Abstract
Background
Whether carcinomas of the ampulla of Vater should be classified with biliary tract tumors and treated in a similar manner remains unknown. We sought to compare the outcomes of similarly staged periampullary adenocarcinomas (AAs) and analyze the chemotherapy responsiveness of AAs.
Patients and methods
A total of 905 patients with resected periampullary adenocarcinomas were identified from a prospective surgical registry from 1988 to 2010. A second cohort of 64 metastatic AA patients from 1992 to 2009 who received either front-line fluoropyrimidine-based or gemcitabine-based chemotherapy was also identified.
Results
Overall survival (OS) for AAs was similar to survival with duodenal adenocarcinomas, but was significantly different from both extrahepatic biliary and pancreatic adenocarcinomas (P < 0.001 for each comparison). In multivariate analysis, AAs had a significantly improved OS in comparison with extrahepatic biliary adenocarcinomas (HR = 1.97, P = 0.006). Fluoropyrimidine-based as opposed to gemcitabine-based chemotherapy for metastatic AAs resulted in a significant improvement in time to progression (P = 0.001) but only a trend toward benefit for OS (P = 0.07) in multivariate analysis.
Conclusions
Differences in the natural history of ampullary and extrahepatic biliary adenocarcinomas exist. Analyses of metastatic ampullary adenocarcinomas suggest that fluoropyrimidine-based chemotherapy may represent a more appropriate front-line chemotherapy approach.
Keywords: ampullary adenocarcinomas, chemotherapy, fluoropyrimidine, gemcitabine, periampullary
introduction
Until recently, carcinoma of the ampulla of Vater has been classified by the World Health Organization of Tumors as a cancer of the extrahepatic biliary tract. However, as distinct epithelia coalesce within the ampulla (duodenal, pancreatic, and biliary) the exact epithelium origin for these tumors is unknown, and in the most recent fourth edition of the World Health Organization of Tumors, ampullary carcinomas are discussed separately from other periampullary carcinomas. Given this uncertainty, the optimal chemotherapy regimen for ampullary adenocarcinomas (AAs) remains undefined.
Single institution series have suggested improved outcomes for AAs in comparison to extrahepatic cholangiocarcinomas [1]. Though no single study has compared stage stratified outcomes for AAs versus extra hepatic cholangiocarcinomas, a comparison of two separate studies evaluating the SEER database suggests superior 5-year relative survival (RS) for AAs compared with extrahepatic biliary adenocarcinomas when stratified by disease stage: localized, 45% versus 34%; and regional, 31% versus 18%, respectively [2, 3].
In contrast to extrahepatic biliary adenocarcinomas, AAs demonstrate differing histological subtypes: intestinal, pancreaticobiliary, or mixed [4]. These histological subtypes have demonstrated prognostic relevance in some [5–7] but not all studies [8–10]. Given the histopathological heterogeneity within AAs and their differing clinical outcomes from other extrahepatic biliary tract adenocarcinomas, conclusive data regarding the use of chemotherapy for ampullary cancer are not available.
The purpose of this study was twofold. First, we sought to better determine the difference between extrahepatic biliary cancers and AAs in a large dataset that would enable a comparison of similarly staged patients. In addition, to evaluate the impact of either gemcitabine-based or fluoropyrimidine-based chemotherapy on patients with metastatic AA.
patients and methods
periampullary adenocarcinoma cohort
From a prospectively maintained surgical database, all patients with a periampullary adenocarcinoma (primary from ampulla of Vater, duodenum, pancreas, or extrahepatic bile duct) who underwent definitive resection for locoregional disease at the University of Texas MD Anderson Cancer Center (UTMDACC) from September 1988 to March 2010 were identified. A total of 905 patients staged I, II, or III represent the analyzed population. Tumor stage was determined according to the American Joint Committee on Cancer staging system sixth edition.
