Abstract
Here, authors present a review on clinical presentation and management of exposure of phosgene gas after reviewing the literature by searching with keywords phosgene exposure on Google, Cochrane, Embase and PubMed with a background of experience gained from 10 patients who were admitted to our institute after an accidental phosgene exposure in February 2011 nearby a city in India. Phosgene is a highly toxic gas, occupational workers may have accidental exposure. The gas can also be generated inadvertently during fire involving plastics and other chemicals and solvents containing chlorine, which is of concern to emergency responders. Phosgene inhalation may cause initially symptoms of respiratory tract irritation, patients feel fine thereafter, and then die of choking a day later because of build up of fluid in the lungs (delayed onset non-cardiogenic pulmonary edema). Phosgene exposure is associated with significant morbidity and mortality. Patients with a history of exposure should be admitted to the hospital for a minimum of 24 h for observation because of the potential for delayed onset respiratory failure and acute respiratory distress syndrome.
Keywords: Acute respiratory distress syndrome, N-acetyl-cysteine, phosgene
BACKGROUND
Phosgene is a highly toxic, colourless gas at room temperature and standard pressure that condenses at 0°C to a fuming liquid. Its molecular formula is COCl2. Phosgene is more than three times dense of air and therefore, concentrated emission plumes tend to settle to the ground and collect in low areas. At a concentration of about 0.5 ppm in the air, this odor has been described as similar to that of new-mown hay or cut green corn, after adaptation this recognition odour would require concentration of 1.5 ppm. At higher concentrations, the odor may be strong, stifling, and unpleasant. Impurities can discolor liquid phosgene and cause it to turn a pale yellow to green color.[1] In water, phosgene is sparingly soluble and decomposes to hydrochloric acid and carbon dioxide. Hence, wet phosgene is very corrosive.[2] It is freely soluble in most liquid hydrocarbons, benzene, toluene, and glacial acetic acid. It is formed by thermal decomposition of chlorinated compounds. Phosgene is non-combustible.
Phosgene first gained reputation worldwide during the World War I, when it was used in chemical warfare. It was the principal agent used, accounting for approximately 80% of the 100,000 gas-induced casualties.[3,4,5] Phosgene is primarily used as a building block in various pharmaceutical and organic industries. Most commercially produced phosgene is used captively at the production sites in the manufacture of other chemicals. The manufacture of isocyanates consumes about 85% of the world's phosgene production.[2] The primary use of phosgene is in the production of toluene diisocyanate, a pre-cursor of the polyurethane resins used to make foams, elastomers, and coatings. Phosgene is also used in the manufacture of herbicides, pesticides, dyes, and pharmaceuticals. In addition to its industrial production, suspected sources of atmospheric phosgene are fugitive emissions, thermal decomposition of chlorinated hydrocarbons, and photo-oxidation of chloroethylenes. Phosgene exposure can occur in fires involving certain chlorinated organic compounds found in many household solvents, paint removers, and dry cleaning fluids or wool, Polyvinyl chloride, and other plastics.[6] Phosgene at a concentration of 1 ppm in the air causes little or no immediate irritation, but after a latent period of some hours, it may cause severe pulmonary edema and at concentrations of 4-10 ppm in air it causes irritation of the respiratory tract and eyes.[7] Lethal dose of phosgene in humans is approximately 500 ppm/min of exposure or exposure at 3 ppm for 170 min is equally as fatal as exposure at 30 ppm for 17 min.[8] Exposure occurs by inhalation and the fact that phosgene is only a slightly water-soluble gas and that due to this, significant irritation of upper respiratory tract and eyes may not occur, leading to prolonged exposure. Phosgene exerts its toxicity through the acylation of proteins as well as through the release of hydrochloric acid. The amino, hydroxyl and sulfhydryl groups in proteins appear to be the target for acylation, leading to marked inhibition of several enzymes related to energy metabolism and a breakdown of the blood-air barrier.
Here, authors present a review on clinical presentation and management of exposure to phosgene gas with background experience gained from 10 patients who were admitted to our institute after an accidental phosgene exposure in February 2011 nearby a city in India.
CASE SERIES
In this series, 10 patients who were admitted after exposure to phosgene at our institute are described. The lag time between exposures of phosgene to admission was 10-19 h. All patients initially after exposure to phosgene had experienced choking like sensation and cough. Ocular symptoms such as redness and lacrimation developed after 2-3 h in 30%, followed by breathlessness in all cases. Around 50% had diffuse chest pain, and 50% had 2-3 episodes of vomiting. On examination, around 80% were tachypneic, 60% were hypotensive and 60% had diffuse bilateral coarse crepitations.
