Abstract
Objective:
The objective of this study was to clarify the generation and gender differences in the association between central obesity and the accumulation of metabolic risk factors (RFs) in the Japanese population.
Material and methods:
A total of 12 389 subjects (age: 18–80 years) without receiving medication for diabetes, dyslipidemia or hypertension were enrolled in this study and divided according to age and gender. In each group, we performed analyses as follows: (1) a receiver operating characteristic (ROC) analysis to evaluate the utility of the waist circumference (WC) for detecting subjects with multiple RFs of metabolic syndrome (MS); (2) a cross-sectional study to examine the relationship between the WC and the odds ratio (OR) for detecting those subjects and (3) a longitudinal study to examine how longitudinal changes (Δ) in WC over a 1-year period affected the values of each metabolic RF.
Results:
With age, the WC cutoff values yielding the maximum Youden index for detecting subjects with multiple RFs increased only in women, and the areas under the curves of the ROC analysis of WC for detecting those subjects decreased in both genders. The positive correlation between the WC and the OR for detecting subjects with multiple RFs became weaker with age, especially in women. In the longitudinal study, the significant correlation between ΔWC and Δ each metabolic RF, except for hypertension, and between ΔWC and Δ the number of RFs became weaker with age in women, whereas the significant correlation between ΔWC and Δ the number of RFs was not affected with age in men. In women aged ⩾60 years, none of the changes in each metabolic RF were significantly associated with ΔWC.
Conclusions:
Aging attenuates the association of central obesity with the accumulation of metabolic RFs, especially in women.
Keywords: aging, central obesity, metabolic risk factors, cross-sectional and longitudinal study, Japanese population
Introduction
The prevalence of metabolic syndrome (MS), which is composed of a cluster of cardiovascular risk factors (RFs) such as central obesity, high blood pressure, impaired lipid metabolism and hyperglycemia, has been growing globally. Individuals with MS have a higher risk of type 2 diabetes and cardiovascular disease (CVD).1, 2, 3 As the morbidity and mortality of CVD is rapidly increasing world-wide,4 establishing appropriate screening for MS is essential to prevent the initiation and progression of CVD. As many studies have demonstrated that the waist circumference (WC) has a close relationship with the visceral fat area (VFA) on computed tomography,5, 6 WC is almost always used as one criterion for the diagnosis of MS, and this measure is typically used as a simplified measure of the VFA.7, 8, 9 A Japanese committee also established criteria for the diagnosis of MS in the Japanese population (Japanese criteria) in cooperation with the International Diabetes Federation (IDF), in which the WC cutoff values were fixed as 85 and 90 cm for men and women, respectively.10 However, the IDF subsequently established novel WC criteria, including WC cutoff values of 90 and 80 cm for men and women in Asians, respectively,11 which totally differ from those used in the Japanese criteria. Because of this inconsistency, the WC cutoff values in the Japanese criteria have been disputed. To settle this issue, multiple lines of study have been designed to determine the optimal WC cutoff values for the Japanese criteria;12, 13, 14, 15, 16 however, no consensus has yet been reached. Moreover, generation differences in the optimal WC cutoff values for the detection of subjects with multiple RFs of MS have been reported;17, 18 therefore, several reports have concluded that sex- and age-specific cutoff values for MS are needed for the Japanese population. In addition, the associations between central obesity and each metabolic RF have recently been reported to differ considerably according to age.19, 20 However, to the best of our knowledge, few reports have examined whether aging affected the central obesity-induced accumulation of RFs and the association between the longitudinal change in WC and change in each metabolic RF in the Japanese population.
With the above-described background in mind, we hypothesized that aging may affect the association between central obesity and the accumulation of metabolic RFs, leading to the onset of CVD. To validate our hypothesis, we divided all subjects according to age and (1) re-evaluated the utility of WC for the detection of multiple RFs of MS in a large cohort using receiver operating characteristic (ROC) analyses; (2) performed a cross-sectional study regarding the association of age with central obesity-induced accumulation of metabolic RFs such as hypertension, dyslipidemia and a high fasting plasma glucose (FPG) level and (3) conducted a longitudinal study to examine how longitudinal changes (Δ) in WC and body weight (BW) over a 1-year period affected the values of each metabolic RF.
Materials and methods
Participants
A total of 41 490 subjects underwent health check-ups between 2008 and 2010 at the Shakaihoken Takaoka hospital (Takaoka, Japan) and in Itoigawa city, Niigata Prefecture. Of these subjects, we recruited 12 389 subjects (7611 men and 4778 women) between the ages of 18–80 years who had received annual health examinations at least twice over a 1-year period, who were not receiving medication for diabetes, hyperlipidemia or hypertension and whose blood sample had been drawn after an overnight 12-h fast. The study subjects were divided into four age groups according to generation (<40, 40–49, 50–59 and ⩾60 years).
Collection of clinical information, anthropometric measurements and metabolic RFs
Anthropometric measurements of individuals wearing light clothing and without shoes were conducted by well-trained examiners. Body height and weight were measured using an automated scale (model THP-SE3476; KANSAI SEIKI Co., Kusatsu, Japan). Height was measured to the nearest 0.1 cm and weight was measured in an upright position to the nearest 0.1 kg. Body mass index (BMI) was calculated by dividing BW (kg) by height squared (m2). The WC measurements were obtained at the end of normal expiration and were measured to the nearest 0.1 cm at the umbilical level, which is the Japanese standard method, using a flexible anthropometric tape. Blood pressure was measured twice in a sitting position using an automated device (Colin Model BP-203RV; Colin, Tokyo, Japan) after the subject had rested for at least 5 min. The average of two readings was used for the blood pressure value. After a 12-h overnight fast, blood samples were drawn to measure the plasma glucose (PG) level, triglyceride (TG) and high-density lipoprotein (HDL) cholesterol levels. Self-reported questionnaires based on a ‘specific health examination'21 were used to investigate the drinking status and whether the subjects were currently receiving medical treatment for hyperlipidemia, hypertension or diabetes or were current smokers. Using the information obtained from the questionnaire, we estimated the drinking status according to the frequency of alcohol intake (everyday or sometimes or rarely (does not drink alcohol)) and the amounts of all alcoholic beverages consumed. The usual daily intake of alcohol was assessed in units of ‘gou' (a traditional Japanese unit of measurement, corresponding to 23 g of ethanol) and then converted to grams of ethanol per day. Based on this information, we defined ‘nondrinker' as a subject who does not drink alcohol or drinks alcohol only rarely, ‘occasional drinkers' as subjects who sometimes drink alcohol and ‘current drinkers' as subjects who drink alcohol everyday. In addition, current drinkers were divided into four groups according to the amounts of alcohol consumed: <23 g per day, 23–45 g per day, 46–68 g per day or ⩾69 g per day because the amount of alcohol consumption has been reported to be linearly associated with the onset of hypertension22, 23 and dyslipidemia.24 Moreover, to examine the longitudinal change in the anthropometric measurements and metabolic RFs, we handled these data in 2008 or 2009 as a baseline data and in 2009 or 2010 as 1-year follow-up data.
Sample size calculation
To conduct a longitudinal study to examine how longitudinal changes in WC and BW over a 1-year period affected the values of each metabolic RF, we calculated the required sample size based on the outcome of several previous studies25, 26 as follows. According to these studies, we estimated the change in the BW and WC during 1 year required to achieve significant longitudinal changes in the components of MS over the course of 1 year. When these estimated values were used to perform a post hoc power analysis (using a power=80% and an α=5%) to calculate the required sample size, 153 men and 76 women were found to be needed to identify longitudinal changes in the components of MS during the 1-year follow-up period.
