Abstract
Oxidative stress is increased in chronic kidney disease, owing to an imbalance between the oxidative and antioxidant pathways as well as a state of persistent hyperhomocysteinemia. The enzymes glutathione S-transferases (GSTs) and methylenetetrahydrofolate reductase (MTHFR) are implicated in the regulation of these pathways. This study investigates the association between polymorphisms in the Glutathione S-transferase Mu 1 (GSTM1), glutathione S-transferase theta 1 (GSTT1), and MTHFR genes and end-stage renal disease (ESRD) of unknown etiology in patients in Mexico. A Case-control study included 110 ESRD patients and 125 healthy individuals. GSTM1 and GSTT1 genotypes were determined using the multiplex polymerase chain reaction (PCR). The MTHFR C677T polymorphism was studied using a PCR/restriction fragment length polymorphism method. In ESRD patients, GSTM1 and GSTT1 null genotype frequencies were 61% and 7% respectively. GSTM1 genotype frequencies differed significantly between groups, showing that homozygous deletion of the GSTM1 gene was associated with susceptibility to ESRD of unknown etiology (P = 0.007, odds ratios = 2.05, 95% confidence interval 1.21-3.45). The MTHFR C677T polymorphism genotype and allele distributions were similar in both groups (P > 0.05), and the CT genotype was the most common genotype in both groups (45.5% and 46.6%). Our findings suggest that the GSTM1 null polymorphism appears to be associated with the ESRD of unknown etiology in patients in Mexico.
Keywords: End-stage renal disease, glutathione S-transferases, methylenetetrahydrofolate reductase, polymorphism
Introduction
Chronic kidney disease (CKD) is a global public health problem. The causes of CKD are heterogeneous, ranging from infectious diseases and metabolic multisystemic disease to congenital and genetic disorders. This variety of possible etiologies makes it difficult to identify the mechanisms involved in its pathogenesis.[1] In many cases, the etiology remains unknown.
The annual incidence of end-stage renal disease (ESRD) in Mexico is 346 cases per million people (pmp), with prevalence of 929 pmp.[2] An estimated 8.5% of the Mexican population have CKD,[3] and close to 60,000 individuals are on dialysis.[4]
Oxidative stress plays a key role in the pathogenesis and progression of CKD. The balance between the oxidative and antioxidant pathways is regulated by many factors. The glutathione S-transferases (GSTs) are a group of enzymes that participate in the biotransformation and detoxification of xenobiotics and endogenous substances; they catalyze the conjugation of glutathione with electrophilic xenobiotics. The GST superfamily is encoded by 16 genes, and at least seven different types of GST have been identified thus far.[5]
Glutathione S-transferase Mu 1 (GSTM1) (Online Mendelian Inheritance in Man, OMIM 138350) and glutathione S-transferase theta 1 (GSTT1) (OMIM 600436) are highly expressed in the human kidney, and they are located on chromosomes 1p13.3 and 22q11, respectively. Null genotypes of both genes, homozygous deletions, lead to a lack of expression of their respective enzymes. Therefore, the null polymorphisms of GSTM1 and GSTT1 result in a decreased antioxidant defense,[6] high accumulation of reactive oxygen metabolites, and consequent loss of renal function.[7]
A few studies have demonstrated the association of genetic polymorphisms of GST with the development of CKD, mostly in diabetes, without any conclusive results.[8,9,10] However, patients with ESRD have total serum homocysteine three times higher than the general population.[11] Hyperhomocysteinemia is common among hemodialysis patients and may result from genetic defects in enzymes involved in homocysteine metabolism.[12] Recent studies have associated the methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms with the diabetic nephropathy, and hyperhomocysteinemia with microalbuminuria.[13] Therefore, the presence of the MTHFR C677T (rs1801133) polymorphism might influence the clinical course and progression of CKD. This study investigates the association between GSTM1, GSTT1, and MTHFR polymorphisms in the context of ESRD of unknown etiology in Mexican patients.
Subjects and Methods
Study design
This was a case-control study designed to investigate the association of GST and MTHFR polymorphisms in ESRD patients. All patients and healthy subjects provided written informed consent to participate in this study, in adherence with the Declaration of Helsinki and the Mexican regulations for health and research. This study was approved by the Internal Review Board of the Hospital Civil “Fray Antonio Alcalde” in Guadalajara, Jalisco, Mexico.