In this series, arterial blood gas (ABG) showed type 1 respiratory failure with metabolic acidosis in 60% of the patients. Other parameters such as PCO2 and Bicarbonate levels were within normal limits. None of our case had deranged value of hematocrits, random blood glucose, and renal function tests. However, leucocytosis (in 30% cases), which might have occurred due to systemic inflammatory response syndrome, hyponatremia (in 30%), and hypokalemia (40%) was observed [Table 1]. Chest X-rays showed bilateral fluffy infiltrates with normal cardiac shadow suggestive of non-cardiogenic pulmonary edema [Figures 1 and 2]. Electrocardiogram (ECG) showed sinus tachycardia in all patients. Other investigations such as Troponin T and N-terminal prohormone of brain natriuretic peptide were carried out to exclude heart failure as a cause of breathlessness, were within normal limits.
Table 1.
Describes the clinical presentation, investigations, treatment, and outcome of patients who are the victims of the accidental phosgene exposure
Figure 1.
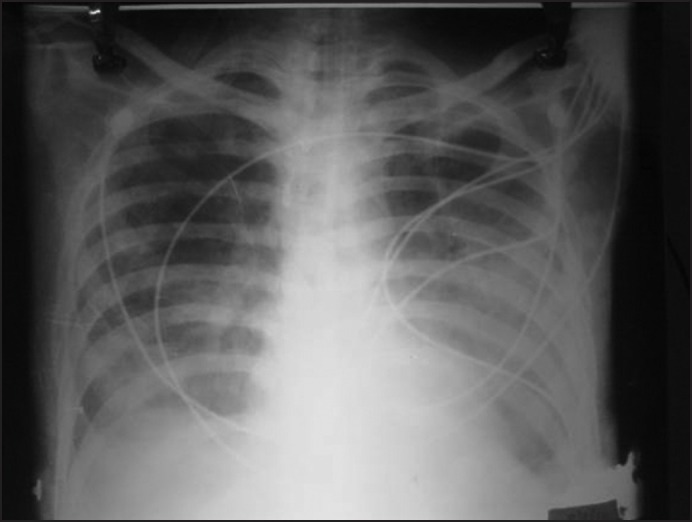
Chest X-rays Posteroanterior view patient (3) showed bilateral fluffy infiltrates with normal cardiac shadow suggestive of non-cardiogenic pulmonary edema
Figure 2.
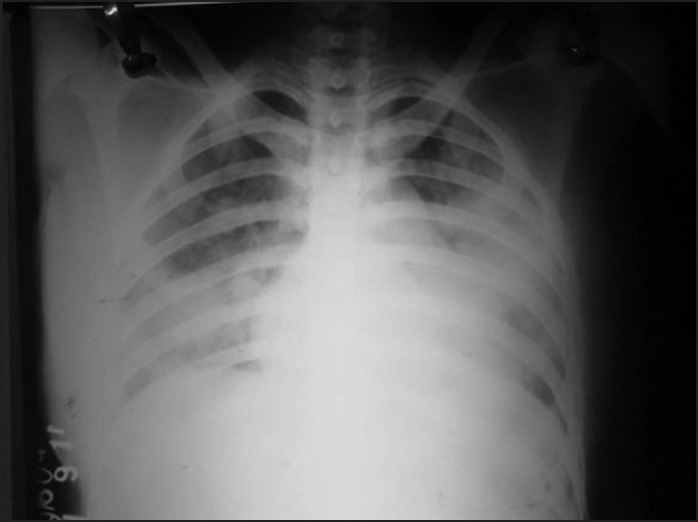
Chest X-rays Posteroanterior view patient (6) showed bilateral fluffy infiltrates with normal cardiac shadow suggestive of non-cardiogenic pulmonary edema
In our series, all patients were treated with N-acetyl-cysteine (NAC) (1-10 mL of the 20% solution or 2-20 mL of the 10% solution via Nebulization every 2-6 h) along with intravenous corticosteroids. Vasopressors were required in those cases, who were initially remaining hypotensive despite intravenous fluid administration. Patients who were hypotensive also had developed acute respiratory distress syndrome (ARDS) as a result, were put on mechanical ventilation. Those cases that had leucocytosis, intravenous antibiotics were given considering the simultaneous respiratory infection might be the cause for leucocytosis, and antibiotics were also given prophylactically to patients who needed ventilator support. Diuretics were administered to case nine who had severe breathlessness with normal blood pressure, bilateral infiltrates in chest X-ray that could not be categorized into ARDS.