Ethics statement
The study was approved by the Ethics Committee of the University of Toyama. Informed consent was not obtained from the study participants for the following reasons. According to the Japanese Ethical Guidelines for Epidemiological Research,27 in the case of observational research using existing and anonymized data provided by another institution, informed consent does not necessarily need to be obtained from the research subjects. In the present study, we used existing and anonymized data from health check-ups performed at the Shakaihoken Takaoka hospital and in Itoigawa city, Niigata Prefecture, between 2008 and 2010, and we were not involved in performing the actual health check-ups or data collection. In addition, we have an agreement with the head of these institutions regarding the transfer of data, and the anonymous data was provided under this agreement. We uploaded our study to the homepage of the First Department of Internal Medicine, Faculty of Medicine, Toyama University,28 and announced its existence to the study participants who received annual health examinations at the Shakaihoken Takaoka Hospital (Takaoka, Japan) and in Itoigawa City, Niigata Prefecture. Moreover, the Ethics Committee of the University of Toyama agreed that the data collection method and data analysis used in our study were appropriate ethically according to the Japanese Ethical Guidelines for Epidemiological Research (permission number 22–79).
Diagnostic criteria for MS
According to the IDF criteria,11 MS was defined among men and women as a WC in excess of 90 cm and 80 cm, respectively, in addition to having two or more of the following components: (1) TG ⩾1.7 mmol l−1, (2) HDL cholesterol <1.03 mmol l−1 in men and <1.29 mmol l−1 in women, (3) systolic BP ⩾130 mm Hg and/or diastolic BP ⩾85 mm Hg, or (4) FPG ⩾5.6 mmol l−1.
Statistical analysis
All the data were expressed as the mean±s.d. The statistical analyses were performed using JMP for Windows, Version 9.0 (SAS Institute, Cary, NC, USA) and SPSS for Windows, version 19.0 (SPSS Inc., Chicago, IL, USA). Differences in the clinical features between the four groups according to age were examined using an ANOVA. ROC analyses were performed to determine the appropriate cutoff points for WC and BMI for the identification of subjects with two or more RFs of MS. The diagnostic abilities of the tests were compared using the areas under the curves (AUC), and the significance of the difference between two areas was assessed using the method described by Hanley and McNeil.29 The Youden index (sensitivity+specificity-1) is an integrative indicator of sensitivity and specificity.30 Variables with skewed distributions were logarithmically (natural) transformed for further analysis. The relationships between WC or BW and each of the metabolic RF were investigated using a simple regression analysis. Correlation coefficients were compared after Fisher's z-transformation. In Figure 1, we divided the subjects into eight groups according to 5 cm WC increments (<70 cm, 70–75 cm, 75–80 cm, 80–85 cm, 85–90 cm, 90–95 cm, 95–100 cm and ⩾100 cm) and calculated the odds ratio (OR) of detecting subjects with multiple RFs of MS using a multiple logistic regression analysis adjusted for age, the degree of alcohol consumption (nondrinker, 0; occasional drinker, 1; current drinker consuming <23 g per day, 2; current drinker consuming 23–45 g per day, 3; current drinker consuming 46–68 g per day, 4; or current drinker consuming ⩾69 g per day, 5), and smoking status (nonsmoker, 0; current smoker, 1), with<70 cm WC group as the reference. Results with P-values <0.05 were considered statistically significant.
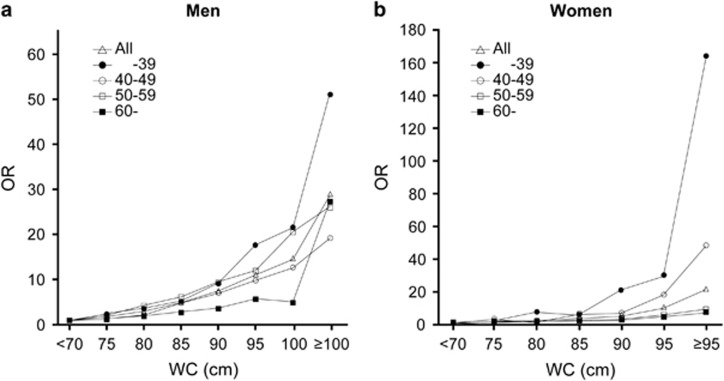
Figure 1.
OR adjusted for age, the degree of alcohol consumption and smoking status for detecting the subjects with two or more RFs of metabolic syndrome according to generation and WC values categories, with WC <70 cm as a reference level, in men (a) and women (b), respectively. Open triangles: all ages, closed circles: <40, open circles: 40–49, open squares: 50–59, closed squares: ⩾60. WC: waist circumference.
Results
Demographic, hemodynamic, and metabolic characteristics of the study subjects according to age group
The clinical characteristics of the subjects in the present study are shown in Table 1. The average age, WC and BMI were 47.5±10.6 years, 83.5±8.6 cm and 23.3±3.2 kg m−2, respectively, in men and 48.2±10.0 years, 78.3±9.0 cm and 21.7±3.2 kg m−2, respectively, in women. The overall prevalence of central obesity and MS were 20.9 and 12.0%, respectively, in men and 40.8 and 10.5%, respectively, in women. The overall prevalence of an elevated BP, an elevated FPG level, a reduced HDL cholesterol level and an elevated TG level were 36.3, 44.7, 9.8% and 24.9%, respectively, in men and 24.3, 20.4, 12.7 and 5.8%, respectively, in women.
Table 1. Demographic, hemodynamic and metabolic characteristics of study population according to age group.
| Variable | All |
Age group (years) |
||||
|---|---|---|---|---|---|---|
| <40 | 40–49 | 50–59 | ⩾60 | P-values ANOVA | ||
| Men | ||||||
| n | 7611 | 2146 | 2217 | 2090 | 1158 | |
| Age (years) | 47.5±10.6 | |||||
| Prevalence of metabolic syndrome (%)a | 12.0 | 10.7 | 12.2 | 13.5 | 11.1 | 0.025 |
| Central obesity (%)a | 20.9 | 19.8 | 22.0 | 22.3 | 18.5 | 0.019 |
| Waist circumference(cm) | 83.5±8.6 | 82.7±9.6 | 84.0±8.6 | 84.0±7.8 | 83.1±7.7 | <0.0001 |
| Body mass index (kg m−2) | 23.3±3.2 | 23.4±3.6 | 23.5±3.2 | 23.1±2.9 | 22.8±2.7 | <0.0001 |
| Systolic blood pressure (mm Hg) | 123.4±16.1 | 120.7±14.6 | 122.5±15.5 | 124.7±17.0 | 127.8±17.0 | <0.0001 |
| Diastolic blood pressure (mm Hg) | 74.2±11.1 | 73.0±10.4 | 76.6±11.2 | 78.2±11.0 | 78.2±10.4 | <0.0001 |
| Elevated blood pressure (%)b | 36.3 | 27.9 | 34.9 | 40.2 | 47.8 | <0.0001 |
| Fasting plasma glucose (mmol l−1) | 5.6±0.9 | 5.4±0.8 | 5.6±0.9 | 5.8±1.1 | 5.7±0.9 | <0.0001 |
| Elevated fasting plasma glucose (%)c | 44.7 | 33.0 | 43.6 | 54.2 | 51.1 | <0.0001 |
| High-density lipoprotein cholesterol (mmol l−1) | 1.4±0.4 | 1.4±0.3 | 1.4±0.4 | 1.4±0.4 | 1.5±0.4 | 0.0045 |
| Reduced high-density lipoprotein cholesterol (%)d | 9.8 | 8.9 | 10.5 | 10.4 | 9.2 | 0.239 |
| Triglycerides (mmol l−1) | 1.4±1.1 | 1.4±1.1 | 1.5±1.1 | 1.5±1.1 | 1.3±0.8 | <0.0001 |
| Elevated triglycerides (%)e | 24.9 | 22.1 | 26.7 | 27.9 | 21.2 | <0.0001 |
| Current smokers (%) | 70.8 | 70.2 | 74.8 | 75.5 | 55.6 | <0.0001 |
| Current drinker (%)f | 35.6 | 25.4 | 37.1 | 43.0 | 38.3 | <0.0001 |
| Women | ||||||
| n | 4778 | 1042 | 1580 | 1534 | 622 | |
| Age (years) | 48.2±10.0 | |||||
| Prevalence of metabolic syndrome (%)a | 10.5 | 4.8 | 8.9 | 12.8 | 18.8 | <0.0001 |
| Central obesity (%)a | 40.8 | 23.7 | 37.4 | 48.5 | 59.2 | <0.0001 |
| Waist circumference(cm) | 78.3±9.0 | 74.9±8.7 | 77.7±8.9 | 79.7±8.6 | 81.7±9.0 | <0.0001 |
| Body mass index (kg m−2) | 21.7±3.2 | 21.0±3.4 | 21.7±3.4 | 21.9±3.1 | 22.2±2.9 | <0.0001 |
| Systolic blood pressure (mm Hg) | 117.9±16.3 | 112.4±14.4 | 116.0±15.6 | 120.7±16.5 | 125.5±16.5 | <0.0001 |
| Diastolic blood pressure (mm Hg) | 70.9±10.5 | 67.4±9.7 | 70.2±10.4 | 72.9±10.5 | 73.7±10.3 | <0.0001 |
| Elevated blood pressure (%)b | 24.3 | 12.0 | 19.6 | 31.6 | 38.4 | <0.0001 |
| Fasting plasma glucose (mmol l−1) | 5.2±0.6 | 5.1±0.4 | 5.1±0.5 | 5.3±0.7 | 5.4±0.7 | <0.0001 |
| Elevated fasting plasma glucose (%)c | 20.4 | 11.0 | 16.6 | 26.7 | 30.7 | <0.0001 |
| High-density lipoprotein cholesterol (mmol l−1) | 1.7±0.4 | 1.7±0.4 | 1.7±0.4 | 1.7±0.4 | 1.7±0.4 | 0.0062 |
| Reduced high-density lipoprotein cholesterol (%)d | 12.7 | 14.7 | 11.1 | 12.8 | 13.3 | 0.0528 |
| Triglycerides (mmol l−1) | 0.9±0.5 | 0.8±0.4 | 0.9±0.5 | 1.0±0.5 | 1.0±0.5 | <0.0001 |
| Elevated triglycerides (%)e | 5.8 | 2.4 | 5.0 | 7.3 | 10.0 | <0.0001 |
| Current smoker (%) | 13.4 | 22.1 | 14.1 | 10.2 | 4.8 | <0.0001 |
| Current drinker (%)f | 4.7 | 5.3 | 6.5 | 3.6 | 2.3 | <0.0001 |
Data are means±s.d. or %. Among the four groups according to age, the P-values were calculated using an ANOVA.
