Abstract
Women are often subjected to serum human chorionic gonadotropin (HCG) testing prior to diagnostic and therapeutic interventions. A positive result leads to further testing to rule out pregnancy and avoid possible fetal teratogenicity. The impact of chronic kidney disease (CKD) on HCG testing has not been studied. We report a series of 5 women out of 62 with CKD, who had a positive HCG test on routine pre-transplant screening at a single transplant center. We analyzed their case records retrospectively. Despite aggressive investigation, their elevated HCG levels remained unexplained. The positive test contributed to delays in transplantation and increased overall cost of treatment.
Keywords: Human chorionic gonadotropin, kidney disease, transplantation
Introduction
Human chorionic gonadotropin (HCG) is a heterodimeric hormone secreted by the trophoblastic tissue of the placenta as well as certain tumors. Small amounts of HCG have been reported to be secreted by the pituitary gland in non-pregnant females and males.[1] Various commercial assays are available in the market to detect HCG in both serum and urine. These tests are commonly used to diagnose pregnancy. They are also used in the diagnosis, management and follow-up of ectopic pregnancy, trophoblastic diseases and germ cell tumors. In the absence of pregnancy and neoplasia, elevated HCG in females has always been a cause of concern. Studies have attributed this to false positivity due to the presence of heterophilic antibodies[2,3] or a physiologic increase in HCG in post-menopausal females.[4,5,6] Low positive HCG values have led to empirical chemotherapy in some and delay or cancellation of important medical procedures in others.[6,7]
We report a series of non-pregnant female patients with chronic renal failure with positive serum HCG over a period of 1 year from our center.
Materials and Methods
The project was approved by the Institutional Review Board of the NSLIJ Health System. The database of all patients who underwent renal transplant at our center from July 2007 through July 2008 was reviewed. A total of 171 patients with end-stage renal disease were screened for renal transplantation during that year, 109 males and 62 females. Case charts of all the female patients with positive HCG (>5 IU/L) were studied in detail. We retrospectively analyzed the case charts of these 5 patients.
Results
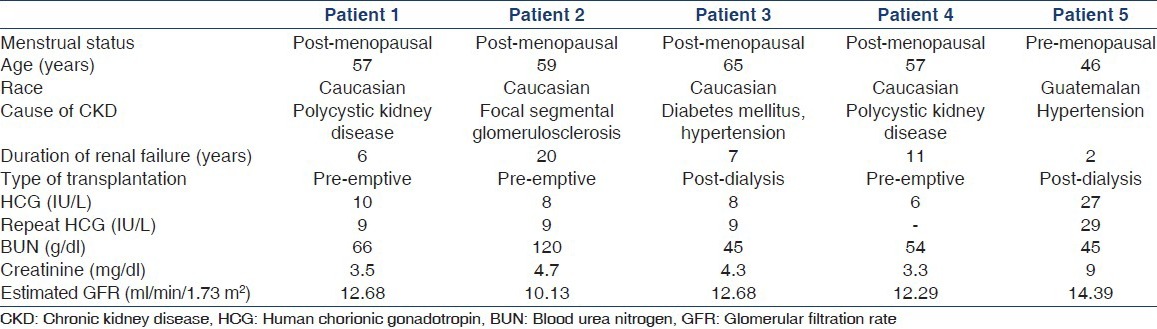
The mean age of female patients was 53.7 years (range 23-79 years). A total of 30 patients were more than 55 years of age. For pre-transplant screening, all the female patients underwent serum HCG screening to rule out pregnancy, irrespective of their ages. HCG screening was carried out using “HCG plus kit” from Roche Pharmaceuticals, USA (0-5 IU/L are considered normal values for the test). Five out of 62 (8%) female patients had a positive HCG test (≥5 IU/L). The test was repeated in 4 four out of five patients to rule out any discrepancy. These patients were then referred to the gynecology and surgery departments. Physical and radiological examinations ruled out pregnancy as well as malignancies in all patients. A total of 3 patients underwent successful renal transplantation while one is still on the waiting list. One patient was transferred on request to another center. The characteristics of these patients are described in Table 1. Two patients were dialysis-dependent while the remaining was being worked up for pre-emptive transplant. Four patients were post-menopausal while one was premenopausal. The mean value of HCG in post-menopausal patients was 8.4 (range 6-10). However, HCG was slightly higher in premenopausal patient. Epstein-Barr virus serologies were positive in all patients; two were cytomegalovirus positive. No other common environmental exposures, which could have led to the production of heterophilic antibody, could be identified in them.
Table 1.