Sadly enough, in our series, four patients died despite all efforts, but two patients who were put early on ventilator survived and in them duration of ventilator requirement was less than 4 days. Those who died were on a ventilator for more than 4 days. During 6 months follow-up, none of our patients had reported to have any chronic sequela. The full description of all patients from initial presentation to outcome is summarised in Table 1.
Clinical presentation of phosgene exposure
Inhalation is the primary exposure route for phosgene. Signs and symptoms depend on the route of exposure; inhalation being the primary exposure route for phosgene. The symptom complex of phosgene inhalation at high concentrations are described in three phases, (1) an initial bio protective phase, (2) a symptom-free latent period, and (3) a terminal phase characterized by pulmonary edema.[9]
Bio protective phase
In the initial phase, high concentrations (>3 ppm) may result in a vagal reflex action that causes frequent, shallow respiration, and decreased respiratory vital capacity and volume. This, in turn, leads to a increase in arterial CO2 pressure and decreased blood pH. After cessation of exposure, the reflex syndrome shows a tendency to regress. The initial phase consists of pain in the eyes and throat and tightness in the chest, often with shortness of breath, wheezing, and coughing; hypotension, bradycardia and rarely sinus arrhythmias can occur.
Clinical latent phase
In the second phase, which may last for several hours post-exposure, clinical signs, and symptoms are generally lacking (Schneider and Diller, 1989; Diller, 1985). However, histologic examination reveals the beginning of an edematous swelling, with blood plasma increasingly entering the pulmonary interstitium and alveoli. This may result in damage to the alveolar type I cells and a rise in hematocrit. In exposed humans, the individual is unaware of these processes; thus, this phase is termed as “clinical latent phase.” The length of this phase varies inversely with the inhaled dose.
Terminal phase
In this phase, phosgene toxicity leads to the accumulation of fluid in the lungs resulting into the edematous lung parenchyma. Respiratory insufficiency, indicated by tachypnea, dyspnea, tachycardia, cyanosis, and decreased pO2. The severity of the edema increases potentially, resulting in decreased gas exchange as the fluid gradually rises from the alveoli to the proximal segments of the respiratory tract. Agitated respiration may cause the protein-rich fluid to take a frothy consistency. A severe edema may result in an increased concentration of hemoglobin in the blood and congestion of the alveolar capillaries. Pathological findings include extensive degenerative changes in the epithelium of trachea, bronchi, and bronchioli, and hemorrhagic edematous focal pneumonia.[10]
In massive exposures, immediate death sometimes result from occlusion of the pulmonary circulation secondary to intravascular hemolysis and thrombus formation.[11] At sufficiently high exposure the respiratory symptoms may be accompanied by hypovolemia, hypotension, and hemoconcentration. In general, this phase peaks approximately 24 h after an acute exposure and assuming lethality does not occur, recedes over the next 3-5 days. However in several cases, infectious pneumonitis develops 3-5 weeks after exposure. Mortality is high in such cases and it does occur within 24-48 h after exposure. The danger period usually is 6-24 h after exposure with the development of peribronchial edema, pulmonary congestion, and alveolar edema, all leading to death from anoxia. The delay is ascribed to slow intrapulmonary hydrolysis of phosgene to hydrogen chloride and chloride.[11]
Skin contact can result in lesions similar to those from frostbite or burns, direct contact with liquid phosgene can cause severe burns.[12] In a case series of 52 patients of phosgene poisoning reported by Kuzelova the predominant symptoms were respiratory tract irritation in 94%; malaise, nausea, and vomiting in 54%; headache in 40%; burning and lacrimation of eyes and other-30%. Three cases had pulmonary edema.[13]
Of note, Methyl Isocyanate poisoning is an important differential diagnosis of such case because both are generally required for the manufacture of various polymer industries and the toxicity to methyl isocyanate is clinically indistinguishable to that of phosgene, but the former is more fatal.
Pathophysiological mechanism of inhalational toxicity
Phosgene decomposes to form hydrochloric acid and carbon monoxide. Hydrochloric acid proceeds to cause epithelial damage and necrosis in bronchi, small bronchioles, and capillaries. This leads to increased permeability of the alveolar and capillary basement membranes with resultant pulmonary edema. Sometimes bronchopneumonia and occasionally lung abscess may develop after inhalation as delayed complications.[14] In a study, the mechanism of phosgene poisoning is demonstrated by the significant increase in tracheal pressure, the rate of lung weight gain, levels of leukotrienes C4, D4, and E4, lipid peroxidation (thiobarbituric acid-reactive substances), and oxidized glutathione over a time following exposure to phosgene.[15]
Investigations
There is no specific laboratory test for confirmatory diagnosis of phosgene gas exposure; however, various means of monitoring pulmonary status should be undertaken. These include a chest x-ray, oxygen saturation, and ABG, and volume status assessment (initially via vital signs and examination of mucous membranes).