Metabolic syndrome and central obesity were defined according to International Diabetes Federation (IDF) criteria (Alberti et al.11).
Elevated blood pressure (BP) was diagnosed if systolic BP was ⩾130 mm Hg or diastolic BP was ⩾85 mm Hg.
Elevated fasting plasma glucose (FPG) was diagnosed if FPG was ⩾5.6 mmol/l.
Reduced high-density lipoprotein (HDL) cholesterol was diagnosed if HDL cholesterol was <1.03 mmol l−1 in men and <1.29 mmol l−1 in women.
Elevated triglycerides (TG) was diagnosed if TG was ⩾1.7 mmol l−1.
Current drinkers were regarded as individuals who drink some amount of alcohol on a daily basis.
When we divided all the participants into four strata according to age, the prevalence of MS increased with age (P=0.025) and reached the peak in the 50–59-year group in men. On the other hand, the prevalence of MS increased with age (P<0.0001) and reached the peak in the ⩾60-year group in women (Table 1). A similar tendency was observed for the prevalence of central obesity (P<0.0001) (Table 1). The prevalence of an elevated BP, an elevated FPG and an elevated TG level increased with age in both genders (P<0.0001), whereas the prevalence of a reduced HDL cholesterol level was not influenced by age in both genders.
Estimation of WC and BMI cutoff level for detecting subjects with multiple RFs of MS
Next, we plotted the ROC curve to evaluate the utility of the WC and BMI for the detection of subjects with multiple RFs of MS (Table 2). According to the ROC curve, the WC and BMI cutoff values yielding an 80% sensitivity for detecting subjects with multiple RFs of MS for all ages were 80.5 cm and 22.0 kg m−2 in men and 76.5 cm and 20.9 kg m−2 in women, respectively (Table 2). Our data were almost identical to the results of previous studies.12, 13, 14, 15, 16
Table 2. Areas under the curve (AUC) of ROC for the detection of multiple risk factors of metabolic syndrome.
|
Anthropometric indexes |
Presence/absence for the multiple risk factors |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | Mean | (s.d.) | n | AUC | Cutoff value corresponding to 80% sensitivity | Specificity corresponding to 80% sensitivity (%) | Cutoff value corresponding to maximal sensitivity plus specificity | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | aJ value |
| WC | |||||||||||||
| Men | All | 83.5 cm | 8.6 | 911/6700 | 0.696 | 80.5 cm | 45.3 | 83.1 cm | 68.4 | 60.3 | 48.1 | 78.0 | 0.3 |
| <40 | 82.7 cm | 9.6 | 229/1917 | 0.737 | 80.3 cm | 50.1 | 82.0 cm | 76.6 | 59.1 | 38.8 | 88.2 | 0.4 | |
| 40–49 | 84.0 cm | 8.6 | 271/1946 | 0.699 | 81.1 cm | 49.7 | 81.2 cm | 79.4 | 49.9 | 46.0 | 81.8 | 0.3 | |
| 50–59 | 84.0 cm | 7.8 | 283/1807 | 0.673 | 80.5 cm | 40.9 | 83.7 cm | 67.4 | 59.3 | 54.4 | 71.6 | 0.3 | |
| ⩾60 | 83.1 cm | 7.7 | 128/1030 | 0.647* | 79.1 cm | 37.1 | 82.2 cm | 66.4 | 54.8 | 50.1 | 70.5 | 0.2 | |
| Women | All | 78.2 cm | 9.0 | 504/4274 | 0.731 | 76.5 cm | 49.3 | 79.2 cm | 72.3 | 62.7 | 25.5 | 92.7 | 0.3 |
| <40 | 74.9 cm | 8.7 | 50/992 | 0.822 | 77.1 cm | 71.2 | 77.5 cm | 79.8 | 71.3 | 18.6 | 97.7 | 0.5 | |
| 40–49 | 77.7 cm | 8.9 | 140/1440 | 0.760 | 75.1 cm | 46.4 | 79.8 cm | 76.6 | 67.5 | 23.7 | 95.6 | 0.4 | |
| 50–59 | 79.7 cm | 8.6 | 197/1337 | 0.657 | 76.0 cm | 36.0 | 80.8 cm | 62.0 | 61.7 | 27.8 | 87.2 | 0.2 | |
| ⩾60 | 81.7 cm | 9.0 | 117/505 | 0.652* | 78.5 cm | 38.5 | 85.0 cm | 52.5 | 70.8 | 38.4 | 81.1 | 0.2 | |
| BMI | |||||||||||||
| Men | All | 23.3 kg m−2 | 3.2 | 911/6700 | 0.684 | 22.0 kg m−2 | 44.2 | 23.2 kg m−2 | 65.2 | 62.4 | 48.3 | 76.9 | 0.3 |
| <40 | 23.4 kg m−2 | 3.6 | 229/1917 | 0.738 | 22.5 kg m−2 | 52.3 | 24.1 kg m−2 | 63.7 | 73.4 | 44.8 | 85.7 | 0.4 | |
| 40–49 | 23.5 kg m−2 | 3.2 | 271/1946 | 0.704 | 22.3 kg m−2 | 47.6 | 23.2 kg m−2 | 69.6 | 61.2 | 49.0 | 78.9 | 0.3 | |
| 50–59 | 23.1 kg m−2 | 2.9 | 283/1807 | 0.662 | 21.7 kg m−2 | 38.9 | 23.7 kg m−2 | 54.3 | 69.0 | 55.8 | 67.7 | 0.2 | |
| ⩾60 | 22.8 kg m−2 | 2.7 | 128/1030 | 0.641 | 21.4 kg m−2 | 36.9 | 22.9 kg m−2 | 61.9 | 59.9 | 51.3 | 69.7 | 0.2 | |
| Women | All | 21.7 kg m−2 | 3.2 | 504/4274 | 0.736 | 20.9 kg m−2 | 50.1 | 22.5 kg m−2 | 64.1 | 72.9 | 29.4 | 92.0 | 0.4 |
| <40 | 21.0 kg m−2 | 3.4 | 50/992 | 0.820 | 21.2 kg m−2 | 66.9 | 24.8 kg m−2 | 59.5 | 91.9 | 37.6 | 96.5 | 0.5 | |
| 40–49 | 21.7 kg m−2 | 3.4 | 140/1440 | 0.762 | 21.0 kg m−2 | 52.9 | 22.8 kg m−2 | 66.3 | 76.4 | 27.0 | 94.5 | 0.4 | |
| 50–59 | 21.9 kg m−2 | 3.1 | 197/1337 | 0.690 | 20.7 kg m−2 | 39.9 | 23.1 kg m−2 | 54.2 | 76.9 | 35.9 | 87.6 | 0.3 | |
| ⩾60 | 22.2 kg m−2 | 2.9 | 117/505 | 0.685 | 20.8 kg m−2 | 37.0 | 23.3 kg m−2 | 56.9 | 75.1 | 44.2 | 83.4 | 0.3 | |
Abbreviations: AUC, area under receiver-operator characteristics curve; BMI, body mass index; NPV, negative predictive value; Multiple risk factors, having two or more risk factors of metabolic syndrome defined by IDF (2005); PPV, positive predictive value; ROC, receiver operating characteristic curve analysis; WC, waist circumference
*P<0.05 compared with AUC in <40-year group.
