Abstract
Background
Activation of nicotinic receptors with nicotine has been shown to reduce post-surgical pain in clinical and preclinical studies. Choline is a selective agonist at α7-type nicotinic receptors that does not have addictive or sympathetic activating properties. It is anti-nociceptive in animal studies. We conducted a double-blind randomized trial of oral choline supplementation with lecithin to aid in the treatment of pain after gynaecological surgery.
Methods
Sixty women having open gynaecological surgery were randomly assigned to receive 20 g of lecithin before surgery or placebo. Plasma choline concentration and tumour necrosis factor (TNF) were measured. Pain report was the primary outcome measure.
Results
We achieved a small but statistically significant increase in choline after surgery with oral supplementation. Plasma TNF was not decreased and pain report was not different between groups at rest or with movement. There were no adverse effects of treatment.
Conclusions
Oral supplementation with lecithin during the perioperative period resulted in very slow absorption and thus only a small increase in plasma choline was achieved. This concentration was inadequate to reduce TNF as has been shown in other studies. The absence of an anti-inflammatory effect was likely related to our failure to demonstrate efficacy in pain reduction.
Keywords: analgesia, anti-inflammatory agents, nutritional requirements, pain, pain measurement
Editor's key points.
There is a need for adjuvant analgesics to improve postoperative analgesia and reduce opioid consumption.
Basic science studies have demonstrated a potential anti-inflammatory and analgesic effect of choline.
This study examined effects of oral choline on postoperative pain and tumour necrosis factor (TNF) levels.
Neither pain nor TNF was affected, despite a small increase in systemic levels of choline.
While further study may be warranted, oral choline supplementation does not confer any analgesic benefit.
Seventy to eighty per cent of the 23 million Americans who undergo surgical procedures each year experience moderate-to-severe pain, despite ‘state of the art’ treatment.1–3 Extended hospitalization, compromised prognosis, and higher morbidity and mortality are consequences of inadequately managed acute postoperative pain.4 Opioid agonists are the current mainstay of pain treatment after surgery, but opioid therapy is severely limited by side-effects at effective doses. There is some evidence that the advent of patient-controlled analgesia and epidural analgesia has reduced the incidence of unacceptable pain relief,5 but the situation is still far from perfect for many patients. For many patients, there is no dose of opioid alone that adequately treats acute postoperative pain without unacceptable side-effects. For this reason, although patients have access to large doses of opioids via patient-controlled analgesia pumps, they choose to accept moderate-to-severe pain rather than taking more opioid.6–8
Multimodal pain therapy combines administration of adjuvant analgesic drugs with opioids to reduce the required dose, and thus side-effects that occur when each drug is used alone. Non-specific activation of nicotinic acetylcholine receptors with nicotine has analgesic efficacy as an adjuvant for postoperative pain,6,9–12 but its use is limited by side-effects such as nausea, autonomic dysfunction, and concerns about addiction to nicotine. Choline is a nutritional supplement that selectively activates α7 nicotinic acetylcholine receptors13,14 and potentially α8,α9-containing nicotinic receptors15 but not the α4β2 subtypes that are associated with nausea and addictive properties or the α3β4 subtypes that are expressed in the autonomic nervous system. α7-containing nicotinic receptors are expressed in the central and peripheral nervous system and on immune cells where their activation reduces the production of inflammatory cytokines via a pathway that involves the transnucleation of nuclear factor kappa b and decreased production of tumour necrosis factor (TNF).16 Systemically administered choline has been shown to have analgesic efficacy in preclinical trials potentially through a reduction in surgically induced inflammation.17–23
In this double-blind, randomized trial, we tested the hypotheses that oral choline supplementation can raise plasma choline concentration, mitigate the inflammatory response to surgery via the TNF pathway, and thus result in analgesia. To test this hypothesis, we enrolled 60 women who planned open gynaecological surgery into a double-blinded randomized trial of choline supplementation.
