Abstract
Background
We investigated the safety and efficacy of bevacizumab combined with gemcitabine followed by infusional 5-fluorouracil (5-FU) in patients with advanced pancreas cancer (APCA).
Design
Patients with untreated APCA received bevacizumab 10 mg/kg, gemcitabine 1000 mg/m2 over 100 min, and 5-FU 2400 mg/m2 over 48 h on days 1 and 15 of each 28-day cycle. The primary end point was the proportion of patients with progression-free survival (PFS) at 6 months from initiation of therapy. If PFS at 6 months was ≥41%, the regimen would be considered promising.
Results
Forty-two patients were enrolled in the study; of which, 39 were evaluable for primary end point. PFS at 6 months was 49% (95% CI 34% to 64%). Median PFS was 5.9 months (95% CI 3.5 to 8.1) and median overall survival (OS) was 7.4 months (95% CI 4.7 to 11.2). Partial response and stable disease occurred in 30% and 45% of patients, respectively. Treatment-related hypertension and normal baseline albumin correlated with an improved response rate, PFS and OS. Grade 3 to 4 toxicities included fatigue (14%), hypertension (5%), and venous thrombosis (5%).
Conclusions
The study met its primary end point. Further investigation of anti-VEGF therapy in combination with fluoropyrimidine-based therapy is warranted in APCA. Treatment-related hypertension and normal baseline albumin may predict for the efficacy of bevacizumab and should be investigated in prospective studies.
Keywords: bevacizumab, 5-fluorouracil, gemcitabine, pancreas cancer, VEGF
introduction
Pancreas cancer (PCA) is the fourth leading cause of cancer death in the United States [1]. Prognosis remains dismal, with a 5-year survival of <5% for all stages [2]. Surgical resection followed by adjuvant therapy offers the only chance for cure; however, <15% of patients present with resectable disease [3]. Cytotoxic chemotherapy with gemcitabine has been the standard of care and the backbone of experimental regimens in advanced pancreas cancer (APCA) for over a decade based on a modest clinical benefit over bolus 5-fluorouracil (5-FU) [4]. Since the late 1990s, minimal progress has been made to improve survival for these patients with gemcitabine-based combination regimens [5–12]. Recently published phase III data show a significant survival benefit for the combination of 5-FU, oxaliplatin, and irinotecan (FOLFIRINOX) over gemcitabine in patients with untreated metastatic pancreas cancer [13]; however, because of the significant toxicity associated with FOLFIRINOX, the regimen is only appropriate for a select subset of patients. The chemoresistance of pancreas cancer has led to a continuing search for new therapeutic targets.
Vascular endothelial growth factor (VEGF) is a pro-angiogenic growth factor implicated in the pathogenesis of many cancers [14, 15]. In PCA, VEGF promotes tumor growth, invasion, and metastases via activation of the MAPK pathway [16], and also functions as an autocrine growth factor for PCA cells [17, 18]. Overexpression of VEGF and its receptors occurs in >90% of PCA and correlates with poor prognosis [19–23]. Preclinical data suggest that inhibition of VEGF attenuates PCA growth and metastasis [24–26]. Thus, VEGF represents an attractive therapeutic target in human PCA.
Bevacizumab (Avastin®, Roche/Genentech, Inc., South San Francisco, USA) is a recombinant humanized monoclonal antibody that binds VEGF-A, blocking its interaction with its receptors. Bevacizumab improves outcomes in combination with chemotherapy in a number of advanced malignancies [27–32], however, its role in APCA remains controversial, and current recommendations for its use do not extend outside the investigational setting [33]. Preclinical data and promising results from early clinical studies [24, 25, 34–36] suggested investigation of antiangiogenic therapies in APCA. While phase III studies adding bevacizumab to gemcitabine [37] or gemcitabine and erlotinib [38] failed to confirm an overall survival (OS) benefit in APCA, bevacizumab improved PFS when added to gemcitabine and erlotinib [38]. Correlative work from this trial [39] suggested that pretreatment plasma levels of VEGFA and VEGFR2 may emerge as important predictive biomarkers to identify patients who are most likely to benefit from antiangiogenic therapy. These data suggest that VEGF may remain a valid target in appropriately selected patients with APCA.
Preliminary clinical evidence suggested that gemcitabine given at a fixed dose rate (FDR) of 10 mg/m2/min had an advantage over standard gemcitabine in patients with APCA [40]. The results of a follow-up three-arm phase III trial reported only a trend toward a survival benefit for FDR gemcitabine over standard gemcitabine, however that study was possibly underpowered [11]. 5-FU has single-agent activity in PCA [41] and phase III data suggest that 5-FU and gemcitabine are equivalent in the adjuvant setting [42]. Of greater interest, gemcitabine has been shown to act as a potential biomodulator of 5-FU activity [43]. Preclinical studies confirm that the sequential administration of prolonged infusion of gemcitabine followed by 5-FU exposure for 24 h results in significant synergistic antitumor activity in cancer cell lines [44]. In early clinical trials of patients with advanced gastrointestinal and genitourinary cancers [45, 46], the combination of prolonged-infusion gemcitabine followed by a fluoropyrimidine showed promising clinical activity. Finally, 5-FU has known clinical synergy with bevacizumab in colon cancer [29–31, 48] and administration of 5-FU as a continuous infusion improves survival and response rate over bolus administration [48].
