Holmberg J, Armulik A, Senti KA, Edoff K, Spalding K, Momma S, Cassidy R, Flanagan JG, Frisen J. Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes Dev. 2005;19:462–471. doi: 10.1101/gad.326905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J Cell Biol. 2010;189:619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska M, Dalva MB. Ephrin regulation of synapse formation, function and plasticity. Mol Cell Neurosci. 2012;50:35–44. doi: 10.1016/j.mcn.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Yue X, Shi G, Yue Y, Crockett DP, Blair-Flynn J, Reuhl K, Tessarollo L, Zhou R. Corpus callosum deficiency in transgenic mice expressing a truncated ephrin-A receptor. J Neurosci. 2003;23:10963–10970. doi: 10.1523/JNEUROSCI.23-34-10963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue E, Deguchi-Tawarada M, Togawa A, Matsui C, Arita K, Katahira-Tayama S, Sato T, Yamauchi E, Oda Y, Takai Y. Synaptic activity prompts gamma-secretase-mediated cleavage of EphA4 and dendritic spine formation. J Cell Biol. 2009;185:551–564. doi: 10.1083/jcb.200809151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaherian A, Kriegstein A. A stem cell niche for intermediate progenitor cells of the embryonic cortex. Cereb Cortex. 2009;19((Suppl 1):i70–77. doi: 10.1093/cercor/bhp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao JW, Feldheim DA, Chen DF. Ephrins as negative regulators of adult neurogenesis in diverse regions of the central nervous system. Proc Natl Acad Sci USA. 2008;105:8778–8783. doi: 10.1073/pnas.0708861105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasemeier-Kulesa JC, Bradley R, Pasquale EB, Lefcort F, Kulesa PM. Eph/ephrins and N-cadherin coordinate to control the pattern of sympathetic ganglia. Development. 2006;133:4839–4847. doi: 10.1242/dev.02662. [DOI] [PubMed] [Google Scholar]
- Kayser MS, Nolt MJ, Dalva MB. EphB receptors couple dendritic filopodia motility to synapse formation. Neuron. 2008;59:56–69. doi: 10.1016/j.neuron.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodosevich K, Watanabe Y, Monyer H. EphA4 preserves postnatal and adult neural stem cells in an undifferentiated state in vivo. J Cell Sci. 2011;124:1268–1279. doi: 10.1242/jcs.076059. [DOI] [PubMed] [Google Scholar]
- Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci. 2009;12:15–20. doi: 10.1038/nn.2231. [DOI] [PubMed] [Google Scholar]
- Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr Opin Cell Biol. 2004;16:580–589. doi: 10.1016/j.ceb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Knoll B, Drescher U. Ephrin-As as receptors in topographic projections. Trends Neurosci. 2002;25:145–149. doi: 10.1016/s0166-2236(00)02093-2. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Radial and horizontal deployment of clonally related cells in the primate neocortex: relationship to distinct mitotic lineages. Neuron. 1995;15:311–321. doi: 10.1016/0896-6273(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Noctor S, Martinez-Cerdeno V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- Kullander K, Butt SJ, Lebret JM, Lundfald L, Restrepo CE, Rydstrom A, Klein R, Kiehn O. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science. 2003;299:1889–1892. doi: 10.1126/science.1079641. [DOI] [PubMed] [Google Scholar]
- Lim BK, Matsuda N, Poo MM. Ephrin-B reverse signaling promotes structural and functional synaptic maturation in vivo. Nat Neurosci. 2008;11:160–169. doi: 10.1038/nn2033. [DOI] [PubMed] [Google Scholar]
- Lim YS, McLaughlin T, Sung TC, Santiago A, Lee KF, O'Leary DD. p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron. 2008;59:746–758. doi: 10.1016/j.neuron.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszewicz A, Cortay V, Giroud P, Berland M, Smart I, Kennedy H, Dehay C. The concerted modulation of proliferation and migration contributes to the specification of the cytoarchitecture and dimensions of cortical areas. Cereb Cortex. 2006;16(Suppl 1):i26–34. doi: 10.1093/cercor/bhk011. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz A, Savatier P, Cortay V, Giroud P, Huissoud C, Berland M, Kennedy H, Dehay C. G1 phase regulation, area-specific cell cycle control, and cytoarchitectonics in the primate cortex. Neuron. 2005;47:353–364. doi: 10.1016/j.neuron.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackarehtschian K, Lau CK, Caras I, McConnell SK. Regional differences in the developing cerebral cortex revealed by ephrin-A5 expression. Cereb Cortex. 1999;9:601–610. doi: 10.1093/cercor/9.6.601. [DOI] [PubMed] [Google Scholar]