Methods
Ethical approval for this double-blind, placebo-controlled pilot study was obtained by the Institutional Review Boards at Columbia University Medical Center and The University of California, San Francisco. The trial is registered with clinical trials.gov (NCT00720343) and is conducted under investigator IND 101516 from the FDA.
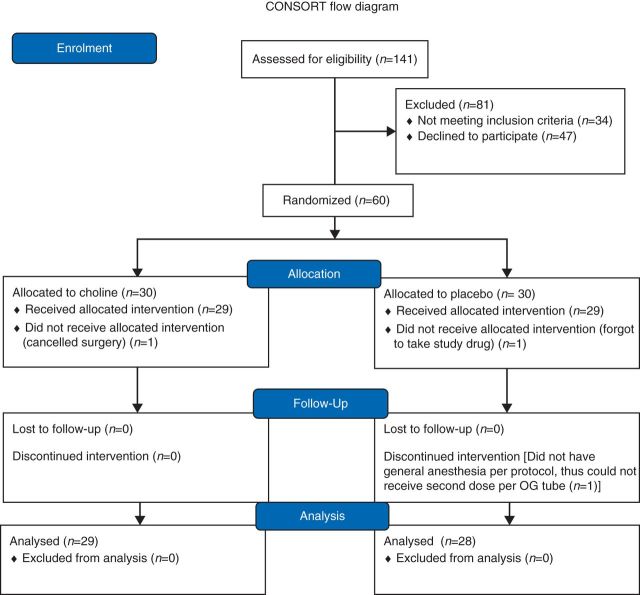
Sixty women between the ages of 18 and 60 were recruited at a preoperative visit at Columbia University Medical Center who planned hysterectomy or myomectomy through a low transverse incision (Fig. 1). Chronic pain or pain medication use, tobacco use, choline or soy intolerance, active psychiatric disease, pregnancy, and lactation were exclusion criteria.
Fig 1.
Consort Diagram.
Each subject was assigned via a random allocation table maintained by the research pharmacy to receive either choline supplementation or placebo study drug (gelatin). Randomization was achieved using a block design. The subject was given a sealed bottle of 10 study tablets that had been prepared by the pharmacy and was instructed to take them with dinner the night before surgery. Choline was provided as GNC Triple Lecithin 1200 choline tablets. Ten tablets contain 12 g of high choline lecithin and 4.2 g phosphatidyl choline. A second dose of study drug was administered by the clinical anaesthesiologist as a slurry through the oral gastric tube that was placed after induction of general anaesthesia. The slurry was prepared by the research pharmacy for use by the clinical anaesthesiologist. Twenty-four grams were chosen as the maximum tolerated dose as oral intake of more than 25 g was found to cause nausea.24
On enrolment, the subjects provided a baseline blood sample for measurement of plasma choline and TNF. Plasma choline concentration was measured at five time points (Fig. 2). (1) The baseline concentration on enrolment, (2) after induction of general anaesthesia but before the second choline dose, (3) at the completion of surgery (reflecting the second choline dose administered at the beginning of surgery through the oral gastric tube), (4) 1 h after surgery coincident with pain scores, and (5) 24 h after surgery coincident with pain scores. TNF was tested on enrolment and 1 h after surgery with choline measurement #4.
Fig 2.