Based on these observations, we designed a phase II study to investigate the efficacy and safety of the combination of bevacizumab combined with FDR gemcitabine followed by infusional 5-FU over 48 h in patients with untreated APCA.
patients and methods
patient eligibility
Eligible patients were required to have biopsy-proven stage III or IV pancreatic adenocarcinoma with measurable disease by RECIST 1.0 [50], Eastern Cooperative Oncology Group (ECOG) performance status 0–1, no prior treatment for metastatic disease, and adequate bone marrow (neutrophils >1500/μl, hemoglobin >9 g/dl, platelets >100 000/μl), kidney [creatinine < 1.5 × upper limit of normal (ULN)], and liver (bilirubin ≤ ULN, AST/ALT ≤ 1.5 × ULN or ≤ 3 × ULN with liver metastases) function. Prior adjuvant chemotherapy (including gemcitabine) was allowed, provided that >4 weeks had elapsed since the end of therapy. Exclusion criteria included prior treatment with VEGF inhibitors, brain metastases, congestive heart failure requiring active therapy, myocardial infarction or stroke within the past 6 months, bleeding diathesis, uncontrolled hypertension or diabetes mellitus, and proteinuria. All patients provided written informed consent before study enrollment.
treatment plan
This was a multicenter phase II study including the Ohio State University and University of Michigan. Patients received bevacizumab followed by gemcitabine then 5-FU on days 1 and 15 of each 28-day cycle. Bevacizumab was administered intravenously at a dose of 10 mg/kg over 30 min. Gemcitabine was administered intravenously at a dose of 1000 mg/m2 over 100 min (FDR, 10 mg/m2/min). 5-FU was given as a continuous 48-h intravenous infusion at a dose of 2400 mg/m2. Treatment was continued until disease progression, intolerable toxicity, intercurrent illness or death preventing further treatment, or patient withdrawal of consent.
dose delays and modifications
Patients were required to have neutrophils ≥1500/μl, platelets ≥100 000/μl, and all other treatment-related toxicity resolved to grade ≤1 in order to begin a treatment cycle. Dose reductions of gemcitabine were corrected to preserve the FDR. Gemcitabine was reduced to 75% of the original dose for grade 3 or 4 thrombocytopenia, febrile neutropenia (grade 4 neutropenia and ≥grade 2 fever), and grade 3 or 4 nonhematologic toxicity, excluding nausea and vomiting controlled with supportive measures. 5-FU was reduced to 75% of the original dose for febrile neutropenia, grade 3 palmar-plantar erythrodysesthesia (hand and foot syndrome), and any grade 3 or 4 nonhematologic toxicity, excluding nausea and vomiting controlled with supportive measures. There were no recommended dose reductions for bevacizumab. If adverse events occurred that required bevacizumab to be held, the dose remained the same once treatment resumed. Bevacizumab was discontinued and patients were removed from study for grade IV hypertension, venous thrombosis, or hemorrhage, arterial thrombosis of any grade, gastrointestinal perforation, wound dehiscence requiring medical or surgical intervention, recurrent toxicities despite dose modifications, or any toxicity felt by the investigator to prohibit safe continuation of therapy.
assessment of toxicity and response
Adverse events were graded according to the NCI-CTCAE v 3.0. Pretreatment assessment included baseline history and physical, complete blood count, serum chemistry including liver functions, urine protein/creatinine ratio, serum βhCG, EKG, and CA 19-9 level. These assessments (excluding EKG) were repeated on day 1 of each subsequent cycle along with toxicity assessment. On day 15 of every cycle, patients had physical examination, toxicity assessment, complete blood count, and serum chemistries. Radiographic assessment of response was carried out at baseline and every 8 weeks using the same imaging modality [computed tomography (CT) or magnetic resonance imaging (MRI)] used to establish baseline tumor measurements. Responses were measured according to RECIST 1.0 [49]. Hypertension was assessed by blood pressure measurement on days 1 and 15 of each cycle. Baseline albumin was measured before initiation of treatment, and subsequent measurements were obtained on days 1 and 15 of each treatment cycle.
statistical methods
The primary end point was the percentage of patients free from disease progression or death at 6 months (24 weeks) from initiation of therapy. Secondary endpoints include overall response rate (ORR) as defined by RECIST 1.0 [49], 6-month and 1-year survival rates, OS, and the frequency and severity of treatment-associated adverse events. Using a Fleming single-stage phase II study design, we planned to enroll 39 evaluable patients to determine whether the true 24-week PFS rate was 0.30 or less versus 0.50 or more [α = 0.10; β = 0.10]. Patients were considered nonevaluable for the primary end point if they died from nontreatment related or nondisease related cause before the 6-month assessment period, or if they were removed from the study for treatment-related toxicity with <6 months of follow-up. If at least 16 evaluable patients (41%) were progression-free at 6 months, the regimen will be recommended for further study. Post hoc subgroup analyses included patients with and without treatment-related hypertension of any CTCAE grade, and patients with normal (≥3.4 g/dl) and low (<3.4 g/dl) baseline albumin. OS, PFS, and ORR were compared between the subgroups. Survival curves were estimated using the Kaplan–Meier method, and 95% confidence intervals for the medians were provided. The group difference in survival was assessed with the log-rank test. Response rates were compared using Fisher's exact test. For all but the primary endpoints, data were analyzed based on the intention-to-treat principle.
results
patient characteristics (Table 1)
Table 1.