- Margolis SS, Salogiannis J, Lipton DM, Mandel-Brehm C, Wills ZP, Mardinly AR, Hu L, Greer PL, Bikoff JB, Ho HY, et al. EphB-mediated degradation of the RhoA GEF Ephexin5 relieves a developmental brake on excitatory synapse formation. Cell. 2010;143:442–455. doi: 10.1016/j.cell.2010.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler KJ, Becker-Barroso E, Martinez A, Llovera M, Wentzel C, Poopalasundaram S, Hindges R, Soriano E, Comella J, Drescher U. A TrkB/EphrinA interaction controls retinal axon branching and synaptogenesis. J Neurosci. 2008;28:12700–12712. doi: 10.1523/JNEUROSCI.1915-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Noctor SC, Kriegstein AR. The role of intermediate progenitor cells in the evolutionary expansion of the cerebral cortex. Cereb Cortex. 2006;16(Suppl 1):i152–161. doi: 10.1093/cercor/bhk017. [DOI] [PubMed] [Google Scholar]
- Martone ME, Holash JA, Bayardo A, Pasquale EB, Ellisman HM. Immunolocalization of the receptor tyrosine kinase EphA4 in the adult rat central nervous system. Brain Res. 1997;771:238–250. doi: 10.1016/s0006-8993(97)00792-0. [DOI] [PubMed] [Google Scholar]
- McConnell SK. Constructing the cerebral cortex: neurogenesis and fate determination. Neuron. 1995;15:761–768. doi: 10.1016/0896-6273(95)90168-x. [DOI] [PubMed] [Google Scholar]
- Miller B, Chou L, Finlay BL. The early development of thalamocortical and corticothalamic projections. J Comp Neurol. 1993;335:16–41. doi: 10.1002/cne.903350103. [DOI] [PubMed] [Google Scholar]
- Miller FD, Gauthier AS. Timing is everything: making neurons versus glia in the developing cortex. Neuron. 2007;54:357–369. doi: 10.1016/j.neuron.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Miller K, Kolk SM, Donoghue MJ. EphA7-ephrin-A5 signaling in mouse somatosensory cortex: developmental restriction of molecular domains and postnatal maintenance of functional compartments. J Comp Neurol. 2006;496:627–642. doi: 10.1002/cne.20926. [DOI] [PubMed] [Google Scholar]
- Miyashita-Lin EM, Hevner R, Wassarman KM, Martinez S, Rubenstein JL. Early neocortical regionalization in the absence of thalamic innervation. Science. 1999;285:906–909. doi: 10.1126/science.285.5429.906. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, Ogawa M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development. 2004;131:3133–3145. doi: 10.1242/dev.01173. [DOI] [PubMed] [Google Scholar]
- Mori T, Buffo A, Gotz M. The novel roles of glial cells revisited: the contribution of radial glia and astrocytes to neurogenesis. Curr Top Dev Biol. 2005;69:67–99. doi: 10.1016/S0070-2153(05)69004-7. [DOI] [PubMed] [Google Scholar]
- Munarini N, Jager R, Abderhalden S, Zuercher G, Rohrbach V, Loercher S, Pfanner-Meyer B, Andres AC, Ziemiecki A. Altered mammary epithelial development, pattern formation and involution in transgenic mice expressing the EphB4 receptor tyrosine kinase. J Cell Sci. 2002;115:25–37. doi: 10.1242/jcs.115.1.25. [DOI] [PubMed] [Google Scholar]
- Murai KK, Nguyen LN, Irie F, Yamaguchi Y, Pasquale EB. Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nat Neurosci. 2003;6:153–160. doi: 10.1038/nn994. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Johnson JE, O'Leary DDM. Graded and areal expression patterns of regulatory genes and cadherins in embryonic neocortex independent of thalamocortical input. J Neurosci. 1999;19:10877–10885. doi: 10.1523/JNEUROSCI.19-24-10877.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola NA, Viney E, Hilton DJ, Roberts B, Willson T. Molecular cloning of two novel transmembrane ligands for Eph-related kinases (LERKS) that are related to LERK-2. Growth Factors. 1996;13:141–149. doi: 10.3109/08977199609034574. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Nolt MJ, Lin Y, Hruska M, Murphy J, Sheffler-Colins SI, Kayser MS, Passer J, Bennett MV, Zukin RS, Dalva MB. EphB controls NMDA receptor function and synaptic targeting in a subunit-specific manner. J Neurosci. 2011;31:5353–5364. doi: 10.1523/JNEUROSCI.0282-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North HA, Zhao X, Kolk SM, Clifford MA, Ziskind DM, Donoghue MJ. Promotion of proliferation in the developing cerebral cortex by EphA4 forward signaling. Development. 2009;136:2467–2476. doi: 10.1242/dev.034405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary DD. Do cortical areas emerge from a protocortex? Trends Neurosci. 1989;12:400–406. doi: 10.1016/0166-2236(89)90080-5. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, Wilkinson DG. Eph receptors and ephrins in neural development. Curr Opin Neurobiol. 1999;9:65–73. doi: 10.1016/s0959-4388(99)80008-7. [DOI] [PubMed] [Google Scholar]