Characteristics of patients
Discussion
HCG is a heterodimeric glycoprotein hormone composed of α and β subunits. HCG-α is identical to the α-subunit of luteinizing hormone (LH), follicle stimulating hormone (FSH) and thyroid stimulating hormone while β subunit is exclusive to each hormone. Normally produced by syncytiotrophoblast cells of placenta, HCG is also secreted by trophoblastic and gastrointestinal tumors. HCG is produced in very small amounts in men and non-pregnant women, primarily from the anterior pituitary gland.[1] In pregnancy, 30% of HCG is lost by filtration in the glomeruli. The remainder is cleared by other pathways, likely by metabolism in the liver and kidneys.[1] The biological function of HCG is maintaining secretion of progesterone from corpus luteum until the placenta takes over this function. It also promotes male sexual differentiation and stimulates testosterone secretion from the fetal testes.[1] Non-nicked HCG is the biologically active form of HCG. Various other degraded and dissociated forms of HCG are present in the serum and urine, with little or no biological activity.[8]
Besides being used as a marker of pregnancy and certain tumors, HCG screening tests are also carried out before most medical interventions in female patients. This is done to avoid potential harm to a growing fetus in undiagnosed pregnancies. Commercial assays for measurement of HCG are varied. Most immunoassays use different combinations of antibodies against different sites on HCG or its subunits. Some assays detect non-nicked HCG alone, some detect non-nicked HCG and free β HCG while others detect both nicked (biologically inactive) and non-nicked HCG.[8] Though these tests have high sensitivity and specificity, the heterogeneity in the assays may lead to false positive results. Heterophilic antibodies in humans may cross-react with animal immunoglobulin used in the assay and can result in falsely positive tests.[9] These antibodies develop following exposure to serum or tissue antigens of animals and occur in about 3-15% of healthy individuals.[10] As a consequence, the elevated HCG levels in our patients may have been false positives. However, it has been observed that the values in such cases of false positive results range from 100 IU/L to 500 IU/L, a good deal higher than our results.[9] Moreover, the assay used in our patients contained blocking antibodies, which bind these heterophilic antibodies and prevent cross reactions.
Studies[4,5,6] have shown that the serum concentration of HCG increases in post-menopausal females. Researchers have demonstrated that this HCG is secreted from the pituitary gland.[11,12] Suginami and Kawaoi obtained serial sections of pituitary glands at autopsy from women of various chronological ages.[11] They used immunohistochemical staining for localizing HCG-like substance in those sections. They concluded that HCG-like substance was localized in the gonadotrophs of the pituitary glands obtained from post-menopausal females. Stenman et al., in their study, showed that treatment of post-menopausal women with a combination of estrogen and progesterone reduced their serum HCG levels by half.[13] This further points towards the pituitary production of HCG in them.
In studies on female cohorts, HCG levels have been found to be proportionate to increases in FSH after menopause. Snyder et al., noted a significant correlation between HCG, age and FSH.[4] Other authors consider pituitary HCG to be produced with increasing menopausal production of LH, owing to the decreased negative feedback by progesterone.[6,14] Case reports have implied that HCG concentrations ≥5.0 IU/L should not be considered abnormal in post-menopausal women.[5,15] In a study in females more than 55 years of age, HCG levels >5 IU/L in 16/240 (6.7%) were observed. In fact, Snyder et al., in their paper, suggested that the upper limits of normal of serum HCG for post-menopausal women be increased to 14.0 IU/L.[4] Thus, this post-menopausal rise in HCG may explain the elevated HCG in four of our patients.
The role of chronic kidney disease (CKD) as a cause of elevated HCG levels has not been studied. It is well-known that 30% of HCG being produced is cleared by the kidney and an additional fraction metabolized by it.[1] In our cohort of female CKD patients, HCG levels >5 IU/L were observed in 5/62 (8.1%). In the subset of patients with age >55, HCG levels more than 5 IU/ml were noted in 4/30 (13.3%). Thus, the presence of CKD in our patients may have served to elevate the HCG levels further in them. However, the factors, which elevate the HCG levels in only some patients with CKD remain poorly understood. A prospective cross-sectional study on pregnant patients showed that mild renal or hepatic impairment has no effect on maternal serum screening, which includes HCG.[16] Whether more severe renal disease (such as in our patients) has an effect on HCG excretion is yet to be ascertained. Hypogonadism in males with CKD or end stage renal disease has been widely described.[17,18,19,20] Reduced testosterone concentration with the increase in level of gonadotropins, including LH and FSH, in has been attributed to a wide range of factors including aging, chronic inflammation, malnutrition and vascular disease. In these circumstances, a pituitary overproduction of HCG in parallel to FSH/LH, akin to the reports above, also appears plausible.[11,13]
Many women experience delays in their management and postponement of surgical procedures because of elevated HCG. Instances of needless chemotherapy administered to such women because of the presumption of cancer have also been reported.[7] Our patients also had to undergo various other investigations increasing the overall cost and delaying treatment. These included obstetrics/gynecology referrals with follow-up, pelvic ultrasound examinations (one or more) and serial blood testing. The transplant procedure itself was rescheduled in one of the patients on account of the positive HCG.
In summary, healthy non-pregnant females can have elevated HCG either due to false positive results or menopausal status. Large-scale studies are needed to ascertain the prevalence of a positive HCG test by conventional criteria in post-menopausal females in the general population. CKD may affect HCG levels by impairing metabolism. Correcting HCG levels for age and co-morbidities may be prudent in reducing procedural delays and treatment costs in such women.