Patient characteristics (N = 42)
| Characteristic | N (%) |
|---|---|
| Sex | |
| Male | 19 (45) |
| Female | 23 (55) |
| Age (years) | |
| Median | 60 |
| Range | 36 to 79 |
| Race/ethnicity | |
| Caucasian | 37 (88) |
| African American | 3 (7) |
| Other | 2 (5) |
| ECOG performance status | |
| 0 | 15 (36) |
| 1 | 27 (64) |
| Prior adjuvant therapy | 2 (5) |
| Gemcitabine-baseda | 1 (50) |
| Chemoradiationb | 1 (50) |
| Disease stage | |
| III | 2 (5) |
| IV | 40 (95) |
| Site of metastasis (N = 40) | |
| Liver only | 29 (73) |
| Liver + other | 2 (5) |
| Other only | 9 (23) |
| CA19-9 | |
| Normal (≤37 U/ml) | 6 (14) |
| Elevated (>37 U/ml) | 36 (86) |
| Albumin | |
| Normal (≥3.4 g/dl) | 28 (67) |
| Low (<3.4 g/dl) | 14 (33) |
aPatients developed recurrence/metastases while on a clinical trial of gemcitabine ± Saccharomyces cerevisiae vaccine.
bPatients were treated on a clinical trial of 5-FU, cisplatin, interferon-α, and radiation.
Patient characteristics are detailed in Table 1. Forty-two patients (23 F, 19 M) with a median age 60 (range 36 to 79) and ECOG performance status of zero or one were enrolled between January 2007 and October 2008. Two patients (5%) had stage III disease and 40 patients (95%) had stage IV disease. The most common site of metastatic disease was the liver (75%). Two patients had recurrent metastatic disease after prior surgical resection and adjuvant therapy. Most patients (86%) had elevated baseline CA19-9 levels (>37 U/ml). Sixty-seven percent of patients had normal baseline albumin (≥3.4 g/dl) and 33% of patients had low albumin (<3.4 g/dl) before initiation of treatment.
Of the 42 patients enrolled, 39 were evaluable for the primary end point. Two patients were removed as pre-specified from study, before reaching the 6-month assessment point, due to treatment-related toxicity. Of note, both these patients had stable disease and CA19-9 declines of >25% at the time of removal from study. The third patient was removed from study due to noncompliance unrelated to toxicity. Forty patients were assessable for response. All 42 patients were evaluable for survival and toxicity analyses.
toxicity (Table 2)
Table 2.
Toxic effects observed according to the National Cancer Institute Common Toxicity Criteria Version 3.0a (N = 42)
| Grade 1, N (%) | Grade 2, N (%) | Grade 3, N (%) | Grade IV, N (%) | |
|---|---|---|---|---|
| Hematologic | ||||
| Anemia | 19 (45) | 8 (19) | 0 (0) | 1 (2) |
| Thrombocytopenia | 11 (26) | 1 (2) | 1 (2) | 0 (0) |
| Leukopenia | 8 (19) | 3 (7) | 0 (0) | 0 (0) |
| Neutropenia | 4 (10) | 2 (5) | 0 (0) | 0 (0) |
| Lymphopenia | 4 (10) | 2 (5) | 2 (5) | 0 (0) |
| Non-hematologic | ||||
| Fatigue | 6 (14) | 14 (33) | 6 (14) | 0 (0) |
| Vomiting | 23 (55) | 8 (19) | 2 (5) | 0 (0) |
| Nausea | 19 (45) | 5 (12) | 1 (2) | 0 (0) |
| Diarrhea | 13 (33) | 3 (7) | 0 (0) | 0 (0) |
| Elevated ALT | 5 (12) | 1 (2) | 1 (2) | 0 (0) |
| Elevated AST | 6 (14) | 0 (0) | 0 (0) | 0 (0) |
| Mucositis | 6 (14) | 3 (7) | 0 (0) | 0 (0) |
| Altered sense of taste | 11 (26) | 1 (2) | 0 (0) | 0 (0) |
| Hypertension | 5 (12) | 1 (2) | 2 (5) | 0 (0) |
| Fistula formation | 0 (0) | 0 (0) | 1 (2) | 0 (0) |
| Proteinuria | 3 (7) | 1 (2) | 0 (0) | 0 (0) |
| Bleeding | 4 (10) | 2 (5) | 1 (2) | 0 (0) |
| Thrombosis | 0 (0) | 0 (0) | 2 (5) | 0 (0) |
| Headache | 5 (12) | 0 (0) | 0 (0) | 0 (0) |
| Rash | 2 (5) | 3 (7) | 0 (0) | 0 (0) |
| Peripheral sensory neuropathy | 5 (12) | 1 (2) | 0 (0) | 0 (0) |
aMaximum grade per patient.
Toxic effects are outlined in Table 2. The most frequent treatment-related toxicities were vomiting (69%), anemia (66%), fatigue (61%), and nausea (59%). Chemotherapy-related grade 3–4 toxicities were uncommon and included fatigue (14%), vomiting (5%), lymphopenia (5%), nausea (2%), anemia (2%), thrombocytopenia (2%), and ALT elevation (2%). Grade 3 toxicities attributed to bevacizumab were rare and included venous thrombotic events (5%), hypertension (5%), epistaxis (2%), and fistula formation (2%). Only one patient experienced grade 4 toxicity (anemia) and there were no treatment-related deaths. Hypertension was observed in eight patients (19%). Fifteen patients (38%) required treatment delay or dose reduction. Bevacizumab was discontinued in one patient due to progressive renal insufficiency and treatment-related grade 2 proteinuria (urine protein:creatinine ratio 3.4) in the setting of underlying chronic renal insufficiency. Three patients were removed from the study as pre-specified due to treatment-related toxicity including grade 3 venous thrombosis (n = 2) and persistent grade 2 thrombocytopenia (n = 1).
efficacy (Table 3 and Figure 1A)
Table 3.