metastatic ampullary adenocarcinoma cohort
From a separate UTMDACC tumor registry, a total of 138 AA patients with metastatic disease from February 1992 to July 2010 were identified. Thirty-one had stage IV disease and 107 had developed distant metastatic disease following a pancreaticoduodenectomy for AA. For the patients who presented with metastatic disease, only those with an endoscopically visualized mass at the ampulla of Vater, which was biopsy positive for adenocarcinoma, and having a computed tomography (CT) scan demonstrating no evidence of a primary pancreatic mass were included. In addition, all patients were required to have pathology reviewed and confirmed at UTMDACC, treatment with either fluoropyrimidine- or gemcitabine-based chemotherapy as first-line chemotherapy for greater than one month, radiographically visible disease at the time of treatment, and follow-up imaging study completed. A total of 64 patients met these criteria, of whom 25 were also represented in our periampullary adenocarcinoma cohort. Response to chemotherapy was evaluated based on the treating physician's best response assessment. For cases with available pathology (17 cases), histological subtype was intestinal in 4, pancreaticobiliary in 7, and mixed in 6 per histology re-review of the primary resection specimen by a gastrointestinal pathologist (HW). Due to the small number, no further histology subset analyses were conducted.
statistical analysis
For the resected periampullary adenocarcinoma cohort, OS is defined as the time interval between the date of pathological diagnosis and the date of death. For the metastatic AA cohort, OS and time to progression (TTP) are defined from the date of chemo start for distant metastasis to the either date of death or date of disease progression, respectively. The probabilities of OS or progression-free are estimated using the method of Kaplan and Meier and log-rank test was used to assess the difference in OS or TTP among subgroups of patients. Categorical variables were compared using Fisher's exact test and continuous variables were compared by Wilcoxon rank-sum test between groups.
Univariate and multivariate Cox proportional hazards models including all covariates with a P value of <0.1 in a univariate analysis were fit for both OS and TTP. For the resected periampullary adenocarcinoma group potential covariates assessed in the multivariate model were primary tumor site, age, gender, T stage, N stage, surgical margin status, neoadjuvant, and adjuvant treatment. A backward model selection procedure was applied. For the metastatic ampullary cancer group, the potential covariates in our multivariate model included the year of diagnosis, age, gender, tumor differentiation, presence of ampullary adenoma at initial diagnosis, perioperative chemotherapy, time from surgery to distant metastasis, chemo with gemcitabine or fluoropyrimidine, chemotherapy combined with platinum. P values <0.05 were considered to be statistically significant.
This study was approved by the institutional review board of UTMDACC.
results
overall survival for the periampullary adenocarcinoma cohort
In the resected periampullary adenocarcinoma cohort, there were 131 ampullary, 63 duodenal, 41 extrahepatic biliary and 670 pancreatic adenocarcinoma patients, supplementary Table S1, available at Annals of Oncology online.
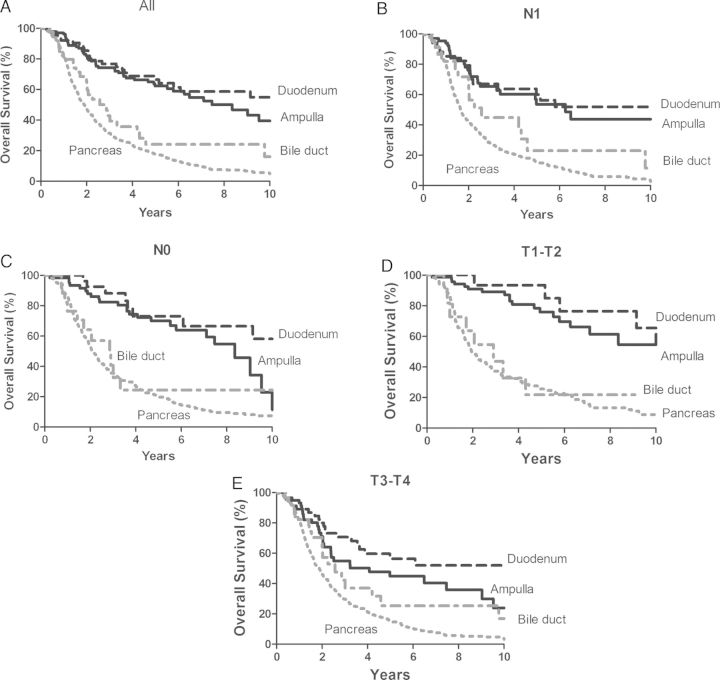
The median OS for all 905 patients was 2.3 years (95% CI 2.1–2.5 years). The estimated 5-year OS and 10-year OS rates of AA patients were 62% and 40%, which were not significantly different from duodenal adenocarcinoma (66% and 55%, P = 0.19). The 5-year OS and 10-year OS rates for the extrahepatic biliary adenocarcinoma cohort were 24% and 16%, and for the pancreatic adenocarcinoma cohort were 17% and 5%, respectively. Both were significantly different from AA patients (both P < 0.001), Figure 1A.