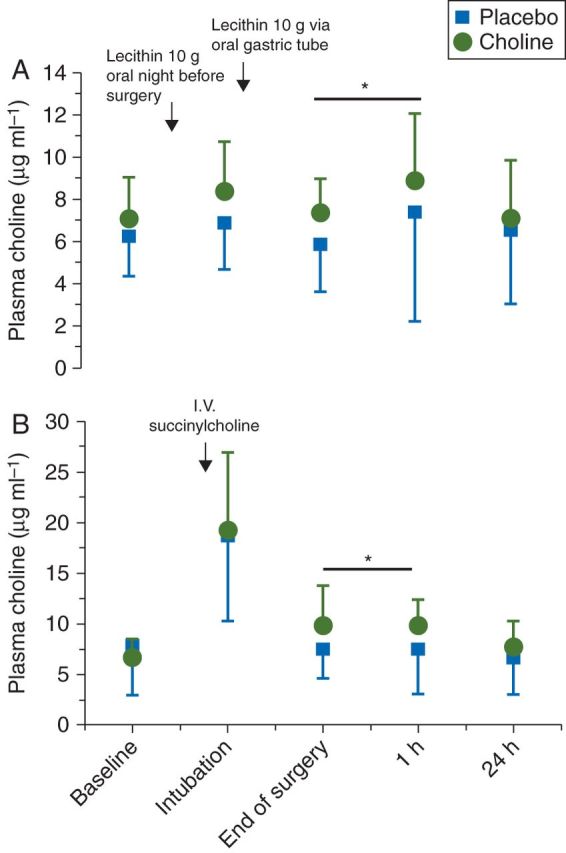
Plasma choline concentrations. Plasma choline (µg ml−1) concentration levels are measured for the placebo and choline supplementation cohorts at baseline, intubation, end of surgery, 1 h after surgery, and 24 h after surgery in patients who (a) were treated with succinylcholine and (b) were treated with a non-depolarizer. Baseline plasma choline concentrations are not significantly different between the two groups. Plasma choline concentrations are significantly higher in the choline cohort at all other measurements (P=0.038).
On the morning of surgery, the subject confirmed that they had taken the study drug as directed. The study protocol specified that subjects who did not take the study drug dropped from the protocol. Study personnel provided the clinical anaesthesiologist with the subject's second dose of study drug slurry which was administered into the oral gastric tube after induction of general anaesthesia and intubation by the anaesthesiologist.
Anaesthesia was provided by clinical anaesthesiologists at Columbia University Medical Center who were not involved with the study. Anaesthesia was induced with propofol and succinylcholine was used to facilitate tracheal intubation. During the study, there was a nation-wide shortage of succinylcholine and some patients had general anaesthesia induced with propofol and rocuronium as noted. Sevoflurane was used for anaesthesia maintenance with 0.1–0.2 mg kg−1 morphine given intraoperatively at the discretion of the anaesthesiologist. Metoclopramide 10 mg was administered i.v. after induction of anaesthesia to facilitate passage of the second dose of the study drug. Ketorolac 15 mg was administered i.v. at the conclusion of surgery.
All subjects received standard postoperative care, with treatment in the postoperative care unit. Patient-controlled analgesia was provided on admission to the recovery room with morphine at 1 mg demand dose, 6 min lock out, and 1 h maximum of 10 mg. The patient had access to additional clinician boluses of 3 mg morphine every 5 min to a maximum of 12 mg morphine or the equivalent hydromorphone at a 5:1 ratio. If pain was inadequately treated with this regimen, there was an option to increase the patient demand dose to 1.5 mg morphine and the 1 h maximum to 15 mg. Ketorolac was dosed 15 mg every 8 h for 24 h.
The primary outcome variable was numerical rating scale (NRS) pain score 1 h after surgery. NRS was queried at rest and with movement (cough) 1 h after surgery and 24 h after surgery and at the postoperative visit 2 weeks later. Opioid medication use, nausea/vomiting NRS scores, and sedation scores and wound integrity were planned secondary outcomes.
Plasma choline was measured with high performance liquid chromatography.25 TNF was measured after stimulation of whole blood with lipopolysaccharide at 0, 0.1, 1, 10, and 100 ng ml−1 according to our previously described protocol.26 TNF concentration was measured using DuoSet Elisa Development kit for human TNF (R&D Systems #DY210) according to the manufacturer's instructions.
Patient wounds were assessed 24 h after surgery and at the postoperative visit which took place normally 2 weeks after surgery. The wound was photographed with a digital camera from 12 in away, draped in blue surgical towels. The photographs were scored by a gynaecologist (R.J.M.) according to a validated rubric that considers serous discharge, purulent discharge, erythema, and wound separation.27 As the incidence of complications was small, only the incidence was compared between groups 24 h after surgery.