Patients
We recruited 110 patients with ESRD of unknown etiology who were referred to the Hospital Civil of Guadalajara “Fray Antonio Alcalde”. ESRD of unknown etiology was defined as ESRD with a glomerular filtration rate of <15 mL/min/1.73 m2 or dialysis persisting for at least 3 months with no identifiable cause and not associated with any known risk factors (e.g., type 2 diabetes mellitus, essential hypertension, glomerulonephritis, infections, drugs, etc.,). A complete medical history was obtained. All patients had been on dialysis for at least 3 months. The reference group included 125 healthy individuals from Guadalajara, Jalisco, Mexico.
Deoxyribonucleic acid (DNA) extraction and genotyping
Genomic DNA was extracted from 10 mL of peripheral venous blood, collected in EDTA, according to the Miller method.[14] GSTM1 primers sequences were: 5′-TATGCAGCTGGGCATGATCT-3′ and 5′-TCAATGACAGCACTCAGAAAACT-3′. GSTT1 primers sequences were 5′-CTGACCTCGTAGCCATCACG-3′ and 5′-ACCCAGGGCATCAGCTTCT-3′.
GSTM1 and GSTT1 genotypes were determined using multiplex polymerase chain reaction (PCR). Three sets of primers were used to amplify a 412-bp fragment of the GSTT1 gene, a 460-bp fragment of the GSTM1 gene, and a 268-bp fragment of the β-globin gene as an amplification control. The PCR amplification of 20 ng of genomic DNA was performed in a total volume of 10 μL, containing 1 × PCR buffer, 1.5 mM MgCl2, 0.1 mM dNTPs, 0.8 pM each primer, and 0.02 U Taq polymerase. PCR conditions required denaturation for 4 min at 94°C; followed by 30 cycles of 30 s at 94°C (denaturation), 1 min at 61°C (annealing), and 1 min at 72°C (elongation); with a final elongation step of 10 min at 72°C. PCR controls (known genotype) were included in every batch of PCR samples. In addition, null genotypes were assayed twice, as an additional quality control. The primer sequences and thermal conditions employed to amplify the MTHFR polymorphism were published previously by Gallegos-Arreola et al.[15] The PCR products were analyzed using electrophoresis in a 6% polyacrilamyde gel (29:1), followed by silver staining.
Statistical analysis
Allele frequencies were determined by counting, and the distribution of genotypes in both groups was compared using the χ2 test or Fisher's exact test. Statistical estimates were assayed using the SPSS v. 20.0 software. Hardy-Weinberg equilibrium was tested using the χ2 test. Odds ratios (ORs) with 95% confidence intervals (95% CIs) were calculated to estimate the associations between genotypes and disease. P <0.05 was considered statistically significant.
Results
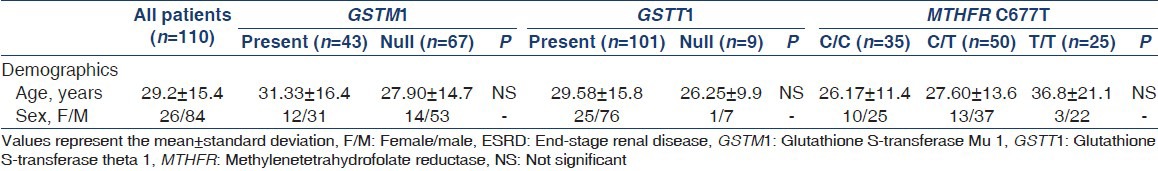
The mean age of patients was 29.24 ± 15.48 (range 15-90) years and 74.5% were younger than 30 years; 76% were male. The mean age at diagnosis was 27.56 ± 15.50 (range 5-90) years. All patients were on dialysis for a mean duration of 2.11 ± 2.09 (range 1-12) years. About 30% were on continuous ambulatory peritoneal dialysis, 38% were on automated peritoneal dialysis, and 32% were on hemodialysis. The demographic characteristics of patients with ESRD are displayed in Table 1. Demographic and laboratory characteristics of the reference group were within normal parameters.