J=sensitivity+specificity−1.
To examine how aging affected the cutoff value and the utility of WC for detecting subjects with multiple RFs of MS, we conducted the ROC analyses in each age group (Table 2). The WC cutoff values yielding the maximum Youden index for detecting subjects with multiple RFs of MS increased with age in women (<40 years vs ⩾60 years, 77.5 cm vs 85.0 cm), whereas these values were comparable among age groups in men. The AUC of the ROC analysis of WC for the detection of multiple RFs of MS significantly decreased with age in both genders. When we compared the AUC in the subjects aged <40 years with that in those aged ⩾60 years, the AUC was significantly smaller in the subjects aged ⩾60 years in both genders (men: AUC for <40 years=0.738, AUC for ⩾60 years=0.641, P-value for <40 years vs ⩾60 years<0.05; women: AUC for <40 years=0.822, AUC for ⩾60 years=0.652, P-value for <40 years vs ⩾60 years<0.05) and the decrease of AUC with age was stronger in women than in men.
Association of WC with the number of metabolic RFs and each component of MS according to age group
To further examine the effect of aging on the association of WC with the OR for detecting subjects with multiple RFs, using a multiple logistic regression analysis, the ORs for detecting those subjects according to WC values categorized by 5-cm increments in men and women among each age group were calculated (Figure 1). Based on a 70-cm WC in both genders, the OR (95% CI) for detecting subjects with multiple RFs in the <40-year age group increased with WC and became 53.4 (22.8–147.9) in men with a WC ⩾100 cm and 134.1 (41.8–536.9) in women with a WC ⩾95 cm; however, these central obesity-induced increments in the OR weakened with advancing age in both gender (for example, OR=26.9 (7.3–134.4) in men ⩾60 years with a WC ⩾100 cm, OR=6.7 (2.8–16.9) in women ⩾60 years with a WC ⩾95 cm) (Figure 1). In addition, when we compared the OR for a 5-cm WC increase for the detection of subjects with multiple metabolic RFs between the <40-year age group and the ⩾60-year age group, in both genders, the OR in the older group (men: OR=1.42 (95% CI, 1.30–1.54), women: OR=1.42 (95% CI, 1.27–1.60)) was apparently lower than that in the younger group (men: OR=1.68 (95% CI, 1.58–1.80), women: OR=2.11 (95% CI, 1.83–2.46)). Table 3 shows the simple correlation coefficients between WC and the values of each component of MS for each age group. We observed a significant positive association between the WC and each component of MS including the systolic BP (r=0.39, P<0.001 in men and r=0.35, P<0.001 in women), the diastolic BP (r=0.37, P<0.001 in men and r=0.31, P<0.001 in women) and the log FPG (r=0.26, P<0.001 in men and r=0.20, P<0.001 in women) in the <40-year age group in both genders; however, these associations weakened with advancing age in both genders. When these correlation coefficients for the <40-year age group and the ⩾60-year age group were compared, a significant difference was observed in both genders (P<0.0001) (Table 3). Moreover, we also observed a significant inverse association between the WC and the HDL cholesterol (r=−0.35, P<0.001 in men and r=−0.29, P<0.001 in women) in the <40-year age group; however, this association became stronger in the ⩾60-year age group only in women (r=−0.38, P<0.001). The positive correlation between the WC and log TG decreased with age in men, but not in women (Table 3).
Table 3. Correlation between WC and each components of metabolic syndrome.
| Variables |
Age group (years) |
Comparison of correlation coefficients |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
All |
<40 |
40–49 |
50–59 |
⩾60 |
<40 vs ⩾60 |
50–59 vs ⩾60 |
||||||
| r | P | r | P | r | P | r | P | r | P | aP | aP | |
| Men | ||||||||||||
| Systolic pressure (mm Hg) | 0.29 | <0.001 | 0.39 | <0.001 | 0.33 | <0.001 | 0.22 | <0.001 | 0.20 | <0.001 | <0.001 | 0.435 |
| Diastolic pressure (mm Hg) | 0.30 | <0.001 | 0.37 | <0.001 | 0.30 | <0.001 | 0.23 | <0.001 | 0.22 | <0.001 | <0.001 | 0.547 |
| Log triglycerides | 0.35 | <0.001 | 0.39 | <0.001 | 0.32 | <0.001 | 0.34 | <0.001 | 0.29 | <0.001 | <0.001 | 0.010 |
| HDL cholesterol (mmol l−1) | −0.33 | <0.001 | −0.35 | <0.001 | −0.35 | <0.001 | −0.32 | <0.001 | −0.32 | <0.001 | 0.057 | 0.660 |
| Log fasting plasma glucose | 0.23 | <0.001 | 0.27 | <0.001 | 0.26 | <0.001 | 0.21 | <0.001 | 0.14 | <0.001 | <0.001 | 0.002 |
| Women | ||||||||||||
| Systolic pressure (mm Hg) | 0.34 | <0.001 | 0.35 | <0.001 | 0.33 | <0.001 | 0.26 | <0.001 | 0.20 | <0.001 | <0.001 | 0.036 |
| Diastolic pressure (mm Hg) | 0.31 | <0.001 | 0.31 | <0.001 | 0.30 | <0.001 | 0.24 | <0.001 | 0.20 | <0.001 | <0.001 | 0.164 |
| Log triglycerides | 0.36 | <0.001 | 0.30 | <0.001 | 0.33 | <0.001 | 0.32 | <0.001 | 0.29 | <0.001 | 0.713 | 0.144 |
| HDL cholesterol (mmol l−1) | −0.29 | <0.001 | −0.29 | <0.001 | −0.31 | <0.001 | −0.29 | <0.001 | −0.38 | <0.001 | 0.001 | <0.001 |
| Log fasting plasma glucose | 0.22 | <0.001 | 0.23 | <0.001 | 0.24 | <0.001 | 0.20 | <0.001 | 0.05 | 0.185 | <0.001 | <0.001 |
Abbreviations: HDL cholesterol, high-density lipoprotein cholesterol; r, correlation coefficients using simple correlation analysis between WC and each components of metabolic syndrome; WC, waist circumference.
Correlation coefficients among each age group were compared after Fisher's z-transformation.