Figure 1.
Overall survival (OS) for 905 resected periampullary adenocarcinoma cases stratified by tumor type: (A) all, (B) Lymph node-positive cases, (C) Lymph node-negative cases, (D) T1-T2 cases, (E) T3-T4 cases.
When stratified by N stage, significant differences between ampullary compared with biliary adenocarcinoma (P = 0.003 for N0 group and P = 0.044 for N1 group, respectively) and ampullary compared with pancreatic adenocarcinoma (both P < 0.001) were seen, Figure 1B and C. No significant differences in OS between ampullary and duodenal adenocarcinoma patients (P = 0.08 and 0.67 in the N0 and N1 group, respectively) were identified. When stratified by T stage, patients with T1–T2 AAs had significantly better survival compared with biliary (P < 0.0001) and pancreatic adenocarcinomas (P < 0.0001), but not duodenal adenocarcinomas (P = 0.15). However, while patients with T3–T4 AAs had improved survival compared with those having T3–T4 pancreatic cancers (P < 0.0001), their survival was no better than patients with T3–T4 biliary (P = 0.34) or duodenal adenocarcinomas (P = 0.07), Figure 1D and E.
In the multivariate Cox proportional hazard model for OS, AAs differed from all other periampullary adenocarcinomas: pancreatic adenocarcinoma (HR = 4.13; 95% CI: 2.95–5.77, P < 0.0001), extrahepatic biliary adenocarcinoma (HR = 1.97; 95% CI: 1.22–3.19, P = 0.006), and duodenal adenocarcinoma (HR = 0.62; 95% CI: 0.38–1.00, P = 0.05), Table 1.
Table 1.
Fitted multivariable Cox proportional hazards model for overall survival (OS) in resected periampullary adenocarcinoma patients
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Primary site | |||
| Ampulla (as reference) | 1 | N/A | N/A |
| Duodenum | 0.62 | 0.38–1 | 0.05 |
| Bile duct | 1.97 | 1.22–3.19 | 0.006 |
| Pancreas | 4.13 | 2.95–5.77 | <0.0001 |
| pT stage (T1–T2 as reference) | |||
| T3 | 1.27 | 1.03–1.56 | 0.02 |
| T4 | 2.3 | 1.5–3.52 | 0.0001 |
| pN1 (pN0 as reference) | 1.21 | 1.03–1.43 | 0.02 |
| Neoadjuvant treatment | 0.76 | 0.61–0.93 | 0.008 |
| Adjuvant treatment | 0.73 | 0.6–0.91 | 0.004 |
HR, hazard ratio; CI, confidence interval.
metastatic ampullary adenocarcinoma cohort
First-line chemotherapy for metastatic AA was fluoropyrimidine-based in 40 patients and gemcitabine-based in 24 patients. The clinical characteristics for these two groups are summarized in supplementary Table S2, available at Annals of Oncology online. There were no statistically significant differences seen regarding the time period of treatment (1992–1999 versus 2000–2009), median age, presenting TNM stage, tumor differentiation, or distant metastatic sites between the two groups. For the 50 patients who developed metastatic disease following a pancreaticoduodenectomy, similar rates of prior adjuvant/neoadjuvant chemotherapy use (52 versus 62%, P = 1.00) and median time-to-distant metastases (16.6 months versus 16.8 months, P = 0.98) were seen in the gemcitabine and fluoropyrimidine groups, respectively.
Single-agent chemotherapy was used in eight (20%) patients treated with fluoropyrimidine and nine (38%) patients treated with gemcitabine. The most common chemotherapy combination involved the addition of a platinum agent, occurring in 31 patients (55%) treated with a fluoropyrimidine and 9 patients (38%) treated with gemcitabine, supplementary Table S3, available at Annals of Oncology online.
The response to chemotherapy was evaluated for both the groups and a treatment response was seen in 23 (58%) of patients treated with fluoropyrimidine and in 5 (21%) patients treated with gemcitabine (P = 0.005). For the fluoropyrimidine treated group and the gemcitabine treated group, the rates of stable disease were 30% and 38%, respectively and the rates of progressive disease were 13 and 42%, respectively. Five patients had a complete radiographic response, four treated with fluoropyrimidine-based therapy, and one treated with gemcitabine-based therapy. Metastatic disease was biopsy proven in two of these cases and none of these patients received consolidative radiation or metastectomies. Three of these patients remain disease-free at 8, 30, and 69 months from the start of chemotherapy.