Sample size estimation
Our sample size was determined based on our primary outcome variable, NRS at 1 h after surgery. In our previous trial using a similar population, our control group reported NRS scores of 4.7 (1.8) in our control group. As such, we chose an evenly divided group of 60 subjects to have 80% power to detect a true effect of 1.3 numerical analogue scale for pain (NAS) and 90% power to identify a true effect of 1.5 at P<0.05, based on a two-sided t-test for unpaired data. We chose this reduction in NRS score as being clinically valuable. For comparison, the addition of 1 g acetaminophen after surgery reduces patient pain report ∼1.5 NAS points.28 Using this study design, we would expect to detect a pain relief effect of choline if it were at least as significant as that derived from acetaminophen.
Statistical analysis
The pharmacokinetic properties of oral choline supplementation were evaluated with NONMEM (ICON solutions; Silver Springs, MD, USA) and PLT tools (PLTsoft, San Francisco, CA, USA) using the built-in ADVAN2 routine for a one-compartment model with oral absorption. The model included baseline (endogenous) choline concentration before drug administration. The fact that a subset of patients received an i.v. bolus of succinylcholine allowed for evaluation of absolute oral bioavailability. Each milligram of succinylcholine administered provided 0.36 mg of choline to the central compartment. Each oral dose of study drug contained 4.2 g phosphatidyl choline and thus 569 mg choline. Choline concentrations at each measurement (described in Fig. 2) were evaluated with respect to study group and succinylcholine dosing using repeated-measures analysis of variance (anova) in NONMEM.
The primary outcome variable and all other pain scores, NRS scores for nausea, and sedation were compared between groups with a two-sample t-test with equal or unequal variance as appropriate. The relationship between choline concentration and NRS score and choline concentration and TNF concentration was evaluated with the Pearson correlation coefficients using SAS (SAS Institute, Cary, NC, USA).
Results
Sixty women who planned gynaecological surgery were enrolled between February 2009 and May 2011. Fifty-seven subjects had analysable data. One subject forgot to take the study drug, another cancelled surgery for personal reasons, and a third did not have general anaesthesia and thus did not receive the second drug dose or blood testing after the baseline. Patient characteristic and surgical characteristics did not differ between study groups (Table 1).
Table 1.
Demographic Data. The characteristics data continuous data are median (IQR). Categorical data are %—note ethnicity does not add 100% as Hispanic subjects may have identified as black or white. There are no significant differences between the groups
| Choline (n=29) | Placebo (n=28) | |
|---|---|---|
| Age (yr) | 42 (38–47) | 43 (34–46) |
| Height (m) | 1.63 (1.60–1.68) | 1.63 (1.60–1.68) |
| Weight (kg) | 79 (61–88) | 77 (63–91) |
| Ethnicity | ||
| Hispanic (%) | 28 | 34 |
| Black (%) | 70 | 66 |
| White (%) | 21 | 28 |
| Asian (%) | 7 | 3 |
| Surgery length (h) | 2.9 (2.3–3.2) | 2.9 (2.5–3.6) |
| % Myomectomy | 45% | 66% |
| Interoperative fentanyl (μg) | 150 (144–250) | 175 (150–250) |
| Interoperative morphine (mg) | 10.0 (8.4–12.5) | 10.0 (7.0–10.0) |
Oral administration of phosphitidyl choline-enriched lecithin resulted in an increase in plasma choline concentration at the end of surgery and at the 1 h post-surgical time point corresponding to the primary outcome variable pain score [5.8 (2.2) vs 7.4 (1.7) end of surgery, 7.4 (5.2) vs 8.8 (3.2) 1 h after surgery; anova P=0.038]. Treatment with succinylcholine on induction of general anaesthesia was associated with a large peak in plasma choline concentration that quickly dissipated and was not detectable by 1 h after surgery (Fig. 2, anova P=8.5 E–12). Pharmacokinetic values representing oral choline administration are characterized by a one-compartment model with very slow oral absorption and rapid elimination. The half-time for oral absorption in a patient of median weight is 13.9 h (Table 2). The half-time for elimination is relatively rapid (8.2 min). Oral bioavailability was nearly complete.