Correlations between changes in WC or BW and metabolic RFs according to age group in a longitudinal study
Next, we examined how longitudinal changes (Δ) in WC and BW over a 1-year period affected the longitudinal changes in each RF of MS and the number of metabolic RFs for each age group. The changes (Δ) in the anthropometric indices and each RF of MS during the 1-year follow-up period are shown in Table 4. The 1-year changes in each of these parameters were relatively small in both genders. The Pearson correlation coefficients for the changes in the anthropometric measurements and metabolic RFs are shown in Table 5. First, we calculated the Pearson correlation coefficients between the ΔBW and ΔWC. We found a significant correlation between the ΔBW and ΔWC in both genders and the correlation coefficient was greater in men than in women (r=0.71, P<0.001 in men and r=0.44, P<0.001 in women), suggesting that the change in the visceral fat was more pronounced in men than in women during weight change. In addition, the correlation coefficients between the ΔBW and ΔWC became smaller with age in both genders (men: r for <40 years=0.76, r for ⩾60 years=0.56, P-value for <40 years vs ⩾60 years<0.0001, women: r for <40 years=0.48, r for ⩾60 years =0.37, P-value for <40 years vs ⩾60 years<0.0001). Overall, significant correlations between Δ all RFs of MS and ΔWC were observed in both genders (P<0.01). Then, we examined whether aging affected the association between ΔWC or ΔBW and Δ the number of RFs. The correlation coefficient between ΔWC or ΔBW and Δ the number of RFs was significantly smaller in the ⩾60-year age group than in the <40-year age group in women (P<0.05), but not in men (Table 5). Then, we next examined whether aging affected the association between ΔWC and the changes in each RF of MS. The correlation coefficient between Δlog TG and ΔWC was significantly smaller in the ⩾60-year age group than in the <40-year age group in both genders (P<0.01) (Table 5). The correlation coefficient between ΔBP and ΔWC in men and the correlation coefficients between Δlog FPG and ΔWC and between ΔHDL-c and ΔWC in women were significantly smaller in the ⩾60-year age group than in the <40-year age group (Table 5). When we examined the association between ΔBW and the change in each RF of MS, the correlation coefficients between ΔBW and the change in each metabolic RF except for BP were decreased by age in women, but not in men. Especially, in women aged ⩾60 years, none of the changes in each RF of MS were significantly associated with ΔWC and ΔBW.
Table 4. Changes in anthropometric indices and metabolic risk factors during a 1-year follow-up period.
| Changes in 1-year period |
Age group |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
All |
<40 |
40–49 |
50–59 |
⩾60 |
||||||
| Mean±s.d. | P | Mean±s.d. | P | Mean±s.d. | P | Mean±s.d. | P | Mean±s.d. | P | |
| Men | ||||||||||
| WC (cm) | 0.12±2.98 | 0.382 | 0.24±3.06 | 0.423 | 0.12±3.08 | 0.648 | 0.09±2.78 | 0.720 | −0.02±3.01 | 0.953 |
| BMI (kg m−2) | 0.03±0.81 | 0.504 | 0.07±0.91 | 0.532 | 0.03±0.85 | 0.763 | 0.02±0.71 | 0.866 | 0.02±0.69 | 0.888 |
| BW (kg) | 0.08±2.39 | 0.661 | 0.22±2.71 | 0.527 | 0.05±2.54 | 0.868 | −0.01±2.03 | 0.984 | −0.01±2.03 | 0.977 |
| Systolic blood pressure (mm Hg) | 1.00±11.59 | 0.001 | 0.77±10.97 | 0.087 | 0.94±10.95 | 0.043 | 1.27±12.20 | 0.016 | 1.01±12.76 | 0.153 |
| Diastolic blood pressure (mm Hg) | 0.57±7.76 | 0.002 | 0.63±7.34 | 0.049 | 0.55±7.89 | 0.108 | 0.48±7.75 | 0.166 | 0.67±8.29 | 0.126 |
| Triglyceride (mmol l−1) | 0.01±0.97 | 0.560 | 0.03±1.03 | 0.350 | 0.01±1.00 | 0.677 | −0.01±1.00 | 0.876 | −0.01±0.65 | 0.788 |
| HDL cholesterol (mmol l−1) | 0.00±0.20 | 0.909 | 0.01±0.19 | 0.517 | 0.01±0.19 | 0.621 | −0.01±0.20 | 0.449 | −0.01±0.20 | 0.486 |
| Fasting plasma glucose (mmol l−1) | 0.06±0.55 | 0.001 | 0.04±0.48 | 0.126 | 0.07±0.58 | 0.010 | 0.06±0.58 | 0.059 | 0.07±0.58 | 0.104 |
| Women | ||||||||||
| WC (cm) | 0.05±3.86 | 0.806 | 0.24±3.64 | 0.536 | 0.11±3.77 | 0.736 | −0.04±3.83 | 0.896 | −0.22±4.47 | 0.667 |
| BMI (kg m−2) | 0.03±0.77 | 0.610 | 0.08±0.80 | 0.613 | 0.05±0.75 | 0.685 | −0.01±0.79 | 0.923 | 0.04±0.69 | 0.828 |
| BW (kg) | 0.05±1.91 | 0.797 | 0.23±2.04 | 0.582 | 0.09±1.88 | 0.778 | −0.10±1.89 | 0.733 | −0.02±1.77 | 0.969 |
| Systolic blood pressure (mm Hg) | 0.51±11.43 | 0.127 | 0.20±10.92 | 0.744 | 0.73±10.90 | 0.191 | 0.40±11.88 | 0.513 | 0.77±12.44 | 0.413 |
| Diastolic blood pressure (mm Hg) | 0.19±7.32 | 0.391 | 0.28±7.02 | 0.517 | 0.25±7.30 | 0.500 | 0.06±7.46 | 0.882 | 0.18±7.49 | 0.752 |
| Triglyceride (mmol l−1) | −0.02±0.37 | 0.078 | −0.01±0.38 | 0.416 | −0.02±0.33 | 0.296 | −0.01±0.39 | 0.604 | −0.04±0.39 | 0.143 |
| HDL cholesterol (mmol l−1) | 0.01±0.20 | 0.071 | 0.02±0.21 | 0.196 | 0.03±0.20 | 0.012 | −0.01±0.20 | 0.676 | 0.00±0.20 | 0.899 |
| Fasting plasma glucose (mmol l−1) | 0.02±0.40 | 0.155 | 0.03±0.36 | 0.165 | 0.02±0.36 | 0.318 | 0.02±0.39 | 0.497 | 0.00±0.53 | 0.909 |
Abbreviations: BMI, body mass index; BW, body weight; HDL cholesterol, high-density lipoprotein cholesterol; WC, waist circumference.
P-values between values at baseline and at the 1-year follow-up were analyzed by paired t test. Changes in each variable during a 1-year period was shown.
Table 5. Pearson correlation coefficients among changes in anthropometric indices such as WC or BW and each of metabolic risk factors.