Table 1.
Demographic characteristics of patients with ESRD of unknown etiology
Genotype and allele frequencies of GSTM1, GSTT1, and MTHFR polymorphisms are shown in Table 2. Genotype distributions in the reference group were in agreement with the Hardy-Weinberg equilibrium. GSTM1 genotype frequencies differed significantly between groups; we observed that the homozygous deletion of GSTM1 is a risk factor for ESRD of unknown etiology (P = 0.007, OR = 2.05, 95% CI = 1.21-3.45). Genotypic differences were not observed regarding the GSTT1 polymorphism (P = 0.35). The CT genotype of the MTHFR polymorphism was commonly present in both ESRD patients and healthy participants (45.5% vs. 46.6%, respectively). Genotype and allele distributions did not differ significantly between groups (P > 0.05). The association between genotype combination and risk of ESRD of unknown etiology was significant among GSTM1/GSTT1 null carriers (P = 0.01, OR = 1.94, 95% CI = 1.14-3.32). GSTM1 and GSTT1 genotype and allele frequencies were also compared with other populations, confirming their genetic variability among populations [Figure 1].
Table 2.
Genotype and allele frequencies of MTHFR C677T, GSTM1, and GSTT1 polymorphisms in patients with ESRD of unknown etiology and healthy participants
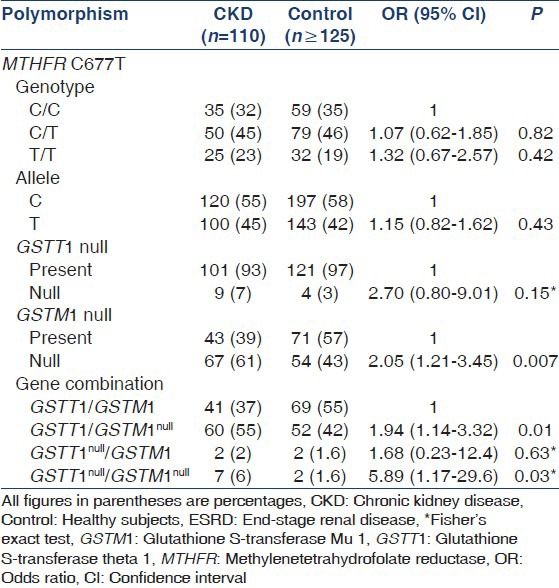
Figure 1.
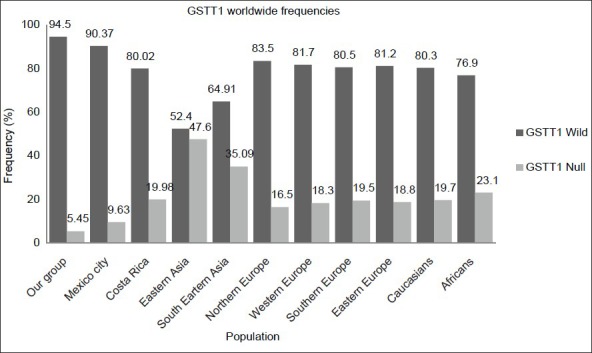
Glutathione S-transferase Mu 1 worldwide allele frequencies (wild and null), including 10 populations in addition to our group
Discussion
CKD patients are exposed to oxidative stress as a consequence of an increase in reactive oxygen species or a decrease in antioxidant defense, which in turn leads to the progressive deterioration of kidney function.[16] The GSTs constitute a superfamily of enzymes that prevent oxidative stress damage. GST polymorphisms may influence responses to damage induced by oxidative stress, and therefore may be involved in the development and progression of CKD. GSTM1 and GSTT1 null polymorphisms are caused by the deletion of the gene, and have been studied the most.[17]
Recent studies have demonstrated increased expression of GSTs in epithelial cells of the proximal tubule during the early stage of diabetes, likely in response to oxidative stress triggered by hyperglycemia or other toxic effects of glucose.[18] It has also been reported that the GSTM1+ genotype is associated with better survival in elderly peritoneal dialysis patients in the Chinese population.[19] Lin et al., reported that the GSTM1 null genotype approximately doubled the risk for all-cause mortality among hemodialysis patients; patients without GSTM1 activity are more susceptible to oxidative stress and are at greater risk for death compared with those who possess GSTM1 activity. In addition, Lin et al., observed that, among maintenance hemodialysis patients, the GSTM1 null genotype was associated with a significantly lower antioxidant capacity than the GSTM+ genotype.[20]