- Oricchio E, Nanjangud G, Wolfe AL, Schatz JH, Mavrakis KJ, Jiang M, Liu X, Bruno J, Heguy A, Olshen AB, et al. The Eph-receptor A7 is a soluble tumor suppressor for follicular lymphoma. Cell. 2011;147:554–564. doi: 10.1016/j.cell.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Frisen J, Barbacid M. Aberrant axonal projections in mice lacking EphA8 (Eek) tyrosine protein kinase receptors. EMBO J. 1997;16:3106–3114. doi: 10.1093/emboj/16.11.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascall JC, Brown KD. Intramembrane cleavage of ephrinB3 by the human rhomboid family protease, RHBDL2. Biochem Biophys Res Commun. 2004;317:244–252. doi: 10.1016/j.bbrc.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- Pitulescu ME, Adams RH. Eph/ephrin molecules—a hub for signaling and endocytosis. Genes Dev. 2010;24:2480–2492. doi: 10.1101/gad.1973910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- Prakash N, Vanderhaeghen P, Cohen-Cory S, Frisen J, Flanagan J, Frostig R. Malformation of the functional organization of somatosensory cortex in adult ephrin-A5 knock-out mice revealed by in vivo functional imaging. J Neurosci. 2000;20:5841–5847. doi: 10.1523/JNEUROSCI.20-15-05841.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privat A. Postnatal gliogenesis in the mammalian brain. Int Rev Cytol. 1975;40:281–323. doi: 10.1016/s0074-7696(08)60955-9. [DOI] [PubMed] [Google Scholar]
- Qiu R, Wang X, Davy A, Wu C, Murai K, Zhang H, Flanagan JG, Soriano P, Lu Q. Regulation of neural progenitor cell state by ephrin-B. J Cell Biol. 2008;181:973–983. doi: 10.1083/jcb.200708091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Limits of neurogenesis in primates. Science. 1985;227:1054–1056. doi: 10.1126/science.3975601. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rakic P. Principles of neural cell migration. Experientia. 1990;46:882–891. doi: 10.1007/BF01939380. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Richter M, Murai KK, Bourgin C, Pak DT, Pasquale EB. The EphA4 receptor regulates neuronal morphology through SPAR-mediated inactivation of Rap GTPases. J Neurosci. 2007;27:14205–14215. doi: 10.1523/JNEUROSCI.2746-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe AW, Pallas SL, Hahm JO, Sur M. A map of visual space induced in primary auditory cortex. Science. 1990;250:818–820. doi: 10.1126/science.2237432. [DOI] [PubMed] [Google Scholar]
- Rudolph J, Zimmer G, Steinecke A, Barchmann S, Bolz J. Ephrins guide migrating cortical interneurons in the basal telencephalon. Cell Adh Migr. 2010;4:400–408. doi: 10.4161/cam.4.3.11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, O'Leary DD. Potential of visual cortex to develop an array of functional units unique to somatosensory cortex. Science. 1991;252:1556–1560. doi: 10.1126/science.2047863. [DOI] [PubMed] [Google Scholar]
- Senturk A, Pfennig S, Weiss A, Burk K, Acker-Palmer A. Ephrin Bs are essential components of the Reelin pathway to regulate neuronal migration. Nature. 2011;472:356–360. doi: 10.1038/nature09874. [DOI] [PubMed] [Google Scholar]
- Sestan N, Rakic P, Donoghue MJ. Independent parcellation of the embryonic visual cortex and thalamus revealed by combinatorial Eph/ephrin gene expression. Curr Biol. 2001;11:39–43. doi: 10.1016/s0960-9822(00)00043-9. [DOI] [PubMed] [Google Scholar]
- Sharma J, Angelucci A, Sur M. Induction of visual orientation modules in auditory cortex. Nature. 2000;404:841–847. doi: 10.1038/35009043. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Rakic P. Development of the human central nervous system. In: Haymaker W, Adams RD, editors. Histology and histopathology of the nervous system. Springfield (IL): C.C. Thomas; 1982. pp. 3–145. [Google Scholar]
- Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- Sturrock RR, Smart IH. A morphological study of the mouse subependymal layer from embryonic life to old age. J Anat. 1980;130:391–415. [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr Cell cycle parameters and patterns of nuclear movement in the neocortical proliferative zone of the fetal mouse. J Neurosci. 1993;13:820–833. doi: 10.1523/JNEUROSCI.13-02-00820.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J Neurosci. 1995;15:6046–6057. doi: 10.1523/JNEUROSCI.15-09-06046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr Mode of cell proliferation in the developing mouse neocortex. Proc Natl Acad Sci USA. 1994;91:375–379. doi: 10.1073/pnas.91.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabykin V, Stoykova A, Usman N, Gruss P. Cortical upper layer neurons derive from the subventricular zone as indicated by Svet1 gene expression. Development. 2001;128:1983–1993. doi: 10.1242/dev.128.11.1983. [DOI] [PubMed] [Google Scholar]
- Torii M, Hashimoto-Torii K, Levitt P, Rakic P. Integration of neuronal clones in the radial cortical columns by EphA and ephrin-A signalling. Nature. 2009;461:524–528. doi: 10.1038/nature08362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M, Levitt P. Dissociation of corticothalamic and thalamocortical axon targeting by an EphA7-mediated mechanism. Neuron. 2005;48:563–575. doi: 10.1016/j.neuron.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Torres R, Firestein BL, Dong H, Staudinger J, Olson EN, Huganir RL, Bredt DS, Gale NW, Yancopoulos GD. PDZ proteins bind, cluster, and synaptically colocalize with Eph receptors and their ephrin ligands. Neuron. 1998;21:1453–1463. doi: 10.1016/s0896-6273(00)80663-7. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziel D, Muhlfriedel S, Zarbalis K, Wurst W, Levitt P, Bolz J. Miswiring of limbic thalamocortical projections in the absence of ephrin-A5. J Neurosci. 2002;22:9352–9357. doi: 10.1523/JNEUROSCI.22-21-09352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino FM, Schwartz ML, Raballo R, Nilson J, Rhee J, Zhou M, Doetschman T, Coffin JD, Wyland JJ, Hung Y-TE. Changes in cerebral cortex size are governed by fibroblast growth factor during embryogenesis. Nat Neorosci. 1999;2:246–253. doi: 10.1038/6350. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen P, Lu Q, Prakash N, Frisen J, Walsh CA, Frostig RD, Flanagan JG. A mapping label required for normal scale of body representation in the cortex. Nat Neurosci. 2000;3:358–365. doi: 10.1038/73929. [DOI] [PubMed] [Google Scholar]
- von Melchner L, Pallas SL, Sur M. Visual behaviour mediated by retinal projections directed to the auditory pathway. Nature. 2000;404:871–876. doi: 10.1038/35009102. [DOI] [PubMed] [Google Scholar]
- Walsh C, Cepko CL. Clonal dispersion in proliferative layers of developing cerebral cortex. Nature. 1993;362:632–635. doi: 10.1038/362632a0. [DOI] [PubMed] [Google Scholar]
- Walsh C, Cepko CL. Clonally related cortical cells show several migration patterns. Science. 1988;241:1342–1345. doi: 10.1126/science.3137660. [DOI] [PubMed] [Google Scholar]
- Walsh C, Cepko CL. Widespread dispersion of neuronal clones across functional regions of the cerebral cortex. Science. 1992;255:434–440. doi: 10.1126/science.1734520. [DOI] [PubMed] [Google Scholar]
- Wu SX, Goebbels S, Nakamura K, Kometani K, Minato N, Kaneko T, Nave KA, Tamamaki N. Pyramidal neurons of upper cortical layers generated by NEX-positive progenitor cells in the subventricular zone. Proc Natl Acad Sci USA. 2005;102:17172–17177. doi: 10.1073/pnas.0508560102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Eom TY, Stanco A, Kim WY, Rao S, Snider WD, Anton ES. Cdc42 and Gsk3 modulate the dynamics of radial glial growth, inter-radial glial interactions and polarity in the developing cerebral cortex. Development. 2010;137:4101–4110. doi: 10.1242/dev.048637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokote H, Fujita K, Jing X, Sawada T, Liang S, Yao L, Yan X, Zhang Y, Schlessinger J, Sakaguchi K. Trans-activation of EphA4 and FGF receptors mediated by direct interactions between their cytoplasmic domains. Proc Natl Acad Sci USA. 2005;102:18866–18871. doi: 10.1073/pnas.0509741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun ME, Johnson RR, Antic A, Donoghue MJ. EphA family gene expression in the developing mouse neocortex: regional patterns reveal intrinsic programs and extrinsic influence. J Comp Neurol. 2003;456:203–216. doi: 10.1002/cne.10498. [DOI] [PubMed] [Google Scholar]
- Zimmer G, Kastner B, Weth F, Bolz J. Multiple effects of ephrin-A5 on cortical neurons are mediated by SRC family kinases. J Neurosci. 2007;27:5643–5653. doi: 10.1523/JNEUROSCI.0954-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


