Abstract
Posterior reversible encephalopathy syndrome (PRES) is an uncommon neurological disorder, characterised by the rapid onset of neurological deficits and characteristic neuroimaging findings—cerebral oedema with a typical preference for the posterior white matter. We report a case of a 59-year-old woman with an untreated HIV infection and hypertension with a PRES diagnosis and a rare involvement of the basal ganglia and brainstem, with microhemorrhages. HIV infection, particularly if untreated, is associated with an inflammatory status and therefore endothelial damage and dysfunction that might have an important role in predisposing acute hypertensive crisis and PRES.
Background
Posterior reversible encephalopathy syndrome (PRES) is a clinicoradiological condition first described by Hinchey et al.1 2 This syndrome has been named as reversible posterior leukoencephalopathy syndrome, reversible posterior cerebral oedema syndrome and hyperperfusion encephalopathy. While it is presently accepted as PRES, it has been questioned. The risk of irreversible neurological damage and mortality referred is in the literature as 15%.
The authors highlight the early recognition of atypical patterns leading to timely diagnosis, avoiding unnecessary research and treatments, and, even more important, the progression to massive cerebral ischaemia or death, while correcting the precipitating factor.
Case presentation
We present a case of a 59-year-old woman infected with HIV1, diagnosed in 2010 (CDC stage C3 with oesophageal candidiasis), without antiretroviral therapy due to lack of adherence, living in an environment of low socioeconomic status. She also had a history of IgA nephropathy with chronic renal failure (no previous treatment but ACE inhibitors) and uncontrolled arterial hypertension, leading to several admissions in the emergency department.
She was again admitted with a 1-month history of bilateral frontal pulsatile headache, radiating to the posterior region, associated with nausea, vomiting and blurred vision. She was hypertensive (210/110 mm Hg) and neurological examination showed left homonymous haemianopsia and diminished cutaneous plantar reflexes.
A laboratory workout revealed renal failure (creatinine=1.93 mg/dL; blood urea nitrogen=67 mg/dL), anaemia (haemoglobin=9.7 g/dL) and thrombocytopenia (95 000/dL).
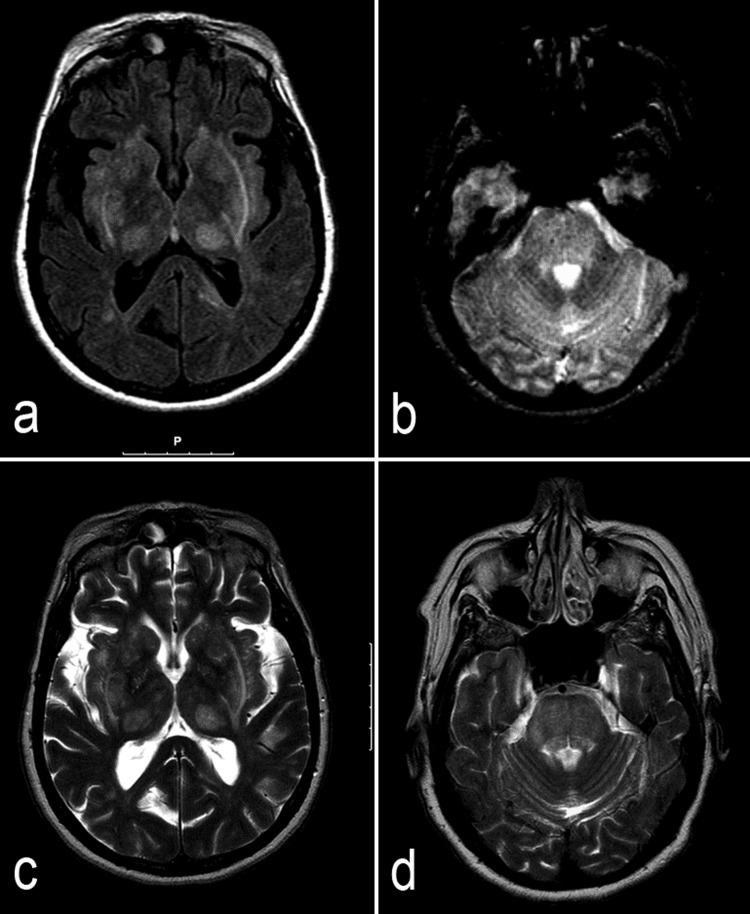
She underwent a brain CT scan and MRI, to exclude opportunistic diseases, the latter (figure 1A, B) showing diffuse symmetrical lesions of the basal ganglia, thalamus, internal and external capsules, brain stem and cerebellar white matter. There was pons expansion with petechial haemorrhages. There was a clear gap between the neurological examination and the MRI findings.