Table 2.
Pharmacokinetic model for oral choline
| Value | 95% confidence interval | |
|---|---|---|
| Volume (litre) | 3.2 | 2.3–4.0 |
| Clearance (h−1) | 5.1 | 3.5–7.7 |
| Ka (h−1) | 0.05 | 0.02–0.09 |
| Oral bioavailability (%) | 100 |
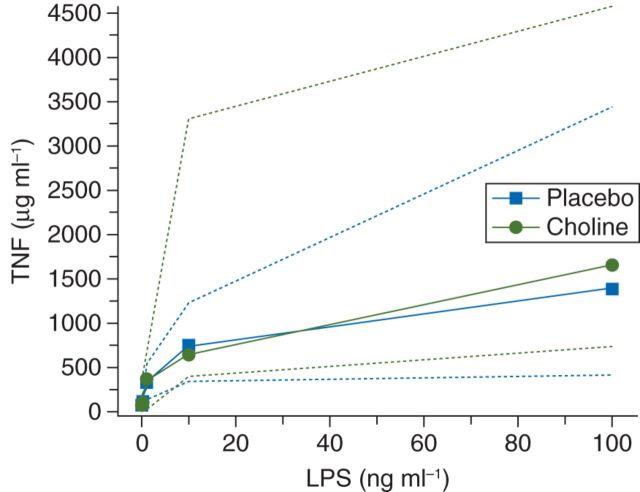
TNF measured at the end of surgery was not lower in patients who received the oral choline supplementation (Fig. 3). There was no correlation between plasma choline and TNF concentrations (Pearson's correlation coefficient=−0.17, P=0.22).
Fig 3.
Tumour necrosis response. TNF release from macrophages with lipopolysaccharide (LPS) stimulation. As concentration of LPS (ng ml−1) is increased, TNF (µg ml−1) release is also increased. There is no difference in TNF response in patients treated with choline.
Postoperative pain at rest or with cough was not different in patients treated with choline at any time point (Fig. 4). The amount of morphine used in the first hour after surgery was similar between the groups [4.0 mg (1.0–8.0)—placebo, 3.6 mg (1.0–6.0)—choline] as was the cumulative morphine at 24 h [48 mg (33–62)—placebo, 49 mg (31–71)—choline]. There was no correlation between TNF concentration and postoperative pain (Pearson's correlation coefficient=−0.05, P=0.78) or plasma choline concentration and postoperative pain report (Pearson's correlation coefficient=−0.05, P=0.78). There was no difference in the reported nausea or incidence of vomiting between treatment groups at any time point. There was no difference in patient satisfaction according to study group allocation.
Fig 4.
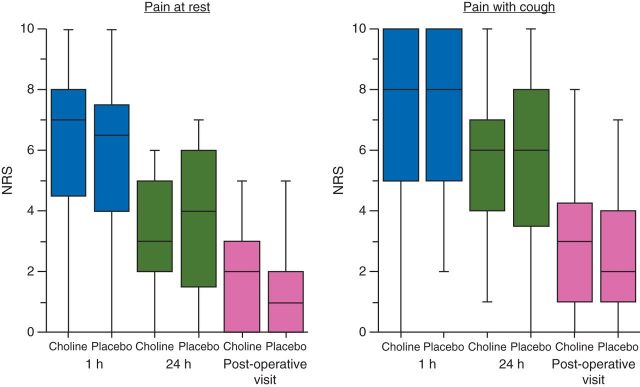
Postoperative pain response NRS scores (0–10) for pain at rest and at movement (simulated with a cough) in placebo and choline supplementation cohorts. Scores are self-reported and taken 1 h after surgery, 24 h after surgery, and at the postoperative visit (∼1 week after surgery).
Two of 29 subjects treated with choline had a wound complication at 24 h, both with tissue separation, erythema, and serous exudate. One was treated with a wound vacuum. In the placebo group, six of the 28 subjects had wound complications, including tissue separation erythema and serous exudate. All complications had resolved by the postoperative visit at 2 weeks. There was no difference in the incidence of wound complications at 24 h or at the postoperative visit between groups (P=0.14).