Chest X-ray typically shows signs of pulmonary edema with enlargement of the hila as the earliest finding (4-8 h after exposure) and/or ill-defined patchy infiltrates. Other investigations such as ECG, Troponin T and NT pro BNP may be required to differentiate between pulmonary edema and ARDS. Owing to the possibility of delayed pulmonary edema, persons exposed to significant levels should be observed for 24 h. Chest radiography, physical findings, and ABG are the primary parameters to be monitored.[16]
Treatment
Management is principally supportive, until date no antidote is available for phosgene toxicity.[17] As in the management of any poisoning, the decontamination has paramount role here; initially, the patient should be removed from the exposed environment and stripped of his/her clothes. If any area of the skin or eyes has been exposed, thorough irrigation with tepid water should be performed. In areas where direct skin contact has occurred, one should perform a thorough rinse and wash with soap and water. After exposure by inhalation, physical exertion should be avoided and strict bed rest enforced for between 24 h and 72 h, particularly, if the exposure dose was unknown or above 25 ppm/min.
When pulmonary edema develops the patient should be managed with anticipation of respiratory failure. They should initially be maintained by oxygen therapy as needed. If the patient continues to be hypoxic, intubation may be necessary with or without positive end-expiratory pressure. Pulmonary edema should be managed with special attention to maintain a net negative fluid balance. Diuretics should be avoided since the pulmonary edema is not secondary to fluid overload. If necessary, hemodynamics should be monitored through a central line or Swan-Ganz catheter. Antibiotics should not be started empirically, but should be reserved for the cases in which there is clinical evidence of pneumonia or bronchitis. The effective treatment of phosgene-induced lung injury involves early post-exposure intervention that can reduce free radical species responsible for lipid peroxidation, correct the imbalance in the glutathione redox state, and prevent the release of biological mediators such as leukotrienes, which are accountable for increased permeability. Bronchodilators may improve existing bronchospasm. In animal studies, beneficial effects have been shown with the administration of numerous drugs, including leukotrienes antagonists, ibuprofen,[18] colchicine, cyclophosphamide,[19] terbutaline, aminophylline, and isoproterenol. Steroids were thought to be effective in reducing inflammation secondary to irritant effects of phosgene gas, but corticosteroids have not been proved to be beneficial. However, anecdotal reports of systemic corticosteroid use in humans with chlorine exposure was found effective,[20,21] had encouraged us to try them in anticipation of benefit. Specific treatments have been studied in the past such as use of hexamethylenetetramine (HMT) and NAC.[22,23] HMT, once considered a specific antidote, has been proved to be effective only if administered in a prophylactic manner. There has been no evidence of benefit from HMT in acute phosgene exposure. NAC is thought to “trap” phosgene and convert it to a less harmful metabolite. It has also been postulated that NAC's antioxidant properties play a role via the decrease in direct toxicity to pulmonary parenchyma[20] However, in vivo; it has not been proven effective in reducing morbidity and mortality with the administration of NAC.[22,23] Nebulised sodium bicarbonate treatment may be beneficial theoretically.
Outcome
Most people who recover after an exposure to phosgene make a complete recovery within 1-3 weeks. However, chronic bronchitis and emphysema have been reported as delayed sequela of phosgene exposure.[24] Exposure to phosgene has been reported to result in Reactive Airway Dysfunction Syndrome, a chemically-or irritant induced type of asthma.[25] Follow-up examinations of soldiers who had been exposed to phosgene during the World War I showed in 6-12% of cases: Chronic bronchitis, emphysema, pulmonary fibrosis, bronchial asthma, pulmonary tuberculosis, neurasthenia, and dementia praecox.[24]
After reviewing the literature and experience gained from the management of 10 cases of phosgene inhalation, the authors would like to recommend that NAC and bronchodilators are to be given in all cases that have breathlessness. Supportive treatment in the form of oxygen inhalation, and vasopressors in hypotensive may be required. Antibiotics administration should be considered in patients who have leucocytosis and in those who have been put on mechanical ventilation. Consider early institution of mechanical ventilation in those patients who have developed ARDS. Asymptomatic patients should be kept under observation for at least 24 h and chest X-ray performed before discharge. Literature also says the beneficial effect of leukotrienes and anti-inflammatory agent like ibuprofen. Though, the role of corticosteroid is dubious, but the mechanism by which it reduces inflammation might have some beneficial effect in inhalation poisoning; therefore, we used corticosteroids and recommend it in all symptomatic cases of phosgene inhalation until more information is not available.