|
Age group (years) |
Comparison of correlation coefficients |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
All |
<40 |
40–49 |
50–59 |
⩾60 |
<40 vs ⩾60 |
50–59 vs ⩾60 |
||||||
| r | P | r | P | r | P | r | P | r | P | aP | aP | |
| Men | ||||||||||||
| Correlation between ΔWC and change in BW and each of metabolic risk factors | ||||||||||||
| Δ Systolic pressure | 0.14 | <0.001 | 0.14 | <0.001 | 0.16 | <0.001 | 0.15 | <0.001 | 0.08 | 0.005 | 0.012 | 0.004 |
| Δ Diastolic pressure | 0.14 | <0.001 | 0.16 | <0.001 | 0.15 | <0.001 | 0.13 | <0.001 | 0.09 | 0.003 | 0.001 | 0.075 |
| Δ Log triglycerides | 0.19 | <0.001 | 0.19 | <0.001 | 0.21 | <0.001 | 0.19 | <0.001 | 0.14 | <0.001 | 0.012 | 0.015 |
| Δ HDL cholesterol | −0.18 | <0.001 | −0.18 | <0.001 | −0.17 | <0.001 | −0.21 | <0.001 | −0.14 | <0.001 | 0.121 | 0.002 |
| Δ Log FPG | 0.11 | <0.001 | 0.09 | <0.001 | 0.15 | <0.001 | 0.10 | <0.001 | 0.09 | 0.003 | 1.000 | 0.657 |
| Δ Number of RFs | 0.13 | <0.001 | 0.11 | <0.001 | 0.15 | <0.001 | 0.13 | <0.001 | 0.11 | <0.001 | 0.478 | 0.901 |
| Δ BW | 0.71 | <.001 | 0.76 | <0.001 | 0.74 | <0.001 | 0.68 | <0.001 | 0.56 | <0.001 | <0.001 | <0.001 |
| Correlation between ΔBW and change in each of metabolic risk factor | ||||||||||||
| Δ Systolic pressure | 0.18 | <0.001 | 0.17 | <0.001 | 0.20 | <0.001 | 0.17 | <0.001 | 0.18 | <0.001 | 0.491 | 0.618 |
| Δ Diastolic pressure | 0.18 | <0.001 | 0.19 | <0.001 | 0.20 | <0.001 | 0.17 | <0.001 | 0.18 | <0.001 | 0.782 | 0.717 |
| Δ Log triglycerides | 0.26 | <0.001 | 0.28 | <0.001 | 0.28 | <0.001 | 0.23 | <0.001 | 0.25 | <0.001 | 0.104 | 0.306 |
| Δ HDL cholesterol | −0.20 | <0.001 | −0.21 | <0.001 | −0.19 | <0.001 | −0.23 | <0.001 | −0.18 | <0.001 | 0.127 | 0.022 |
| Δ Log FPG | 0.14 | <0.001 | 0.12 | <0.001 | 0.18 | <0.001 | 0.10 | <0.001 | 0.13 | <0.001 | 0.651 | 0.182 |
| Δ Number of RFs | 0.17 | <0.001 | 0.15 | <0.001 | 0.20 | <0.001 | 0.15 | <0.001 | 0.16 | <0.001 | 0.497 | 0.551 |
| Women | ||||||||||||
| Correlation between ΔWC and change in BW and each of metabolic risk factors | ||||||||||||
| Δ Systolic pressure | 0.05 | 0.000 | 0.06 | 0.053 | 0.04 | 0.081 | 0.05 | 0.038 | 0.07 | 0.090 | 0.806 | 0.579 |
| Δ Diastolic pressure | 0.05 | 0.001 | 0.05 | 0.095 | 0.07 | 0.004 | 0.03 | 0.249 | 0.05 | 0.242 | 0.878 | 0.507 |
| Δ Log triglycerides | 0.09 | <0.001 | 0.12 | <0.001 | 0.10 | <0.001 | 0.09 | <0.001 | 0.02 | 0.545 | 0.003 | 0.015 |
| Δ HDL cholesterol | −0.04 | 0.003 | −0.07 | 0.035 | −0.04 | 0.105 | −0.06 | 0.017 | 0.00 | 0.937 | 0.037 | 0.018 |
| Δ Log FPG | 0.05 | 0.001 | 0.08 | 0.008 | 0.00 | 0.932 | 0.12 | <0.001 | −0.04 | 0.362 | <0.001 | <0.001 |
| Δ Number of RFs | 0.06 | <.001 | 0.08 | 0.007 | 0.07 | 0.007 | 0.07 | 0.007 | −0.01 | 0.826 | 0.004 | 0.004 |
| Δ BW | 0.44 | <0.001 | 0.48 | <0.001 | 0.46 | <0.001 | 0.42 | <0.001 | 0.37 | <0.001 | <0.001 | 0.024 |
| Correlation between ΔBW and change in each of metabolic risk factor | ||||||||||||
| Δ Systolic pressure | 0.13 | <0.001 | 0.13 | <0.001 | 0.14 | <0.001 | 0.14 | <0.001 | 0.07 | 0.066 | 0.059 | 0.019 |
| Δ Diastolic pressure | 0.10 | <0.001 | 0.09 | 0.003 | 0.11 | <0.001 | 0.10 | <0.001 | 0.08 | 0.061 | 0.579 | 0.265 |
| Δ Log triglycerides | 0.16 | <0.001 | 0.23 | <0.001 | 0.16 | <0.001 | 0.17 | <0.001 | 0.06 | 0.123 | <0.001 | <0.001 |
| Δ HDL cholesterol | −0.09 | <0.001 | −0.08 | 0.007 | −0.13 | <0.001 | −0.11 | <0.001 | −0.03 | 0.519 | 0.075 | 0.002 |
| Δ Log FPG | 0.08 | <0.001 | 0.11 | 0.001 | 0.04 | <0.001 | 0.16 | <0.001 | −0.05 | 0.211 | <0.001 | <0.001 |
| Δ Number of RFs | 0.10 | <0.001 | 0.07 | 0.016 | 0.13 | <0.001 | 0.11 | <0.001 | 0.05 | 0.232 | 0.032 | 0.424 |
Abbreviations: BW, body weight; FPG, fasting plasma glucose; HDL cholesterol, high-density lipoprotein cholesterol; MS, metabolic syndrome; RFs, risk factors were defined according to IDF criteria; WC, waist circumference.
r, correlation coefficients using simple correlation analysis between change (Δ) in WC or BW and change in (Δ) each metabolic risk factor.
Correlation coefficients among each age group were compared after Fisher's z-transformation.
Discussion
In this study, (1) the prevalences of MS and central obesity increased with age, especially in women; (2) the WC cutoff values yielding the maximum Youden index for the detection of subjects with multiple RFs of MS increased with age in women, but not in men and the AUC of the ROC analysis of WC for the detection of those subjects significantly decreased with age in both genders, especially in women; (3) the positive correlation between the WC and the OR for the detection of subjects with multiple RFs of MS became weaker with age, especially in women; (4) in a longitudinal study, the significant correlation between ΔWC and Δ each metabolic RF, except for hypertension, and between ΔWC and Δ the number of RFs became weaker with age in women, whereas the significant correlation between ΔWC and Δ the number of RFs was not affected with age in men.
Vyssoulis et al.19 and other groups have reported generation differences in the association between WC and each RF of MS in the cross-sectional study similar to our findings.19, 20 However, to the best of our knowledge, the age-specific correlations between longitudinal changes in metabolic RFs and changes in the WC have not been previously evaluated, and the present study is the first to examine whether age-specific associations exist between longitudinal changes in the WC and changes in metabolic RFs.
We found that the association between central obesity and the accumulation of metabolic RF weakened with advancing age. The accumulation of visceral fat resulting in central obesity is reportedly more prone to low-grade inflammation in adipose tissue,31 inducing proinflammatory cytokines and leading to the accumulation of metabolic RFs such as impaired glucose tolerance, dyslipidemia, and hypertension, as well as the onset of CVD. On the other hand, aging has also been reported to cause low-grade inflammation.32 Vyssoulis et al.19 examined the high-sensitive C-reactive protein (hs-CRP) level, a marker of low-grade inflammation, in all the study participants and the association between the hs-CRP, the WC and each RF of MS in each of the age groups. They reported that with age, the association between the hs-CRP level and each of the metabolic RFs increased, whereas the associations between the hs-CRP level and the WC weakened. Therefore, they concluded that as an age-related increase in low-grade inflammation, rather than central obesity, affected the onset of each of the components of MS, the associations between central obesity and each of the components of MS weakened with age. In addition, BP and PG reportedly increase with advancing age because of the accumulation of vascular damage33 and a decrease in insulin secretion,34, 35, 36 respectively. Moreover, another report demonstrated that the cardiovascular risk associated with obesity reportedly decreases markedly with age.37 Therefore, our finding that the associations between central obesity and the accumulation of metabolic RF became weaker with age may be caused by age-related increases in low-grade inflammation, the accumulation of vascular damage and a decrease in insulin secretion.