Discussion
Oral administration of a choline-rich supplement in the perioperative period resulted in a small but significant increase in plasma choline concentration rather than the expected decrease after surgery.29,30 However, increased choline concentration was not correlated with lower plasma TNF concentration or lower pain scores at any time after surgery. These results taken together suggest that perioperative oral supplementation with choline would not be an effective strategy to reduce pain and opioid requirements in the perioperative period. The relatively small increase in plasma choline concentration in response to two large oral doses is surprising, given the results of prior pharmacokinetic studies. Choline supplementation has been studied for a variety of indications and has been given as a diet of choline-enriched foods, choline chloride, and as lecithin enriched in phosphatidyl choline.24,31–34 The largest sustained increases in plasma choline were produced with phosphatidyl choline supplementation.24,35 In general, patients tolerate about 25 g of oral lecithin without side-effects, while higher doses may be associated with reduced appetite, vomiting, and diarrhoea.36 We chose as our dose that which we thought would be the maximum tolerated oral dose. Perioperative dosing might not be as efficient as that achievable under controlled conditions in a metabolic unit; however, our pharmacokinetic analysis suggested that despite the potential effects of surgery on bowel function, we achieved nearly 100% oral bioavailability. The half-life of absorption was slow, while the half-life of elimination (likely redistribution) was relatively rapid leading to only a small increase in plasma drug concentration. It is possible that the perioperarive state resulted in slowed absorption that could not keep up with redistribution/elimination. It would likely be more efficient to increase choline in this setting through i.v. administration that would be more easily controlled.
Given the very small increase in plasma choline associated with study drug administration, we can conclude either that this increase was too small to cause the decrease in plasma TNF response that was found in pre-clinical studies, by ourselves and others, or that there is a species difference. Given that the small increase in choline concentration did not induce a reduction in TNF concentration (as a prototypical inflammatory mediator affected by α7 nicotinic activation), it is not surprising that there was no difference in pain outcomes.
Declaration of interest
None declared.
Funding
This work was supported by the United States National Institute of Alternative Medicine Grant—AT004708 (P.F.).
References
- 1.Owen H, McMillan V, Rogowski D. Postoperative pain therapy: a survey of patients' expectations and their experiences. Pain. 1990;41:303–7. doi: 10.1016/0304-3959(90)90007-Z. [DOI] [PubMed] [Google Scholar]
- 2.Svensson I, Sjostrom B, Haljamae H. Assessment of pain experiences after elective surgery. J Pain Symptom Manage. 2000;20:193–201. doi: 10.1016/s0885-3924(00)00174-3. [DOI] [PubMed] [Google Scholar]
- 3.Thomas T, Robinson C, Champion D, et al. Prediction and assessment of the severity of post-operative pain and of satisfaction with management. Pain. 1998;75:177–85. doi: 10.1016/s0304-3959(97)00218-2. [DOI] [PubMed] [Google Scholar]
- 4.Stephens J, Laskin B, Pashos C, et al. The burden of acute postoperative pain and the potential role of the COX-2-specific inhibitors. Rheumatology (Oxford) 2003;42(Suppl. 3):iii40–52. doi: 10.1093/rheumatology/keg497. [DOI] [PubMed] [Google Scholar]
- 5.Dolin SJ, Cashman JN, Bland JM. Effectiveness of acute postoperative pain management: I. Evidence from published data. Br J Anaesth. 2002;89:409–23. [PubMed] [Google Scholar]