The null/low polymorphisms of the GSTM1 and GSTT1 genes have been associated with the risk of developing ESRD in North Indian patients.[17] In addition, Singh et al., reported that patients with transplant therapy demonstrated an increasing trend toward carrying the GSTM1 null genotype (51.3%) versus healthy controls (40.4%) with a risk of about 1.5-fold (P = 0.035), and patients with a variant genotype of GSTM1 were at the higher risk of transplant rejection.[21]
The frequencies of important functional mutations and alleles result in broad ethnic variation. The frequencies reported for homozygous GSTM1 deletions are approximately 50% in Caucasian, 21.7% in Nigerian, 43% in French, and 58.3% in Chinese populations.[22] The GSTT1 null polymorphism is present in 13-26% of Asians and in 35%-52% of Caucasians.[23] The combined deletion frequency of both genes, GSTM1 and GSTT1, is 8% in Malaysia, 6% in North America, and 8% in Egypt.[24]
We compared the GSTM1 and GSTT1 frequencies obtained from this study with those reported elsewhere [Figures 1 and 2].[25,26] The GSTT1 comparison frequencies revealed that there are statistically significant differences among other populations (P < 0.0001), but not in Mexico City (P = 0.05). The GSTM1 frequencies in the Western Mexican population were statistically different from South-east Asian (P = 0.004), Northern European (P = 0.0025), Caucasian (P = 0.032), and African populations (P < 0.0001).
Figure 2.
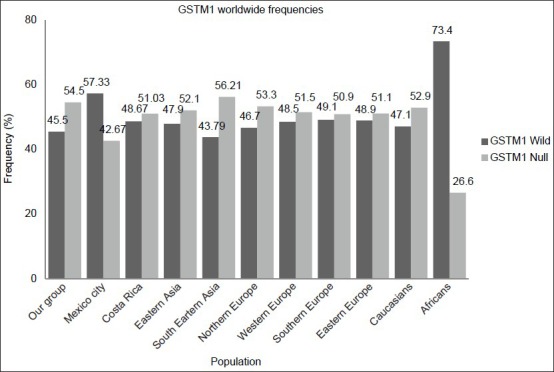
Glutathione S-transferase theta 1 worldwide allele frequencies (wild and null), including 10 populations in addition to our group
Regarding the genetic distribution of GSTT and GSTM among ESRD patients, there are a few studies reported mainly caused by diabetic nephropathy in Asian population.[8,10,17,19,20] The GSTT null frequency is quite low in our experimental population compared with North Indians[17] (7% vs. 58.7%, P < 0.0001); however, the GSTM null is more frequent in our group (60.6% vs. 46.7%, P = 0.02). Although these differences may be explained by the genetic structure of our population, it is important to consider that the etiology of ESRD may vary among these patient groups.
It is also important to highlight the limitations of this study, which detected only the presence or absence of the GSTM1 and GSTT1 gene; gene dosage effects could not be assessed. Another limitation is that the small population size. In addition, the enzyme activities of GST and MTHFR were not determined, and markers of oxidative stress, such as serum vitamin C, malondialdehyde, carbonyl, and reduced glutathione concentrations, were not measured, neither were homocysteine levels. We observed an association between ESRD of unknown etiology and the GSTM1 null deletion, but not GSTT1 or MTHFR C677T polymorphisms, in Mexican individuals in an attempt to associate the clinical variables with the genetic variants. However, additional controlled studies involving enzymes other than GSTM1 are required to elucidate whether genetic factors participate in the modulation of glomerular filtration rate.