In this study, we also observed that the dissociation between BW or WC and the accumulation of metabolic RFs with age were stronger in women than in men. Previous reports have demonstrated that during weight reduction, the men lost more visceral fat than the women, whereas subcutaneous fat loss was more pronounced in women than in men.38 It was also reported that the greater reduction in visceral fat seen in men was accompanied by a more pronounced improvement in the metabolic risk profile.38 Although we did not measure the VFA in the present study, we observed that the correlation coefficient between the ΔWC and ΔBW over 1-year period were greater in men than in women in the longitudinal study. As WC has been reported to have the closest relationship with VFA,5, 6 our results as mentioned above may reflect that men tended to change more visceral fat than women during BW change, which is compatible with a previous report.38 Moreover, several cross-sectional studies examining the correlation between WC and VFA5, 6 demonstrated that the regression coefficient for VFA vs WC was greater in men than in women, indicating that the change in VFA associated with the change in WC was more pronounced in men than in women. From these findings, we speculated that the gender difference in the dissociation between BW or WC and the accumulation of metabolic RFs with age in our study may be caused by the gender differences in changes in visceral fat during weight change and in the relationship between visceral fat and WC.
Several reports have examined the WC and the prevalence of MS according to generation;16, 39 these studies found that the prevalence of central obesity and MS increased with age in women, but not in men, which were compatible with our data. This finding seems to support the hypothesis that aging has an inevitable impact on a woman's body composition.40 The estrogen level of pubescent females causes the accumulation of fat in the hips and thighs, but as the estrogen level begins to decrease with age, the accumulated fat starts to be redistributed in the abdomen and/or waist; thus, the WC begins to increase when puberty comes to an end, even in healthy women.40 This process may be independent of the development of obesity. In Japan, research performed by a Japanese underwear company demonstrated that the WC size starts to increase after ∼40 years in women.41 In consideration of these observations, it is understandable that the mean WC and the prevalence of MS increased with age in women.
Several reports have examined appropriate age-specific WC cutoff points for the detection of subjects with multiple RFs of MS.17, 18 They reported that the cutoff value was smaller in subjects under the age of 40 years than in higher age groups in women but not in men. These findings were identical to our data. Furthermore, they also reported that the AUC of the ROC curve of WC for the detection of multiple RFs of MS tended to decrease with age in both genders. From this finding, the association of WC with the detection of multiple RFs of MS is thought to be stronger among young individuals and to be weaker among elderly individuals. The reason for this may be that in the elderly, MS may also be caused by RFs other than central obesity (such as age-related low-grade inflammation, hyperglycemia and high blood pressure, as mentioned above).
In the present study, the prevalence of MS based on the IDF criteria was 12.0% in men and 10.5% in women. These prevalences were lower than those in a previous study.16 In the present study, to examine the correlations between longitudinal changes in the WC or BW and metabolic RFs, we excluded subjects who were currently receiving medical treatment for hyperlipidemia, hypertension or diabetes because the metabolic RFs in these individuals might be affected by factors other than the change in WC and BW, such as medications. On the other hand, the previous study included the subjects who were currently receiving medical treatment for hyperlipidemia, hypertension or diabetes as study subjects.16 The differences in the study subjects between their report and ours might explain the lower prevalences of MS and its components in the present study.
The Diabetes Prevention Program (DPP) and other studies have shown that weight loss reduces the risk of diabetes, hypertension and dyslipidemia in patients with prediabetes or MS.42, 43, 44 Moreover, it has been reported that lifestyle intervention improved cardiovascular RFs in post-menopausal women.45, 46 Our observations suggest that the improvement of these RFs arising from a decrease in WC or BW per se might be less predictable in older subjects, especially in women. These considerations do not deny the importance of lifestyle modifications for the prevention of diabetes and improvement of each RF of MS, even in elderly obese/overweight women.
The strengths of our study include not only its cross-sectional design but also its longitudinal design and the large population of unmedicated subjects. However, the present study has some potential limitations. First, the subjects enrolled in our study chose to undergo annual health examinations; they were, therefore, likely to be more health-conscious than the average, which might have caused some bias in the current study. Second, although we utilized a longitudinal design, the study was retrospective in nature and the follow-up period was 1 year. We examined whether a follow-up period of 1 year was appropriate for observing longitudinal changes in the components of MS, as mentioned in the Materials and methods section. We estimated the sample size required to identify longitudinal changes in the components of MS during a 1-year follow-up period based on the results of previous studies25, 26 and found that a required sample size of 153 men and 76 women would be necessary. In this study, the smallest number of individuals in any age group was 1158 in men aged ⩾60 years and 622 in women aged ⩾60 years (Table 1). As these numbers were much larger than the sample size required to identify longitudinal changes in the components of MS during a 1-year follow-up period, we believe that a follow-up period of 1 year was appropriate for the presently reported longitudinal study. Third, we only measured the WC at the umbilical level, which is the Japanese standard method, and not at the midpoint between the lower borders of the rib cage and the iliac crest, which is the world-wide standard method. Therefore, our findings might be applicable to the Japanese population, but not for other populations. However, we estimated the WC (world-wide method) using the WC measured at the umbilical level using the method recently reported by Matsushita et al.47 We then used the estimated WC (world-wide method) as the diagnostic criterion for MS and re-analyzed the association between the WC and metabolic RFs in each generation and sex. We also observed the generation and sex differences in the prevalence of MS and the cutoff value for detecting subjects with multiple RFs (Supplementary Tables 1 and 2) and obtained results showing that aging attenuated the association between central obesity and the accumulation of metabolic RFs (data not shown), which were identical to the results obtained when the WC measured at the umbilical level was used.
In conclusion, we clarified that the association between central obesity and the accumulation of metabolic RFs weakened with age in ROC analyses, cross-sectional and longitudinal studies, especially in women. Further, we speculated that these findings may be caused by age-related increases in low-grade inflammation, accumulation of the vascular damage and decrease of the insulin secretion. Therefore, men and younger adult women with obesity/MS should be recommended to adopt positive lifestyle modifications. However, this study was a retrospective study over a 1-year period. A prospective study involving a larger number of subjects is needed to clarify the present findings.