- 6.Hong D, Conell-Price J, Cheng S, et al. Transdermal nicotine patch for postoperative pain management: a pilot dose-ranging study. Anesth Analg. 2008;107:1005–10. doi: 10.1213/ane.0b013e318163204f. [DOI] [PubMed] [Google Scholar]
- 7.Hong D, Flood P, Diaz G. The side effects of morphine and hydromorphone patient-controlled analgesia. Anesth Analg. 2008;107:1384–9. doi: 10.1213/ane.0b013e3181823efb. [DOI] [PubMed] [Google Scholar]
- 8.Olson LC, Hong D, Conell-Price JS, et al. A transdermal nicotine patch is not effective for postoperative pain management in smokers: a pilot dose-ranging study. Anesth Analg. 2009;109:1987–91. doi: 10.1213/ANE.0b013e3181bd1612. [DOI] [PubMed] [Google Scholar]
- 9.Flood P, Daniel D. Pronociceptive actions of isoflurane: a protective role for estrogen. Anesthesiology. 2003;99:476–9. doi: 10.1097/00000542-200308000-00031. [DOI] [PubMed] [Google Scholar]
- 10.Benowitz NL. Nicotine and postoperative management of pain. Anesth Analg. 2008;107:739–41. doi: 10.1213/ane.0b013e3181813508. [DOI] [PubMed] [Google Scholar]
- 11.Yagoubian B, Akkara J, Afzali P, et al. Nicotine nasal spray as an adjuvant analgesic for third molar surgery. J Oral Maxillofac Surg. 2011;69:1316–9. doi: 10.1016/j.joms.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Habib AS, White WD, El Gasim MA, et al. Transdermal nicotine for analgesia after radical retropubic prostatectomy. Anesth Analg. 2008;107:999–1004. doi: 10.1213/ane.0b013e31816f2616. [DOI] [PubMed] [Google Scholar]
- 13.Uteshev VV, Meyer EM, Papke RL. Regulation of neuronal function by choline and 4OH-GTS-21 through alpha 7 nicotinic receptors. J Neurophysiol. 2003;89:1797–806. doi: 10.1152/jn.00943.2002. [DOI] [PubMed] [Google Scholar]
- 14.Papke RL, Bencherif M, Lippiello P. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the alpha 7 subtype. Neurosci Lett. 1996;213:201–4. doi: 10.1016/0304-3940(96)12889-5. [DOI] [PubMed] [Google Scholar]
- 15.Vincler M, Wittenauer S, Parker R, et al. Molecular mechanism for analgesia involving specific antagonism of alpha9alpha10 nicotinic acetylcholine receptors. Proc Natl Acad Sci USA. 2006;103:17880–4. doi: 10.1073/pnas.0608715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, et al. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med. 2008;14:567–74. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagdas D, Sonat FA, Hamurtekin E, et al. The antihyperalgesic effect of cytidine-5′-diphosphate-choline in neuropathic and inflammatory pain models. Behav Pharmacol. 2011;22:589–98. doi: 10.1097/FBP.0b013e32834a1efb. [DOI] [PubMed] [Google Scholar]
- 18.Gurun MS, Parker R, Eisenach JC, et al. The effect of peripherally administered CDP-choline in an acute inflammatory pain model: the role of alpha7 nicotinic acetylcholine receptor. Anesth Analg. 2009;108:1680–7. doi: 10.1213/ane.0b013e31819dcd08. [DOI] [PubMed] [Google Scholar]
- 19.Hamurtekin E, Bagdas D, Gurun MS. Possible involvement of supraspinal opioid and GABA receptors in CDP-choline-induced antinociception in acute pain models in rats. Neurosci Lett. 2007;420:116–21. doi: 10.1016/j.neulet.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 20.Hamurtekin E, Gurun MS. The antinociceptive effects of centrally administered CDP-choline on acute pain models in rats: the involvement of cholinergic system. Brain Res. 2006;1117:92–100. doi: 10.1016/j.brainres.2006.07.118. [DOI] [PubMed] [Google Scholar]