Treatment efficacy
| Endpoint | N (%) |
|---|---|
| PFS at 6 monthsa | 19 (49) (95% CI 34 to 64) |
| Responseb | |
| CR | 0 (0) |
| PR | 12 (30) |
| SD | 18 (45) |
| PD | 10 (25) |
| Albuminc ≥3.4 g/dl | 10 (36)d |
| Albumin <3.4 g/dl | 2 (17)e |
| P-value | 0.2848 |
| Hypertensionf | 5 (63)g |
| No hypertension | 7 (22)h |
| P-value | 0.0386 |
| CA 19-9 maximum reductioni | |
| >25% | 23 (59) |
| >50% | 21 (54) |
| Median PFS (months)j | 5.9 (95% CI 3.5–8.1) |
| Albumine ≥3.4 g/dl | 7.7 |
| Albumin <3.4 g/dl | 2.7 |
| P-value | 0.0124 |
| Hypertensionf | 7.6 |
| No hypertension | 4.9 |
| P-value | 0.38 |
| Median OS (months)j | 7.4 (95% CI 4.7–11.2) |
| Albumine ≥3.4 g/dl | 11.7 |
| Albumin <3.4 g/dl | 3.2 |
| P-value | 0.0017 |
| Hypertensionf | 12 |
| No hypertension | 6 |
| P-value | 0.0166 |
| 6-month survivalj | 60% |
| 1-year survivalj | 36% |
a39 patients were considered evaluable.
b40 patients were considered evaluable.
cBaseline albumin before treatment.
dObjective response rate based on 28 evaluable patients with albumin ≥3.4 g/dl.
eObjective response rate based on 12 patients with albumin <3.4 g/dl.
fGrade ≥1 treatment-related hypertension as defined and graded according to CTCAE v 3.0.
gObjective response rate based on eight evaluable patients with hypertension.
hObjective response rate based on 32 evaluable patients without hypertension.
i36 patients (86%) had baseline elevated CA 19-9.
j42 patients were considered evaluable.
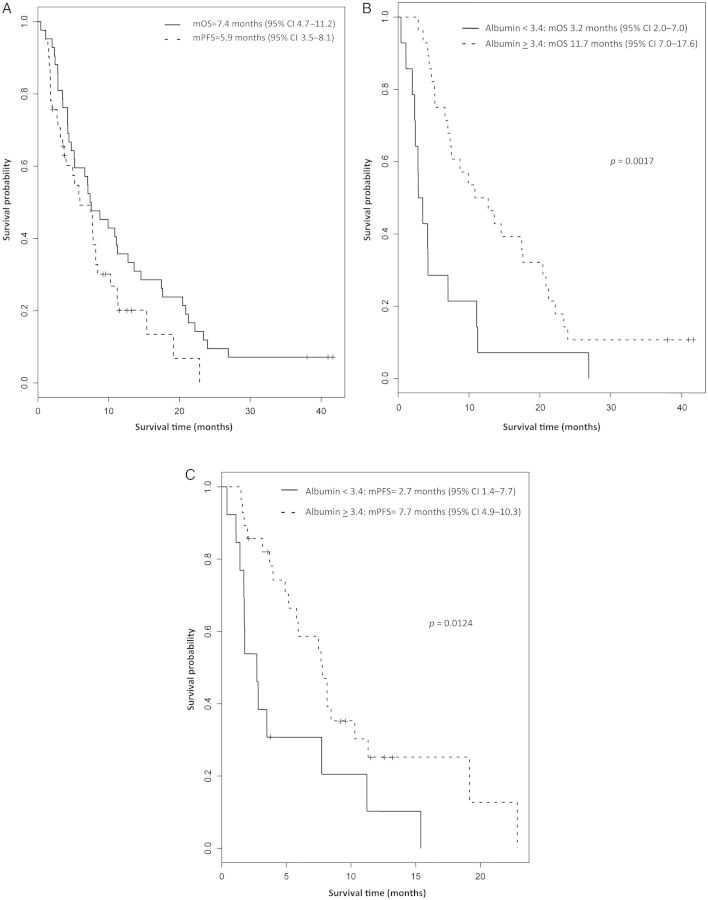
Figure 1.
(A) Kaplan–Meier estimation of overall survival (OS) and progression-free survival (PFS). (B) Kaplan–Meier estimation of OS by pretreatment albumin of >3.4 versus <3.4 g/dl. (C). Kaplan–Meier estimation of PFS by pretreatment albumin of >3.4 versus <3.4 g/dl. mOS, median OS ; mPFS, median PFS.
Treatment efficacy data are summarized in Table 3. Final data analysis is conducted 4 years from enrollment of the first patient. At the time of the final analysis, 3 patients are still alive and 39 patients have died. Freedom from progression is demonstrated in 19 of 39 patients (49%) at 24 weeks (95% CI 34–64%); therefore, the primary end point of the study is met. Partial response (PR) and stable disease (SD) are seen in 30% and 45% of patients, respectively, with a disease control rate (PR + SD) of 75%. Kaplan–Meier survival curves are provided in Figure 1. Median PFS is 5.9 months (95% CI 3.5 to 8.1) and median OS is 7.4 months (95% CI 4.7 to 11.2). Six-month and 1-year survival were 60 and 36%, respectively. Thirty-six patients (86%) had elevated CA 19-9 levels before therapy. Fifty-nine percent of those patients experienced >25% maximum reduction of CA 19-9 levels, and 54% experienced >50% reduction.
potential predictors of clinical outcome (Table 3 and Figure 1B and C)
Data regarding the relationship between hypertension (N = 8), baseline albumin levels and clinical outcomes (ORR, PFS, and OS) are presented in Table 3. Patients with treatment-related hypertension had significantly improved ORR (63 versus 22%; P = 0.0386), and mOS (12 versus 6.1 months, P = 0.0166) and improved PFS (7.6 versus 4.9 months, P = 0.38) that did not reach statistical significance, compared with patients who did not experience treatment-related hypertension. Patients with normal baseline albumin (≥3.4 g/dl, N = 28) at study entry had trend toward improvement in ORR (36 versus 17%, P = 0.2848), and significantly prolonged mPFS (7.7 versus 2.7 months, P = 0.0124) and mOS (11.7 versus 3.2 months, P = 0.0017) compared with patients with low baseline albumin (<3.4 g/dl, N = 14) (Figure 1B and C).
discussion
The prognosis of PCA remains poor with little progress made in the last few decades. In the last decade, phase III studies of gemcitabine in combination with other cytotoxics have yielded no improvement in survival [5–9, 11, 50–53]. This has led to investigation of biologic targets including anti-VEGF therapy.