Acknowledgments
We thank Dr Kenji Matsui, MD, PhD, who is the head of the Office for Research Ethics and Bioethics, National Cerebral and Cardiovascular Center of Japan, for his useful advice regarding the ethical aspects of our study.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Nutrition & Diabetes website (http://www.nature.com/nutd)
Supplementary Material
References
- Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24:e13–e18. doi: 10.1161/01.ATV.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- The Examination Committee of Criteria for ‘Obesity Disease' in Japan, Japan Society for the Study of Obesity New criteria for 'obesity disease' in Japan. Circulation J. 2002;66:987–992. doi: 10.1253/circj.66.987. [DOI] [PubMed] [Google Scholar]
- Kashihara H, Lee JS, Kawakubo K, Tamura M, Akabayashi A. Criteria of waist circumference according to computed tomography-measured visceral fat area and the clustering of cardiovascular risk factors. Circulation J. 2009;73:1881–1886. doi: 10.1253/circj.cj-09-0183. [DOI] [PubMed] [Google Scholar]
- Sparrow D, Borkan GA, Gerzof SG, Wisniewski C, Silbert CK. Relationship of fat distribution to glucose tolerance. Results of computed tomography in male participants of the Normative Aging Study. Diabetes. 1986;35:411–415. doi: 10.2337/diab.35.4.411. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y. Metabolic syndrome--definition and diagnostic criteria in Japan. J Atheroscler Thromb. 2005;12:301. doi: 10.5551/jat.12.301. [DOI] [PubMed] [Google Scholar]
- Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J. The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb. 2005;12:295–300. doi: 10.5551/jat.12.295. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabetic Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- Hara K, Matsushita Y, Horikoshi M, Yoshiike N, Yokoyama T, Tanaka H, et al. A proposal for the cutoff point of waist circumference for the diagnosis of metabolic syndrome in the Japanese population. Diabetes Care. 2006;29:1123–1124. doi: 10.2337/diacare.2951123. [DOI] [PubMed] [Google Scholar]
- Matoba Y, Inoguchi T, Nasu S, Suzuki S, Yanase T, Nawata H, et al. Optimal cut points of waist circumference for the clinical diagnosis of metabolic syndrome in the Japanese population. Diabetes Care. 2008;31:590–592. doi: 10.2337/dc07-0802. [DOI] [PubMed] [Google Scholar]
- Sato A, Asayama K, Ohkubo T, Kikuya M, Obara T, Metoki H, et al. Optimal cutoff point of waist circumference and use of home blood pressure as a definition of metabolic syndrome: the Ohasama study. Am J Hypertens. 2008;21:514–520. doi: 10.1038/ajh.2007.88. [DOI] [PubMed] [Google Scholar]
- Narisawa S, Nakamura K, Kato K, Yamada K, Sasaki J, Yamamoto M. Appropriate waist circumference cutoff values for persons with multiple cardiovascular risk factors in Japan: a large cross-sectional study. J Epidemiol. 2008;18:37–42. doi: 10.2188/jea.18.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura R, Nakagami T, Tominaga M, Yoshiike N, Tajima N. Prevalence of metabolic syndrome and optimal waist circumference cut-off values in Japan. Diabetes Res Clin Practice. 2007;78:77–84. doi: 10.1016/j.diabres.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Matsushita Y, Nakagawa T, Yamamoto S, Takahashi Y, Yokoyama T, Mizoue T, et al. Visceral fat area cutoff for the detection of multiple risk factors of metabolic syndrome in Japanese: the Hitachi Health Study. Obesity (Silver Spring) 2012;20:1744–1749. doi: 10.1038/oby.2011.285. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Hirose H, Kawabe H, Saito I. Characteristics of reference intervals of metabolic factors in healthy Japanese: a proposal to set generation- and gender-specific diagnostic criteria for metabolic syndrome. J Atheroscler Thromb. 2009;16:113–120. doi: 10.5551/jat.e609. [DOI] [PubMed] [Google Scholar]
- Vyssoulis G, Karpanou E, Adamopoulos D, Tzamou V, Stefanadis C, Vischer UM. Effect of age on interdependence and hierarchy of cardiovascular risk factors in hypertensive patients. Am J Cardiol. 2011;108:240–245. doi: 10.1016/j.amjcard.2011.03.035. [DOI] [PubMed] [Google Scholar]
- Oda E, Kawai R. Age- and gender-related differences in correlations between abdominal obesity and obesity-related metabolic risk factors in Japanese. Intern Med. 2009;48:497–502. doi: 10.2169/internalmedicine.48.1765. [DOI] [PubMed] [Google Scholar]
- AMDA International Medical Information Center Questionnaire on specific health examination: Japan, 2008 available at http://amda-imic.com/oldpage/amdact/PDF/eng/spe-he-ex-e.pdf (accessed 27 September 2013).
- Nakanishi N, Makino K, Nishina K, Suzuki K, Tatara K. Relationship of light to moderate alcohol consumption and risk of hypertension in Japanese male office workers. Alcohol Clin Exp Res. 2002;26:988–994. doi: 10.1097/01.ALC.0000021161.94001.33. [DOI] [PubMed] [Google Scholar]
- Ohmori S, Kiyohara Y, Kato I, Kubo M, Tanizaki Y, Iwamoto H, et al. Alcohol intake and future incidence of hypertension in a general Japanese population: the Hisayama study. Alcohol Clin Exp Res. 2002;26:1010–1016. doi: 10.1097/01.ALC.0000021147.31338.C2. [DOI] [PubMed] [Google Scholar]
- Nakashita Y, Nakamura M, Kitamura A, Kiyama M, Ishikawa Y, Mikami H. Relationships of cigarette smoking and alcohol consumption to metabolic syndrome in Japanese men. J Epidemiol. 2010;20:391–397. doi: 10.2188/jea.JE20100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita Y, Nakagawa T, Yamamoto S, Takahashi Y, Yokoyama T, Mizoue T, et al. Effect of longitudinal changes in visceral fat area and other anthropometric indices to the changes in metabolic risk factors in Japanese men: the Hitachi Health Study. Diabetes Care. 2012;35:1139–1143. doi: 10.2337/dc11-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrahmoune H, Herbeth B, Samara A, Marteau JB, Siest G, Visvikis-Siest S. Five-year alterations in BMI are associated with clustering of changes in cardiovascular risk factors in a gender-dependant way: the Stanislas study. Int J Obes (Lond) 2008;32:1279–1288. doi: 10.1038/ijo.2008.94. [DOI] [PubMed] [Google Scholar]
- Ministry of Education C, Sports, Science and Technology and Ministry of Health, Labour and Welfare Japan. Ethical Guidelines for Epidemiological Research: Japan, 2007, available at http://www.lifescience.mext.go.jp/files/pdf/n796_01.pdf (accessed 23 August 2013).
- First Department of Internal Medicine, Faculty of Medicine, Toyama University Home Page (In Japanese). Available at http://www.med.u-toyama.ac.jp/medicin1/ .
- Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- Perkins NJ, Schisterman EF. The inconsistency of ‘optimal' cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163:670–675. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a ‘set up' for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- The DECODE Study Group Age- and sex-specific prevalences of diabetes and impaired glucose regulation in 13 European cohorts. Diabetes Care. 2003;26:61–69. doi: 10.2337/diacare.26.1.61. [DOI] [PubMed] [Google Scholar]
- Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metabol. 2003;284:E7–12. doi: 10.1152/ajpendo.00366.2002. [DOI] [PubMed] [Google Scholar]
- Moller N, Gormsen L, Fuglsang J, Gjedsted J. Effects of ageing on insulin secretion and action. Hormone Res. 2003;60 (Suppl 1:102–104. doi: 10.1159/000071233. [DOI] [PubMed] [Google Scholar]
- Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth A, Steinmetz B. Gender differences in changes in subcutaneous and intra-abdominal fat during weight reduction: an ultrasound study. Obesity Res. 1998;6:393–399. doi: 10.1002/j.1550-8528.1998.tb00370.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi J, Nishimura K, Matoba M, Maekawa N, Mabuchi H. Generation and gender differences in the components contributing to the diagnosis of the metabolic syndrome according to the Japanese criteria. Circulation J. 2007;71:1734–1737. doi: 10.1253/circj.71.1734. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. Adipose tissue distribution and function. Int J Obes. 1991;15 (Suppl 2:67–81. [PubMed] [Google Scholar]
- Yokoyama H, Hirose H, Kanda T, Kawabe H, Saito I. Relationship between waist circumferences measured at the umbilical level and midway between the ribs and iliac crest—a solution to the debate on optimal waist circumference standards in the diagnostic criteria of metabolic syndrome in Japan. J Atheroscler Thromb. 2011;18:735–743. doi: 10.5551/jat.7369. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmarzyk PT, Leon AS, Wilmore JH, Skinner JS, Rao DC, Rankinen T, et al. Targeting the metabolic syndrome with exercise: evidence from the HERITAGE Family Study. Med Sci Sports Exerc. 2003;35:1703–1709. doi: 10.1249/01.MSS.0000089337.73244.9B. [DOI] [PubMed] [Google Scholar]
- Ratner R, Goldberg R, Haffner S, Marcovina S, Orchard T, Fowler S, et al. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program. Diabetes Care. 2005;28:888–894. doi: 10.2337/diacare.28.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, Bradshaw AJ. The future of obesity reduction: beyond weight loss. Nat Rev Endocrinol. 2009;5:319–325. doi: 10.1038/nrendo.2009.78. [DOI] [PubMed] [Google Scholar]
- Nicklas BJ, Dennis KE, Berman DM, Sorkin J, Ryan AS, Goldberg AP. Lifestyle intervention of hypocaloric dieting and walking reduces abdominal obesity and improves coronary heart disease risk factors in obese, postmenopausal, African-American and Caucasian women. J Gerontol Ser A Biol Sci Med Sci. 2003;58:181–189. doi: 10.1093/gerona/58.2.m181. [DOI] [PubMed] [Google Scholar]
- Matsushita Y, Tomita K, Yokoyama T, Mizoue T. Relations between waist circumference at four sites and metabolic risk factors. Obes (Silver Spring) 2010;18:2374–2378. doi: 10.1038/oby.2010.33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.