Our results suggest that the presence of the GSTM1 null genotype, alone or in combination with the GSTT1 null genotype, might be associated with an increase in oxidative stress and susceptibility to ESRD of unknown etiology in the Mexican population, possibly because of the diminished expression of the GSTM1 enzyme, which results in a reduced ability to defend against oxidative stress. Changes in its function might contribute to the development of ESRD through the increase of oxidative stress or the production of free radicals, or might be associated with other factors, such as pollution, nutritional status, smoking, or gene-environment interactions. Additional studies are needed to understand the roles of these genes in the development of ESRD and to confirm these results. In conclusion, the GSTM1 null allele may be is a risk factor for ESRD of unknown etiology in Mexican individuals.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Datta SK, Kumar V, Pathak R, Tripathi AK, Ahmed RS, Kalra OP, et al. Association of glutathione S-transferase M1 and T1 gene polymorphism with oxidative stress in diabetic and nondiabetic chronic kidney disease. Ren Fail. 2010;32:1189–95. doi: 10.3109/0886022X.2010.517348. [DOI] [PubMed] [Google Scholar]
- 2.The United States Renal Data System (USRDS) Atlas of end-stage renal disease. [Last accessed on 2012 Nov 24]. Available from: http://www.usrds.org .
- 3.Amato D, Alvarez-Aguilar C, Castañeda-Limones R, Rodriguez E, Avila-Diaz M, Arreola F, et al. Prevalence of chronic kidney disease in an urban Mexican population. Kidney Int Suppl. 2005;97:S11–7. doi: 10.1111/j.1523-1755.2005.09702.x. [DOI] [PubMed] [Google Scholar]
- 4.Paniagua R, Ramos A, Fabian R, Lagunas J, Amato D. Chronic kidney disease and dialysis in Mexico. Perit Dial Int. 2007;27:405–9. [PubMed] [Google Scholar]
- 5.Salehi Z, Gholizadeh L, Vaziri H, Madani AH. Analysis of GSTM1, GSTT1, and CYP1A1 in idiopathic male infertility. Reprod Sci. 2012;19:81–5. doi: 10.1177/1933719111413302. [DOI] [PubMed] [Google Scholar]
- 6.Sui Y, Han W, Yang Z, Jiang M, Li J. Association of glutathione S-transferase M1 and T1 null polymorphisms with the development of cervical lesions: A meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2011;159:443–8. doi: 10.1016/j.ejogrb.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Galle J. Oxidative stress in chronic renal failure. Nephrol Dial Transplant. 2001;16:2135–7. doi: 10.1093/ndt/16.11.2135. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Kao MT, Chang CC, Chung SY, Chen CM, Tsai JJ, et al. Glutathione S-transferase T1 deletion is a risk factor for developing end-stage renal disease in diabetic patients. Int J Mol Med. 2004;14:855–9. [PubMed] [Google Scholar]
- 9.Fujita H, Narita T, Meguro H, Shimotomai T, Kitazato H, Kagaya E, et al. No association of glutathione S-transferase M1 gene polymorphism with diabetic nephropathy in Japanese type 2 diabetic patients. Ren Fail. 2000;22:479–86. doi: 10.1081/jdi-100100889. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Moon MK, Kim SW, Shin HD, Hwang YH, Ahn C, et al. Glutathione S-transferase M1 gene polymorphism is associated with type 2 diabetic nephropathy. J Korean Diabetes Assoc. 2005;29:315–21. [Google Scholar]
- 11.Wrone EM, Zehnder JL, Hornberger JM, McCann LM, Coplon NS, Fortmann SP. An MTHFR variant, homocysteine, and cardiovascular comorbidity in renal disease. Kidney Int. 2001;60:1106–13. doi: 10.1046/j.1523-1755.2001.0600031106.x. [DOI] [PubMed] [Google Scholar]
- 12.Pastore A, De Angelis S, Casciani S, Ruggia R, Di Giovamberardino G, Noce A, et al. Effects of folic acid before and after vitamin B12 on plasma homocysteine concentrations in hemodialysis patients with known MTHFR genotypes. Clin Chem. 2006;52:145–8. doi: 10.1373/clinchem.2005.056119. [DOI] [PubMed] [Google Scholar]