Acknowledgments
We would like to thank Drs. Abhijit Kontamwar, Sonal Bajaj and Sreevidya Kusunam for their valuable input in this project.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY. Williams Obstetrics. 22nd ed. New York: McGraw-Hill; 2005. Implantation, embryogenesis, and placental development; pp. 39–90. [Google Scholar]
- 2.Cao ZT, Rej R. Are laboratories reporting serum quantitative hCG results correctly? Clin Chem. 2008;54:761–4. doi: 10.1373/clinchem.2007.098822. [DOI] [PubMed] [Google Scholar]
- 3.Cole LA, Khanlian SA. Easy fix for clinical laboratories for the false-positive defect with the Abbott AxSym total beta-hCG test. Clin Biochem. 2004;37:344–9. doi: 10.1016/j.clinbiochem.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Snyder JA, Haymond S, Parvin CA, Gronowski AM, Grenache DG. Diagnostic considerations in the measurement of human chorionic gonadotropin in aging women. Clin Chem. 2005;51:1830–5. doi: 10.1373/clinchem.2005.053595. [DOI] [PubMed] [Google Scholar]
- 5.Alfthan H, Haglund C, Dabek J, Stenman UH. Concentrations of human choriogonadotropin, its beta-subunit, and the core fragment of the beta-subunit in serum and urine of men and nonpregnant women. Clin Chem. 1992;38:1981–7. [PubMed] [Google Scholar]
- 6.Cole LA, Khanlian SA, Muller CY. Detection of perimenopause or postmenopause human chorionic gonadotropin: An unnecessary source of alarm. Am J Obstet Gynecol. 2008;198:275.e1–7. doi: 10.1016/j.ajog.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 7.Cole LA, Khanlian SA. Inappropriate management of women with persistent low hCG results. J Reprod Med. 2004;49:423–32. [PubMed] [Google Scholar]
- 8.Cole LA. Immunoassay of human chorionic gonadotropin, its free subunits, and metabolites. Clin Chem. 1997;43:2233–43. [PubMed] [Google Scholar]
- 9.Hammond CB. False positive hCG. Obstet Gynecol. 2001;98:719–20. doi: 10.1016/s0029-7844(01)01616-7. [DOI] [PubMed] [Google Scholar]
- 10.Olsen TG, Hubert PR, Nycum LR. Falsely elevated human chorionic gonadotropin leading to unnecessary therapy. Obstet Gynecol. 2001;98:843–5. doi: 10.1016/s0029-7844(01)01585-x. [DOI] [PubMed] [Google Scholar]
- 11.Suginami H, Kawaoi A. Immunohistochemical localization of a human chorionic gonadotropin-like substance in the human pituitary gland. J Clin Endocrinol Metab. 1982;55:1161–6. doi: 10.1210/jcem-55-6-1161. [DOI] [PubMed] [Google Scholar]
- 12.Hoermann R, Spoettl G, Moncayo R, Mann K. Evidence for the presence of human chorionic gonadotropin (hCG) and free beta-subunit of hCG in the human pituitary. J Clin Endocrinol Metab. 1990;71:179–86. doi: 10.1210/jcem-71-1-179. [DOI] [PubMed] [Google Scholar]
- 13.Stenman UH, Alfthan H, Ranta T, Vartiainen E, Jalkanen J, Seppälä M. Serum levels of human chorionic gonadotropin in nonpregnant women and men are modulated by gonadotropin-releasing hormone and sex steroids. J Clin Endocrinol Metab. 1987;64:730–6. doi: 10.1210/jcem-64-4-730. [DOI] [PubMed] [Google Scholar]
- 14.Cole LA, Sasaki Y, Muller CY. Normal production of human chorionic gonadotropin in menopause. N Engl J Med. 2007;356:1184–6. doi: 10.1056/NEJMc066500. [DOI] [PubMed] [Google Scholar]
- 15.Odell WD, Griffin J. Pulsatile secretion of human chorionic gonadotropin in normal adults. N Engl J Med. 1987;317:1688–91. doi: 10.1056/NEJM198712313172702. [DOI] [PubMed] [Google Scholar]
- 16.Ying I, Wyatt PR, Nisenbaum R, Ray JG. Effect of mild hepatic or renal impairment on maternal serum screening biochemical measures. J Obstet Gynaecol Can. 2011;33:1218–22. doi: 10.1016/S1701-2163(16)35105-2. [DOI] [PubMed] [Google Scholar]
- 17.Iglesias P, Carrero JJ, Díez JJ. Gonadal dysfunction in men with chronic kidney disease: Clinical features, prognostic implications and therapeutic options. J Nephrol. 2012;25:31–42. doi: 10.5301/JN.2011.8481. [DOI] [PubMed] [Google Scholar]
- 18.Isidori AM, Lenzi A. Risk factors for androgen decline in older males: Lifestyle, chronic diseases and drugs. J Endocrinol Invest. 2005;28:14–22. [PubMed] [Google Scholar]
- 19.Warren MP. Effects of undernutrition on reproductive function in the human. Endocr Rev. 1983;4:363–77. doi: 10.1210/edrv-4-4-363. [DOI] [PubMed] [Google Scholar]
- 20.Cavalli A, Del Vecchio L, Locatelli F. Geriatric nephrology. J Nephrol. 2010;23:S11–5. [PubMed] [Google Scholar]