Fluoropyrimidine-based chemotherapy was associated with a significant improvement in TTP with a median TTP of 9.2 months (range: 1.6–69.3 months) compared with 3.4 months (range 0.8–34.3 months) for gemcitabine based chemotherapy, P = 0.0006, Figure 2A. The fluoropyrimidine group also had a trend toward improved median OS: 19.1 months (range: 2.6–70 months) versus 12.3 months (range: 2.9–55.7 months), respectively, P = 0.06, Figure 2B. When patients were grouped by platinum-based chemotherapy (N = 40, 63%) compared with non-platinum based chemotherapy (N = 24, 38%), there was no difference in either OS or TTP (P = 0.26 and 0.10, respectively.)
Figure 2.
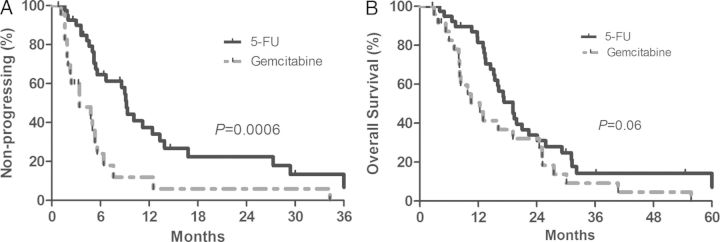
Outcomes for 64 metastatic ampullary adenocarcinoma patients treated with either first-line fluoropyrimidine-based or gemcitabine-based chemotherapy: (A) time to progression (TTP) and (B) OS.
The univariate and multivariate analyses for OS and TTP on metastatic AA patients are shown in supplementary Table S4, available at Annals of Oncology online. In univariate analysis, the use of gemcitabine compared with fluoropyrimidine was a significant factor for TTP (HR = 2.76; 95% CI 1.51–5.04, P = 0.001) and a borderline significant factor for OS (HR = 1.68; 95% CI 0.97–2.92, P = 0.07). The final fitted Cox model for OS and TTP includes chemotherapy treatment (gemcitabine-based or fluoropyrimidine-based) as a covariate, and shows a benefit for the use of fluoropyrimidine-based front-line treatment for TTP (P = 0.001) and a trend favoring benefit from fluoropyrimidine-based treatment for OS (P = 0.07). In the subgroup of 50 localized AA patients who developed distant metastases following pancreaticoduodenectomy, there were similar findings in both univariate and multivariate analyses. The use of gemcitabine-based regimen was a significant poor prognostic factor versus fluoropyrimidine in TTP (HR = 2.52; 95% CI 1.28–4.97, P = 0.007) and a borderline poor factor for OS (HR = 1.82; 95% CI 0.97–3.43, P = 0.06).
discussion
There are limited data and no consensus regarding the appropriate chemotherapy for patients with adenocarcinomas arising from the ampulla of Vater. As AAs have been grouped with biliary tract cancers, they have generally been treated with gemcitabine-based chemotherapy, which is the standard approach for biliary tract cancers. However, our data clearly show that for similarly staged patients, differences in the natural history of ampullary and extrahepatic biliary adenocarcinomas exist. In addition, our analysis of patients with metastatic AAs suggests that fluoropyrimidine-based as opposed to gemcitabine-based chemotherapy may represent a better platform for chemotherapy delivery.
Our 905 patient periampullary adenocarcinoma population represents one of the largest datasets of patients with these cancers. In contrast to other reports which compared overall outcomes for all patients, we were able to compare the outcomes for patients stratified by both nodal status and tumor stage [1]. This is important as an early stage at presentation of AAs has been suggested to explain their improved outcomes since these tumors can obstruct the biliary tree at a small size. Consistent differences existed between these two tumor types when stratified by N or T stage and in multivariate analysis.
Although these findings suggest that AAs have a more favorable biology compared with other periampullary tumor types, they do not address the suspected heterogeneity within AAs. A number of prior studies have attempted to identify prognostically distinct subtypes using differences in histology, cytokeratin expression, mucin expression, microsatellite instability, and intestinal-specific markers [4, 5, 7, 9–13]. In the recent ESPAC-3 study that randomly assigned 434 patients with resected non-pancreatic periampullary adenocarcinoma to either observation or adjuvant chemotherapy, stratifying AA cohort into either intestinal or pancreaticobiliary subtypes demonstrated a significant difference for DFS (45.7 versus 20.6 months, P = 0.01) and a non-significant difference for OS (56 versus 43.1 months, P = 0.28) that favored the intestinal subtype [14].