- 13.Kimura H, Gejyo F, Suzuki S, Miyazaki R. The C677T methylenetetrahydrofolate reductase gene mutation in hemodialysis patients. J Am Soc Nephrol. 2000;11:885–93. doi: 10.1681/ASN.V115885. [DOI] [PubMed] [Google Scholar]
- 14.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallegos-Arreola MP, García-Ortiz JE, Figuera LE, Puebla-Pérez AM, Morgan-Villela G, Zúñiga-González GM. Association of the 677C - >T polymorphism in the MTHFR gene with colorectal cancer in Mexican patients. Cancer Genomics Proteomics. 2009;6:183–8. [PubMed] [Google Scholar]
- 16.Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology (Carlton) 2012;17:311–21. doi: 10.1111/j.1440-1797.2012.01572.x. [DOI] [PubMed] [Google Scholar]
- 17.Agrawal S, Tripathi G, Khan F, Sharma R, Baburaj VP. Relationship between GSTs gene polymorphism and susceptibility to end stage renal disease among North Indians. Ren Fail. 2007;29:947–53. doi: 10.1080/08860220701641314. [DOI] [PubMed] [Google Scholar]
- 18.Fujita H, Haseyama T, Kayo T, Nozaki J, Wada Y, Ito S, et al. Increased expression of glutathione S-transferase in renal proximal tubules in the early stages of diabetes: A study of type-2 diabetes in the Akita mouse model. Exp Nephrol. 2001;9:380–6. doi: 10.1159/000052636. [DOI] [PubMed] [Google Scholar]
- 19.Poon PY, Szeto CC, Kwan BC, Chow KM, Li PK. Relationship between glutathione S-transferase M1 polymorphism and clinical outcomes in Chinese peritoneal dialysis patients. J Nephrol. 2012;25:310–6. doi: 10.5301/jn.5000021. [DOI] [PubMed] [Google Scholar]
- 20.Lin YS, Hung SC, Wei YH, Tarng DC. GST M1 polymorphism associates with DNA oxidative damage and mortality among hemodialysis patients. J Am Soc Nephrol. 2009;20:405–15. doi: 10.1681/ASN.2008020227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh R, Manchanda PK, Kesarwani P, Srivastava A, Mittal RD. Influence of genetic polymorphisms in GSTM1, GSTM3, GSTT1 and GSTP1 on allograft outcome in renal transplant recipients. Clin Transplant. 2009;23:490–8. doi: 10.1111/j.1399-0012.2009.00985.x. [DOI] [PubMed] [Google Scholar]
- 22.Sprenger R, Schlagenhaufer R, Kerb R, Bruhn C, Brockmöller J, Roots I, et al. Characterization of the glutathione S-transferase GSTT1 deletion: Discrimination of all genotypes by polymerase chain reaction indicates a trimodular genotype-phenotype correlation. Pharmacogenetics. 2000;10:557–65. doi: 10.1097/00008571-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Raimondi S, Paracchini V, Autrup H, Barros-Dios JM, Benhamou S, Boffetta P, et al. Meta- and pooled analysis of GSTT1 and lung cancer: A HuGE-GSEC review. Am J Epidemiol. 2006;164:1027–42. doi: 10.1093/aje/kwj321. [DOI] [PubMed] [Google Scholar]
- 24.Alshagga MA, Mohamed N, Nazrun Suhid A, Abdel Aziz Ibrahim I, Zulkifli Syed Zakaria S. Frequencies of glutathione s-transferase (GSTM1, GSTM3 AND GSTT1) polymorphisms in a Malaysian population. Arch Med Sci. 2011;7:572–8. doi: 10.5114/aoms.2011.24123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurose K, Sugiyama E, Saito Y. Population differences in major functional polymorphisms of pharmacokinetics/pharmacodynamics-related genes in Eastern Asians and Europeans: Implications in the clinical trials for novel drug development. Drug Metab Pharmacokinet. 2012;27:9–54. doi: 10.2133/dmpk.dmpk-11-rv-111. [DOI] [PubMed] [Google Scholar]
- 26.Montero R, Araujo A, Carranza P, Mejía-Loza V, Serrano L, Albores A, et al. Genotype frequencies of polymorphic GSTM1, GSTT1, and cytochrome P450 CYP1A1 in Mexicans. Hum Biol. 2007;79:299–312. doi: 10.1353/hub.2007.0037. [DOI] [PubMed] [Google Scholar]