We evaluated a rational combination of bevacizumab combined with FDR gemcitabine followed by infusional 5-FU in PCA. The choice of the combination and its schedule focuses on the biomodulation of infusional 5-FU by a prolonged infusion of gemcitabine and the known synergism between 5-FU and bevacizumab [29–31, 47]. In our study, we observed interesting clinical efficacy and reached the primary study end point with 49% of patients being free of disease progression at 24 weeks. The combination was well tolerated with expected and manageable toxicity. Our observed objective response rate, 1-year survival, OS and progression-free survival (PFS) are interesting compared with historical controls in advanced PCA [5–12, 36–38, 50–55]. Confirming the observed interesting activity is the finding that 59% of patients experienced >25% improvement in CA19-9 levels which has been shown to correlate with a favorable outcome [56–59]. These interesting findings argue for continued investigation of antiangiogenic therapies in PCA.
The choice of a chemotherapeutic backbone may impact the efficacy of antiangiogenic therapy in PCA. In a preclinical study, the antitumor activity of paclitaxel and fluoropyrimidines but not that of gemcitabine caused the release of bone marrow derived circulating endothelial progenitor cells (CEPs) and Tie-2 expressing monocytes (TEMs) as well as the induction of pro-angiogenic growth factors. Anti-angiogenic agents inhibit the CEP and TEM mobilization and proangiogenic signaling and thus enhance significantly the antitumor activity of paclitaxel and fluoropyrimidines but not that of gemcitabine [60]. Additionally, gemcitabine-induced myelosuppression in patients with PCA was found to interfere with the mobilization of proangiogenic cell types targeted by bevacizumab and may further counteract antiangiogenic therapy by substantially reducing the angiogenesis inhibitor TSP-1 [61]. These findings may explain why gemcitabine does not elicit TEM and CEP recruitment and may therefore lack synergy with bevacizumab. This phenomenon is not known to occur with fluoropyrimidines. Clinically, this is reinforced by the fact that in addition to our study, the only other study with a combination of gemcitabine plus bevacizumab in PCA to reach its primary end point included a fluoropyrimidine (Table 4) [54].
Table 4.
Summary of published phase II and III trials of gemcitabine + antiangiogenic therapy in advanced pancreas cancer
| First author | Year | Phase | N | Setting | Investigational therapya | Primary endpoint | P-value | mOS (months) | mPFS (months) | ORR |
|---|---|---|---|---|---|---|---|---|---|---|
| Kindler [36] | 2005 | II | 52 | 1st line | Gemcitabine + bevacizumab | ORR | __ | 8.8 | 5.4 | 21% |
| Spano [62] | 2008 | II | 103 | 1st line | A: Gemcitabine + axitinib B: Gemcitabine | OS | __ | A: 6.9 B: 5.6 | A: 4.2 B: 3.7 | A: 7% B: 3% |
| Javle [55] | 2009 | II | 50 | 1st line | Gemcitabine + capecitabine + bevacizumab | PFS | __ | 9.8 | 5.8 | 22% |
| Van Cutsem [38] | 2009 | III | 607 | 1st line | A: Gemcitabine + bevacizumab + erlotinib B: Gemcitabine + erlotinib + placebo | OS | 0.21 | A: 7.1 B: 6.0 | A: 4.6 B: 3.6 | A:13.5% B: 8.6% |
| Kindler [37] | 2010 | III | 602 | 1st line | A: Gemcitabine + bevacizumab B: Gemcitabine + placebo | OS | 0.95 | A: 5.8 B: 5.9 | A: 3.8 B: 2.9 | A: 13% B: 10% |
| Kindler [55] | 2011 | III | 632 | 1st line | A: Gemcitabine + axitinib B: Gemcitabine + placebo | OS | 0.54 | A: 8.5 B: 8.3 | A: 4.4 B: 4.4 | A: 5% B: 2% |
| Martinb | 2011 | II | 42 | 1st line | FDR gemcitabine + infusional 5-FU + bevacizumab | 6-month PFS | __ | 7.4 | 5.9 | 30% |
mOS, median overall survival, mPFS, median progression free survival; ORR, objective response rate (complete + partial response); ORR, objective response rate (complete + partial response); FDR, fixed dose rate.
aGemcitabine, standard 30 min infusion unless otherwise specified.
bCurrent study.
Several published phase II and III studies investigated antiangiogenic therapy in combination with gemcitabine in PCA [(36–38, 54, 55, 62), Table 4]. All three phase III studies failed to reach their primary end point of OS. However, in the AViTA trial, there was evidence of significant improvements in PFS with the addition of bevacizumab to gemcitabine and erlotinib [38]. More recently, correlative analyses from this trial revealed improved outcomes in bevacizumab-treated patients with baseline elevated plasma levels of VEGFA (OS and PFS) and VEGFR2 (OS) [39] supporting a continued interest in VEGF as a valid therapeutic target in a subset of patients in PCA.