Figure 1.
MRI scans: admission MRI scan axial fluid-attenuated inversion recovery (A) and T2* (B) images showing symmetrical lesions evolving the basal ganglia, thalamus, internal and external capsules and brain stem, with expansion and microhaemorrages in the pons. Follow-up (7 months later) MRI scans: axial T2 images (C and D) showing partial resolution of the admission lesions.
The cerebrospinal fluid sample from the patient was clear, colourless, with 26 cells/µL (all mononuclear), mild proteinorrhachia (0.70 g/dL) and normal glucose. The patient's CD4 cell count was 157 cells/μL (9%) and HIV RNA of 734 000 copies/mL.
Treatment with labetalol and captopril was initiated, followed by ramipril and amlodipine. Blood pressure control and headache disappearence was attained in 24 h while haemianopsia resolved within 72 h.
Outcome and follow-up
Seven months after being discharged from the hospital, she was asymptomatic, with controlled blood pressure, and partial resolution of the brain lesions (figure 1C, D), supporting the diagnosis of PRES.
Discussion
PRES is characterised by headache, seizures, altered mental status, cortical blindness, visual abnormalities and other focal neurological signs associated with characteristic CT or MRI findings.1–3 Cerebral imaging abnormalities are symmetric oedema, often extensive, typically involving the subcortical white-matter and occasionally the cortex in occipital and parietal lobes, at the vertebrobasilar territory, maybe resulting from the absence of baroreceptors in this vascular region.2–4 Less often it can reach other locations such as the basal ganglia, brainstem, frontal lobe and deep white matter like in the capsule, as in the case above.1 4 5 When cerebellum or brainstem are involved, hydrocephalus may occur. Focal areas of restricted diffusion are uncommon (11–26%) and have been associated with an adverse prognosis.2 Microhaemorrhage is present in 15% of the patients.2
Nowadays although this syndrome is better understood, the mechanisms involving brain oedema remain controversial. Pathological studies showed almost no evident infarct but only interstitial oedema, petechial microhaemorrhages and fibrinoid necrosis within the arteriole walls.4 Microscopically, these petechia are ring haemorrhage around capillaries and precapillaries barely occluded by fibrinoid material.5
PRES may be associated with different pathologies. The most frequent precipitants are hypertension (particularly in the presence of eclampsia), acute renal failure, fluid retention, hyperkalaemia, systemic lupus erythematous and the treatment with immunosuppressive agents.1 3–6 Other less common causes like HIV infection or thrombotic thrombocytopenic purpura have been described.3–5
In fact, despite the few cases of PRES described in patients with HIV, they also had other known risk factors like increased blood pressure, making it difficult to determine the exact role of HIV.1 3 7–9 Some cases have been reported with no risk factors, being untreated HIV infection the sole factor, suggesting that infection by itself can precipitate PRES.5 10 Therefore some authors consider that the endothelial damage or dysfunction associated with a long history of HIV infection might have a role in predisposing patients to PRES.2 10 11 On the other hand there are some reports of patients with undetectable viral load and a good immunological status, but with severe hypertension.