At present, AAs are often approached as biliary tract tumors with gemcitabine-based treatment. In the recent phase III ABC-02 study that randomly assigned biliary cancer patients to gemcitabine with or without cisplatin, patients with ampulla of Vater were included [15]. Though only 20 cases were enrolled, the hazard ratios for this subgroup suggested a benefit from combination therapy with gemcitabine and cisplatin in comparison to gemcitabine alone (HR = 0.62, 95% CI 0.21–1.82). Two phase II studies have also evaluated fluoropyrimidine-based therapy for AAs [16, 17]. One phase II study that evaluated CAPOX chemotherapy enrolled 12 patients with metastatic AAs and demonstrated a RR of 33% for this subset [17] . The second phase II study evaluating FAM enrolled only four patients with AAs and did not report separate outcomes for this small subset [16]. Though the recent ESPAC-3 demonstrated no difference in OS between observation and adjuvant chemotherapy (P = 0.25), a difference favoring adjuvant chemotherapy was noted in a secondary multivariate analysis (P = 0.03). No difference between the bolus 5-FU and gemcitabine chemotherapy arms was seen, with both 5-FU and gemcitabine demonstrating similar hazard ratios for both OS (0.79 and 0.70) and DFS (0.68 and 0.69), respectively, in multivariate modeling.
Though limited by a small sample size, our data also suggest that fluoropyrimidine-based therapy may represent a more active front-line systemic chemotherapy compared with gemcitabine-based treatment for patients with AA. Although fluoropyrimidine-treated patients had an improved TTP in comparison to gemcitabine-treated patients in multivariate analysis (P = 0.001), there was only a trend favoring fluoropyrimidine-based treatment for OS (P = 0.07). In order to explore the possibility of differential chemotherapy benefit based upon histological subtypes of AA, we reviewed the histological subtype on all available cases. However, as we were only able to obtain histology on 17 cases (27%), we could not make any conclusions based upon histological subtype.
There are limitations in our study. This was a retrospective study and included patients treated over a long time period. Treatment patterns differed between patients and periampullary tumor types. Though the use of neoadjuvant therapy differed across periampullary tumor types, similar rates were seen for ampullary, duodenal, and extrahepatic bile duct tumors. Analysis of our metastatic ampullary cohort was limited by small size, though this cohort does represent the largest cohort of metastatic AA patients treated with chemotherapy. The choice of chemotherapy agents was based upon each individual treating physician and other factors aside from the analyzed baseline characteristics may have impacted this choice. As the ampulla of Vater is an anatomically small structure with multiple other adjacent tumor types nearby (pancreatic, biliary, and duodenal), determination of the primary site without pathological review of the ampulla of Vater is clinically challenging. To address this difficulty, we applied strict endoscopic and radiographic criteria to all patients who presented with stage IV disease. In addition, as the majority of our metastatic ampullary patients developed metastases following a pancreaticoduodenectomy, we analyzed this population (n = 50) separately. The similar findings in this subgroup, in which histopathological evaluation of the primary tumor provided certainty regarding the primary tumor site, support our selection criteria for stage IV AAs.
Similar to previous data, our results suggest that resected ampullary and extrahepatic biliary adenocarcinomas have distinctly different natural histories. Such results lend credence to the consideration of a non-gemcitabine based chemotherapy approach for AAs. As our evaluation of chemotherapy in AAs is both retrospective in nature and limited by small sample size, our findings suggesting improved outcomes with fluoropyrimidine- over gemcitabine-based chemotherapy should be viewed as hypothesis generating. Prospective studies evaluating optimal chemotherapy for ampullary cancer with stratification based on the histological subtype of AAs are needed.