It has also been proposed that hypertension can be used as a pharmacodynamic biomarker for the efficacy of VEGF signaling inhibition [63, 64]. An association between bevacizumab-related hypertension and improved efficacy has been observed in multiple studies with bevacizumab [65] or axitinib [62] in PCA and bevacizumab in other malignancies [66–72]. We confirmed that treatment-related hypertension may be a useful biomarker that predicts for favorable outcomes of antiangiogenic therapy in PCA. Further investigation is warranted to understand the utility of this finding in the clinical setting. For example, studies suggest a relationship between dose intensity of bevacizumab and hypertension [73, 74].
Previous pharmacokinetic studies of bevacizumab showed that a low baseline albumin results in a 15%–20% increased clearance of bevacizumab [75, 76] and patients with low albumin levels may be exposed to lower levels of bevacizumab, which potentially may lead to inferior clinical outcomes. We evaluated the role of pretreatment albumin levels as a potential predictive biomarker for the efficacy of bevacizumab in PCA and found a significant association between normal or above-normal levels and improved clinical outcomes. In the absence of a control arm, albumin levels may be prognostic and future controlled studies are needed to confirm a potential predictive role. Future controlled studies are needed to confirm a potential predictive role. If indeed predictive, pharmacokinetic dose adjustments of bevacizumab based on albumin levels may need to be studied further.
The interpretation of our study results is limited by the small number of patients and the lack of control arm. Our post hoc subgroup analyses are exploratory in nature and should be interpreted in this limited context.
In conclusion, the combination of bevacizumab with FDR gemcitabine followed by infusional 5-FU is safe and tolerable with promising activity in PCA. Our results suggest that angiogenesis remains a viable target in PCA, provided that antiangiogenic agents are paired with a rational chemotherapy backbone, such as a fluoropyrimidine-based regimen (including FOLFIRINOX), to maximize the potential for synergism. Future studies should also focus on identifying subsets of patients more likely to benefit from bevacizumab in PCA. Baseline plasma VEGFA/VEGFR2 and albumin levels may be important for appropriate patient selection for bevacizumab therapy. Treatment-related hypertension may predict for improved outcomes of bevacizumab therapy. These strategies deserve to be further investigated in randomized controlled clinical trials.
funding
This work was supported by funding from Genentech, Inc. and the Roche Group, grant number AVF3571.
disclosures
T.B.-S. has received consultant fees from Genentech. All other authors have declared no conflict of interest.
Supplementary Material
references
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Weir HK, Thun MJ, Hankey BF, et al. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95:1276–1299. doi: 10.1093/jnci/djg040. doi:10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- 3.Muller MW, Friess H, Koninger J, et al. Factors influencing survival after bypass procedures in patients with advanced pancreatic adenocarcinomas. Am J Surg. 2008;195:221–228. doi: 10.1016/j.amjsurg.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 4.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 5.Berlin JD, Catalano P, Thomas JP, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270–3275. doi: 10.1200/JCO.2002.11.149. doi:10.1200/JCO.2002.11.149. [DOI] [PubMed] [Google Scholar]
- 6.Colucci G, Giuliani F, Gebbia V, et al. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell'Italia Meridionale. Cancer. 2002;94:902–910. doi:10.1002/cncr.10323. [PubMed] [Google Scholar]
- 7.Colucci G, Labianca R, Di Costanzo F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol. 2010;28:1645–1651. doi: 10.1200/JCO.2009.25.4433. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513–5518. doi: 10.1200/JCO.2009.24.2446. [DOI] [PubMed] [Google Scholar]
- 9.Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946–3952. doi: 10.1200/JCO.2005.05.1490. [DOI] [PubMed] [Google Scholar]
- 10.Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605–3610. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poplin E, Feng Y, Berlin J, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:3778–3785. doi: 10.1200/JCO.2008.20.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 13.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog Horm Res. 2000;55:15–35. discussion 35–6. [PubMed] [Google Scholar]
- 15.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. doi:10.1210/er.18.1.4. [DOI] [PubMed] [Google Scholar]
- 16.Itakura J, Ishiwata T, Shen B, et al. Concomitant over-expression of vascular endothelial growth factor and its receptors in pancreatic cancer. Int J Cancer. 2000;85:27–34. doi: 10.1002/(sici)1097-0215(20000101)85:1<27::aid-ijc5>3.0.co;2-8. doi:10.1002/(SICI)1097-0215(20000101)85:1<27::AID-IJC5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Masood R, Cai J, Zheng T, et al. Vascular endothelial growth factor (VEGF) is an autocrine growth factor for VEGF receptor-positive human tumors. Blood. 2001;98:1904–1913. doi: 10.1182/blood.v98.6.1904. doi:10.1182/blood.V98.6.1904. [DOI] [PubMed] [Google Scholar]
- 18.von Marschall Z, Cramer T, Hocker M, et al. De novo expression of vascular endothelial growth factor in human pancreatic cancer: evidence for an autocrine mitogenic loop. Gastroenterology. 2000;119:1358–1372. doi: 10.1053/gast.2000.19578. doi:10.1053/gast.2000.19578. [DOI] [PubMed] [Google Scholar]
- 19.Itakura J, Ishiwata T, Friess H, et al. Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res. 1997;3:1309–1316. [PubMed] [Google Scholar]