Like in this particular case the majority of patients described in the literature had advanced disease (CD4 cell counts <200 cells/uL), leading to the necessary exclusion of an opportunistic disease. In addition to hypertension and HIV infection, renal dysfunction (IgA nephropathy with creatinine clearance=28 mL /m), may also have contributed to PRES.
However, regardless the cause, cerebral vasogenic oedema is typically reversible with the removal of the cause.3–5 On the other hand, a persistent precipitating factor can cause irreversible damage like cortical blindness or death.4 In this case, a cerebral MRI follow-up showed an improvement of white-matter abnormalities suggesting transient oedema rather than infarction.
The clinical history should raise the suspicion of this syndrome. However, the clinical unspecificity makes the diagnosis difficult, and other common diagnoses are questioned before performing a brain image (CT or MRI). In this particular case, first studies were directed towards an opportunistic disease, the presence of severe hypertension which also raised the chance of intracerebral hypertensive haematoma or hypertensive encephalopathy. MRI was essential in the diagnosis of PRES and the rapid favourable response to the antihypertensive treatment supported the diagnosis, as well as the radiological improvement after blood pressure control.
The patient described above presented the classic manifestation of a reversible encephalopathy syndrome, even though with an uncommon radiological pattern.
Learning points.
Widely unknown, this syndrome is often unnoticed and consequently underdiagnosed.
It should be promptly remembered in any patient with HIV with focal neurological deficits, as a reversible but potentially life-threatening syndrome.
Immune activation in the setting of HIV infection probably constitutes a risk for vascular disease.
Footnotes
Contributors: The conception of the article, analysis and interpretation of the clinical data were made by SR and MM. Literature revision was made by SR. Final revision and the investigation was conducted by MF and BM.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Saeed MU, Dacuycuy MA, Kennedy DJ. Posterior reversible encephalopathy syndrome in HIV patients: case report and review of the literature. AIDS 2007;2013:781–2 [DOI] [PubMed] [Google Scholar]
- 2.Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol 2008;2013:1036–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridolfo AL, Resta F, Milazzo L, et al. Reversible posterior leukoencephalopathy syndrome in 2 HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis 2008;2013:19–22 [DOI] [PubMed] [Google Scholar]
- 4.Fernandes FJ, Machado MA, Pedreira AV, et al. Reversible posterior encephalopathy syndrome: case report. Arq Neuropsiquiatr 2002;2013:651–5 [PubMed] [Google Scholar]
- 5.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996;2013:494–500 [DOI] [PubMed] [Google Scholar]
- 6.Kastrup O, Maschke M, Wanke I, et al. Posterior reversible encephalopathy syndrome due to severe hypercalcemia. J Neurol 2002;2013:1563–6 [DOI] [PubMed] [Google Scholar]
- 7.Giner V, Fernández C, Esteban MJ, et al. Reversible posterior leukoencephalopathy secondary to indinavir-induced hypertensivecrisis: a case report. Am J Hypertens 2002;2013:465–7 [DOI] [PubMed] [Google Scholar]
- 8.Choudhary M, Rose F. Posterior reversible encephalopathic syndrome due to severe hypercalcemia in AIDS. Scand J Infect Dis 2005;2013:524–6 [DOI] [PubMed] [Google Scholar]
- 9.Sylvester SL, Diaz LA, Port JD, et al. Reversible posterior leukoencephalopathy in an HIV-infected patient with thrombotic thrombocytopenic purpura. Scand J Infect Dis 2002;2013:706–9 [DOI] [PubMed] [Google Scholar]
- 10.Nightingale S, Wood C, Ainsworth J. The posterior reversible encephalopathy syndrome in HIV infection. BMJ Case Rep. Published online: 25 Jun 2012. doi:10.1136/bcr.01.2012.5647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torriani FJ, Komarow L, Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: the ACTG (AIDS Clinical Trials Group) Study 5152 s. J Am Coll Cardiol 2008;2013:569–76 [DOI] [PMC free article] [PubMed] [Google Scholar]