funding
The Kavanagh Foundation and NCI Core Grant #CA 16672.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Yeo CJ, Sohn TA, Cameron JL, et al. Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg. 1998;227:821–831. doi: 10.1097/00000658-199806000-00005. doi:10.1097/00000658-199806000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albores-Saavedra J, Schwartz AM, Batich K, et al. Cancers of the ampulla of vater: demographics, morphology, and survival based on 5625 cases from the SEER program. J Surg Oncol. 2009;100:598–605. doi: 10.1002/jso.21374. doi:10.1002/jso.21374. [DOI] [PubMed] [Google Scholar]
- 3.Nathan H, Pawlik TM, Wolfgang CL, et al. Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointest Surg. 2007;11:1488–1496. doi: 10.1007/s11605-007-0282-0. discussion 1496–1487 doi:10.1007/s11605-007-0282-0. [DOI] [PubMed] [Google Scholar]
- 4.Kimura W, Futakawa N, Yamagata S, et al. Different clinicopathologic findings in two histologic types of carcinoma of papilla of Vater. Jpn J Cancer Res. 1994;85:161–166. doi: 10.1111/j.1349-7006.1994.tb02077.x. doi:10.1111/j.1349-7006.1994.tb02077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohler I, Jacob D, Budzies J, et al. Phenotypic and genotypic characterization of carcinomas of the papilla of Vater has prognostic and putative therapeutic implications. Am J Clin Pathol. 2011;135:202–211. doi: 10.1309/AJCPCTCUQSYI89YT. doi:10.1309/AJCPCTCUQSYI89YT. [DOI] [PubMed] [Google Scholar]
- 6.Westgaard A, Tafjord S, Farstad IN, et al. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer. 2008;8:170. doi: 10.1186/1471-2407-8-170. doi:10.1186/1471-2407-8-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruemmele P, Dietmaier W, Terracciano L, et al. Histopathologic features and microsatellite instability of cancers of the papilla of vater and their precursor lesions. Am J Surg Pathol. 2009;33:691–704. doi: 10.1097/PAS.0b013e3181983ef7. doi:10.1097/PAS.0b013e3181983ef7. [DOI] [PubMed] [Google Scholar]
- 8.Fischer HP, Zhou H. Pathogenesis of carcinoma of the papilla of Vater. J Hepatobiliary Pancreat Surg. 2004;11:301–309. doi: 10.1007/s00534-004-0898-3. doi:10.1007/s00534-004-0898-3. [DOI] [PubMed] [Google Scholar]
- 9.Zhou H, Schaefer N, Wolff M, et al. Carcinoma of the ampulla of Vater: comparative histologic/immunohistochemical classification and follow-up. Am J Surg Pathol. 2004;28:875–882. doi: 10.1097/00000478-200407000-00005. doi:10.1097/00000478-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Roh YH, Kim YH, Lee HW, et al. The clinicopathologic and immunohistochemical characteristics of ampulla of Vater carcinoma: the intestinal type is associated with a better prognosis. Hepatogastroenterology. 2007;54:1641–1644. [PubMed] [Google Scholar]
- 11.Sessa F, Furlan D, Zampatti C, et al. Prognostic factors for ampullary adenocarcinomas: tumor stage, tumor histology, tumor location, immunohistochemistry and microsatellite instability. Virchows Arch. 2007;451:649–657. doi: 10.1007/s00428-007-0444-1. doi:10.1007/s00428-007-0444-1. [DOI] [PubMed] [Google Scholar]
- 12.Hansel DE, Maitra A, Lin JW, et al. Expression of the caudal-type homeodomain transcription factors CDX 1/2 and outcome in carcinomas of the ampulla of Vater. J Clin Oncol. 2005;23:1811–1818. doi: 10.1200/JCO.2005.03.068. doi:10.1200/JCO.2005.03.068. [DOI] [PubMed] [Google Scholar]
- 13.Santini D, Perrone G, Vincenzi B, et al. Human equilibrative nucleoside transporter 1 (hENT1) protein is associated with short survival in resected ampullary cancer. Ann Oncol. 2008;19:724–728. doi: 10.1093/annonc/mdm576. doi:10.1093/annonc/mdm576. [DOI] [PubMed] [Google Scholar]
- 14.Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine versus observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147–156. doi: 10.1001/jama.2012.7352. doi:10.1001/jama.2012.7352. [DOI] [PubMed] [Google Scholar]
- 15.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. doi:10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 16.Gibson MK, Holcroft CA, Kvols LK, et al. Phase II study of 5-fluorouracil, doxorubicin, and mitomycin C for metastatic small bowel adenocarcinoma. Oncologist. 2005;10:132–137. doi: 10.1634/theoncologist.10-2-132. doi:10.1634/theoncologist.10-2-132. [DOI] [PubMed] [Google Scholar]
- 17.Overman MJ, Varadhachary GR, Kopetz S, et al. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol. 2009;27:2598–2603. doi: 10.1200/JCO.2008.19.7145. doi:10.1200/JCO.2008.19.7145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.