- 20.Luo J, Guo P, Matsuda K, et al. Pancreatic cancer cell-derived vascular endothelial growth factor is biologically active in vitro and enhances tumorigenicity in vivo. Int J Cancer. 2001;92:361–369. doi: 10.1002/ijc.1202. doi:10.1002/ijc.1202. [DOI] [PubMed] [Google Scholar]
- 21.Kuwahara K, Sasaki T, Kuwada Y, et al. Expressions of angiogenic factors in pancreatic ductal carcinoma: a correlative study with clinicopathologic parameters and patient survival. Pancreas. 2003;26:344–349. doi: 10.1097/00006676-200305000-00006. doi:10.1097/00006676-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Karayiannakis AJ, Bolanaki H, Syrigos KN, et al. Serum vascular endothelial growth factor levels in pancreatic cancer patients correlate with advanced and metastatic disease and poor prognosis. Cancer Lett. 2003;194:119–124. doi: 10.1016/s0304-3835(03)00047-8. doi:10.1016/S0304-3835(03)00047-8. [DOI] [PubMed] [Google Scholar]
- 23.Seo Y, Baba H, Fukuda T, et al. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer. 2000;88:2239–2245. doi: 10.1002/(sici)1097-0142(20000515)88:10<2239::aid-cncr6>3.0.co;2-v. doi:10.1002/(SICI)1097-0142(20000515)88:10<2239::AID-CNCR6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 24.Solorzano CC, Baker CH, Bruns CJ, et al. Inhibition of growth and metastasis of human pancreatic cancer growing in nude mice by PTK 787/ZK222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases. Cancer Biother Radiopharm. 2001;16:359–370. doi: 10.1089/108497801753354267. [DOI] [PubMed] [Google Scholar]
- 25.Bruns CJ, Shrader M, Harbison MT, et al. Effect of the vascular endothelial growth factor receptor-2 antibody DC101 plus gemcitabine on growth, metastasis and angiogenesis of human pancreatic cancer growing orthotopically in nude mice. Int J Cancer. 2002;102:101–108. doi: 10.1002/ijc.10681. [DOI] [PubMed] [Google Scholar]
- 26.Buchler P, Reber HA, Ullrich A, et al. Pancreatic cancer growth is inhibited by blockade of VEGF-RII. Surgery. 2003;134:772–782. doi: 10.1016/S0039-6060(03)00296-4. doi:10.1016/S0039-6060(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 27.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 28.Sobrero A, Ackland S, Clarke S, et al. Phase IV study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) in first-line metastatic colorectal cancer. Oncology. 2009;77:113–119. doi: 10.1159/000229787. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs CS, Marshall J, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C study. J Clin Oncol. 2008;26:689–690. doi: 10.1200/JCO.2007.15.5390. [DOI] [PubMed] [Google Scholar]
- 30.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 31.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 32.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. doi:10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 33.Javle M, Hsueh CT. Updates in Gastrointestinal Oncology - insights from the 2008 44th annual meeting of the American Society of Clinical Oncology. J Hematol Oncol. 2009;2:9. doi: 10.1186/1756-8722-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Q, Le X, Abbruzzese JL, et al. Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res. 2001;61:4143–4154. doi: [PubMed] [Google Scholar]
- 35.Liu CD, Tilch L, Kwan D, et al. Vascular endothelial growth factor is increased in ascites from metastatic pancreatic cancer. J Surg Res. 2002;102:31–34. doi: 10.1006/jsre.2001.6307. [DOI] [PubMed] [Google Scholar]
- 36.Kindler HL, Friberg G, Singh DA, et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:8033–8040. doi: 10.1200/JCO.2005.01.9661. [DOI] [PubMed] [Google Scholar]
- 37.Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303) J Clin Oncol. 2010;28:3617–3622. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 39.Van Cutsem E, Jayson G, Dive C, et al. Analysis of Blood Plasma Factors in the AVITA Phase III Randomized Study of Bevacizumab (bev) with Gemcitabine-Erlotinib (GE) in Patients (pts) with Metastatic Pancreatic Cancer (mPC) Eur J Cancer. 2011;45:95. doi:10.1016/S0959-8049(11)70640-2. [Google Scholar]
- 40.Tempero M, Plunkett W, Ruiz Van Haperen V, et al. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402–3408. doi: 10.1200/JCO.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 41.Fung MC, Takayama S, Ishiguro H, et al. Chemotherapy for advanced or metastatic pancreatic cancer: analysis of 43 randomized trials in 3 decades (1974–2002) Gan To Kagaku Ryoho. 2003;30:1101–1111. [PubMed] [Google Scholar]
- 42.Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–1081. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 43.Berlin JD, Alberti DB, Arzoomanian RZ, et al. A phase I study of gemcitabine, 5-fluorouracil and leucovorin in patients with advanced, recurrent, and/or metastatic solid tumors. Invest New Drugs. 1998;16:325–330. doi: 10.1023/a:1006242005837. doi:10.1023/A:1006242005837. [DOI] [PubMed] [Google Scholar]
- 44.Ren Q, Kao V, Grem JL. Cytotoxicity and DNA fragmentation associated with sequential gemcitabine and 5-fluoro-2′-deoxyuridine in HT-29 colon cancer cells. Clin Cancer Res. 1998;4:2811–2818. [PubMed] [Google Scholar]
- 45.Madajewicz S, Hentschel P, Burns P, et al. Phase I chemotherapy study of biochemical modulation of folinic acid and fluorouracil by gemcitabine in patients with solid tumor malignancies. J Clin Oncol. 2000;18:3553–3557. doi: 10.1200/JCO.2000.18.20.3553. [DOI] [PubMed] [Google Scholar]
- 46.Attia S, Morgan-Meadows S, Holen KD, et al. Dose-escalation study of fixed-dose rate gemcitabine combined with capecitabine in advanced solid malignancies. Cancer Chemother Pharmacol. 2009;64:45–51. doi: 10.1007/s00280-008-0844-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005;23:3502–3508. doi: 10.1200/JCO.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 48.Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. Meta-analysis Group in Cancer. J Clin Oncol. 1998;16:301–308. doi: 10.1200/JCO.1998.16.1.301. [DOI] [PubMed] [Google Scholar]