- 21.Kamei J, Ohsawa M, Miyata S, et al. Effects of cytidine 5′-diphosphocholine (CDP-choline) on the thermal nociceptive threshold in streptozotocin-induced diabetic mice. Eur J Pharmacol. 2008;598:32–6. doi: 10.1016/j.ejphar.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Loram LC, Harrison JA, Chao L, et al. Intrathecal injection of an alpha seven nicotinic acetylcholine receptor agonist attenuates gp120-induced mechanical allodynia and spinal pro-inflammatory cytokine profiles in rats. Brain Behav Immun. 2010;24:959–67. doi: 10.1016/j.bbi.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowley TJ, McKinstry A, Greenidge E, et al. Antinociceptive and anti-inflammatory effects of choline in a mouse model of postoperative pain. Br J Anaesth. 2010;105:201–7. doi: 10.1093/bja/aeq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeisel SH, Growdon JH, Wurtman RJ, et al. Normal plasma choline responses to ingested lecithin. Neurology. 1980;30:1226–9. doi: 10.1212/wnl.30.11.1226. [DOI] [PubMed] [Google Scholar]
- 25.Holm PI, Ueland PM, Kvalheim G, et al. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin Chem. 2003;49:286–94. doi: 10.1373/49.2.286. [DOI] [PubMed] [Google Scholar]
- 26.Sloan RP, Shapiro PA, Demeersman RE, et al. Aerobic exercise attenuates inducible TNF production in humans. J Appl Physiol. 2007;103:1007–11. doi: 10.1152/japplphysiol.00147.2007. [DOI] [PubMed] [Google Scholar]
- 27.Wilson AP, Treasure T, Sturridge MF, et al. A scoring method (ASEPSIS) for postoperative wound infections for use in clinical trials of antibiotic prophylaxis. Lancet. 1986;1:311–3. doi: 10.1016/s0140-6736(86)90838-x. [DOI] [PubMed] [Google Scholar]
- 28.Sinatra RS, Jahr JS, Reynolds LW, et al. Efficacy and safety of single and repeated administration of 1 gram intravenous acetaminophen injection (paracetamol) for pain management after major orthopedic surgery. Anesthesiology. 2005;102:822–31. doi: 10.1097/00000542-200504000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Ozarda Ilçöl Y, Ozyurt G, Kilicturgay S, et al. The decline in serum choline concentration in humans during and after surgery is associated with the elevation of cortisol, adrenocorticotropic hormone, prolactin and beta-endorphin concentrations. Neurosci Lett. 2002;324:41–4. doi: 10.1016/s0304-3940(02)00171-4. [DOI] [PubMed] [Google Scholar]
- 30.Ilcol YO, Uncu G, Goren S, et al. Declines in serum free and bound choline concentrations in humans after three different types of major surgery. Clin Chem Lab Med. 2004;42:1390–5. doi: 10.1515/CCLM.2004.259. [DOI] [PubMed] [Google Scholar]
- 31.Buchman AL, Awal M, Jenden D, et al. The effect of lecithin supplementation on plasma choline concentrations during a marathon. J Am Coll Nutr. 2000;19:768–70. doi: 10.1080/07315724.2000.10718076. [DOI] [PubMed] [Google Scholar]
- 32.Stoll AL, Renshaw PF, De Micheli E, et al. Choline ingestion increases the resonance of choline-containing compounds in human brain: an in vivo proton magnetic resonance study. Biol Psychiatry. 1995;37:170–4. doi: 10.1016/0006-3223(94)00120-R. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch MJ, Growdon JH, Wurtman RJ. Relations between dietary choline or lecithin intake, serum choline levels, and various metabolic indices. Metabolism. 1978;27:953–60. doi: 10.1016/0026-0495(78)90139-7. [DOI] [PubMed] [Google Scholar]
- 34.Wurtman RJ, Hirsch MJ, Growdon JH. Lecithin consumption raises serum-free-choline levels. Lancet. 1977;2:68–9. doi: 10.1016/s0140-6736(77)90067-8. [DOI] [PubMed] [Google Scholar]
- 35.Little A, Levy R, Chuaqui-Kidd P, et al. A double-blind, placebo controlled trial of high-dose lecithin in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1985;48:736–42. doi: 10.1136/jnnp.48.8.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Etienne P, Gauthier S, Johnson G, et al. Clinical effects of choline in Alzheimer's disease. Lancet. 1978;1:508–9. doi: 10.1016/s0140-6736(78)90180-0. [DOI] [PubMed] [Google Scholar]