- 49.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. doi:10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 50.Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25:2212–2217. doi: 10.1200/JCO.2006.09.0886. [DOI] [PubMed] [Google Scholar]
- 51.Oettle H, Richards D, Ramanathan RK, et al. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol. 2005;16:1639–1645. doi: 10.1093/annonc/mdi309. [DOI] [PubMed] [Google Scholar]
- 52.Stathopoulos GP, Syrigos K, Aravantinos G, et al. A multicenter phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancer. Br J Cancer. 2006;95:587–592. doi: 10.1038/sj.bjc.6603301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 54.Javle M, Yu J, Garrett C, et al. Bevacizumab combined with gemcitabine and capecitabine for advanced pancreatic cancer: a phase II study. Br J Cancer. 2009;100:1842–1845. doi: 10.1038/sj.bjc.6605099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kindler HL, Ioka T, Richel DJ, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256–262. doi: 10.1016/S1470-2045(11)70004-3. [DOI] [PubMed] [Google Scholar]
- 56.Ko AH, Hwang J, Venook AP, et al. Serum CA19-9 response as a surrogate for clinical outcome in patients receiving fixed-dose rate gemcitabine for advanced pancreatic cancer. Br J Cancer. 2005;93:195–199. doi: 10.1038/sj.bjc.6602687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ziske C, Schlie C, Gorschluter M, et al. Prognostic value of CA 19-9 levels in patients with inoperable adenocarcinoma of the pancreas treated with gemcitabine. Br J Cancer. 2003;89:1413–1417. doi: 10.1038/sj.bjc.6601263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong D, Ko AH, Hwang J, et al. Serum CA19-9 decline compared to radiographic response as a surrogate for clinical outcomes in patients with metastatic pancreatic cancer receiving chemotherapy. Pancreas. 2008;37:269–274. doi: 10.1097/MPA.0b013e31816d8185. [DOI] [PubMed] [Google Scholar]
- 59.Halm U, Schumann T, Schiefke I, et al. Decrease of CA 19-9 during chemotherapy with gemcitabine predicts survival time in patients with advanced pancreatic cancer. Br J Cancer. 2000;82:1013–1016. doi: 10.1054/bjoc.1999.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaked Y, Henke E, Roodhart JM, et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14:263–273. doi: 10.1016/j.ccr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Starlinger P, Brugger P, Schauer D, et al. Myelosuppression of thrombocytes and monocytes is associated with a lack of synergy between chemotherapy and anti-VEGF treatment. Neoplasia. 2011;13:419–427. doi: 10.1593/neo.101508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spano JP, Chodkiewicz C, Maurel J, et al. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Lancet. 2008;371:2101–2108. doi: 10.1016/S0140-6736(08)60661-3. [DOI] [PubMed] [Google Scholar]
- 63.Maitland ML, Ratain MJ. Terminal ballistics of kinase inhibitors: there are no magic bullets. Ann Intern Med. 2006;145:702–703. doi: 10.7326/0003-4819-145-9-200611070-00015. [DOI] [PubMed] [Google Scholar]
- 64.Snider KL, Maitland ML. Cardiovascular toxicities: clues to optimal administration of vascular endothelial growth factor signaling pathway inhibitors. Target Oncol. 2009;4:67–76. doi: 10.1007/s11523-009-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friberg G, Kasza K, Vokes EE, Kindler HL. Early hypertension (HTN) as a potential pharmacodynamic (PD) marker for survival in pancreatic cancer (PC) patients (pts) treated with bevacizumab (B) and gemcitabine (G) J Clin Oncol. 2005;23:3020. [Google Scholar]
- 66.Baka S, Clamp AR, Jayson GC. A review of the latest clinical compounds to inhibit VEGF in pathological angiogenesis. Expert Opin Ther Targets. 2006;10:867–876. doi: 10.1517/14728222.10.6.867. [DOI] [PubMed] [Google Scholar]
- 67.Schneider BP, Wang M, Radovich M, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26:4672–4678. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dahlberg SE, Sandler AB, Brahmer JR, et al. Clinical course of advanced non-small-cell lung cancer patients experiencing hypertension during treatment with bevacizumab in combination with carboplatin and paclitaxel on ECOG 4599. J Clin Oncol. 2010;28:949–954. doi: 10.1200/JCO.2009.25.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ryanne Wu R, Lindenberg PA, Slack R, et al. Evaluation of hypertension as a marker of bevacizumab efficacy. J Gastrointest Cancer. 2009;40:101–108. doi: 10.1007/s12029-009-9104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scartozzi M, Galizia E, Chiorrini S, et al. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009;20:227–230. doi: 10.1093/annonc/mdn637. [DOI] [PubMed] [Google Scholar]
- 71.Goodwin R, Ding K, Seymour L, et al. Treatment-emergent hypertension and outcomes in patients with advanced non-small-cell lung cancer receiving chemotherapy with or without the vascular endothelial growth factor receptor inhibitor cediranib: NCIC Clinical Trials Group Study BR24. Ann Oncol. 2010;21:2220–2226. doi: 10.1093/annonc/mdq221. [DOI] [PubMed] [Google Scholar]
- 72.Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103:763–773. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49:186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 74.Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA. 2011;305:487–494. doi: 10.1001/jama.2011.51. [DOI] [PubMed] [Google Scholar]
- 75.Lu JF, Bruno R, Eppler S, et al. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol. 2008;62:779–786. doi: 10.1007/s00280-007-0664-8. [DOI] [PubMed] [Google Scholar]
- 76.Gaudreault J, Lieberman G, Kabbinavar E, et al. Pharmacokinetics of Bevacizumab (BV) in colorectal cancer (CRC) Clin Pharmacol Ther. 2001;69:25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.