ACKNOWLEDGMENTS
We owe thanks to patients and their relatives for cooperation and support without, which this undertaking would not have been possible.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Hardy E. Kirk-Othmer Encyclopaedia of Chemical Technology. 2nd Ed. Supplement. 1971. Phosgene; pp. 674–83. [Google Scholar]
- 2.Chemical Profiles: Phosgene. Washington D.C. 20460, USA: United States Environmental Protection Agency; 1985. Dec, U.S. Environmental Protection Agency (EPA) p. 4. [Google Scholar]
- 3.Eckert WG. Mass deaths by gas or chemical poisoning. A historical perspective. Am J Forensic Med Pathol. 1991;12:119–25. doi: 10.1097/00000433-199106000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Bradley BL, Unger KM. Phosgene inhalation: A case report. Tex Med. 1982;78:51–3. [PubMed] [Google Scholar]
- 5.Goldfrank LR, Flomenbaum NE, Lewin NA, Weisman RS, Howland MA, Hoffman RS. Goldfrank's Toxicologic Emergencies. 5th ed. East Norwalk, CT: Appleton and Lange; 1994. pp. 1–20. [Google Scholar]
- 6.Doig AT, Challen PJ. Respiratory hazards in welding. Ann Occup Hyg. 1964;7:223–31. doi: 10.1093/annhyg/7.3.223. [DOI] [PubMed] [Google Scholar]
- 7.Grant WM. 2nd ed. Springfield, Illinois: Charles C. Thomas; 1974. Toxicology of the Eye; pp. 825–6. [Google Scholar]
- 8.Cincinnati, OH 45240-1634: CD-ROM; 2006. American Conference of Governmental Industrial Hygienists. Documentation of the TLV's and BEI's with Other World Wide Occupational Exposure Values. [Google Scholar]
- 9.Diller WF. The methenamine misunderstanding in the therapy of phosgene poisoning. Arch Toxicol. 1980;46:199–206. doi: 10.1007/BF00310435. [DOI] [PubMed] [Google Scholar]
- 10.Dreisbach RH. 12th ed. Norwalk, CT: Appleton and Lange; 1987. Handbook of Poisoning; p. 162. [Google Scholar]
- 11.Gosselin RE, Smith RP, Hodge HC. 5th ed. Baltimore: Williams and Wilkins; 1984. Clinical Toxicology of Commercial Products; pp. II–96. [Google Scholar]
- 12.Sullivan JB, Krieger GR, editors. Baltimore, MD: Williams and Wilkins; 1992. Hazardous Materials Toxicology-Clinical Principles of Environmental Health; p. 793. [Google Scholar]
- 13.Kuzelova M, Kos J, Kunor V, Merhaut J. Prac Lek. 1975;27:115–7. [Google Scholar]
- 14.Lewis RJ. 9th ed. 1-3. New York, NY: Van Nostrand Reinhold; 1996. Sax's Dangerous Properties of Industrial Materials; p. 2684. [Google Scholar]
- 15.Sciuto AM, Strickland PT, Kennedy TP, Gurtner GH. Protective effects of N-acetylcysteine treatment after phosgene exposure in rabbits. Am J Respir Crit Care Med. 1995;151:768–72. doi: 10.1164/ajrccm.151.3.7881668. [DOI] [PubMed] [Google Scholar]
- 16.Rom WN, editor. 2nd ed. Boston, MA: Little, Brown and Company; 1992. Environmental and Occupational Medicine; p. 531. [Google Scholar]
- 17.Ellenhorn MJ, Schonwald S, Ordog G, Wasserberger J. 2nd ed. Baltimore, MD: Williams and Wilkins; 1997. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning; p. 1302. [Google Scholar]
- 18.Sciuto AM, Stotts RR, Hurt HH. Efficacy of ibuprofen and pentoxifylline in the treatment of phosgene-induced acute lung injury. J Appl Toxicol. 1996;16:381–4. doi: 10.1002/(SICI)1099-1263(199609)16:5<381::AID-JAT355>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Sciuto AM. Assessment of early acute lung injury in rodents exposed to phosgene. Arch Toxicol. 1998;72:283–8. doi: 10.1007/s002040050503. [DOI] [PubMed] [Google Scholar]
- 20.Chester EH, Kaimal J, Payne CB, Jr, Kohn PM. Pulmonary injury following exposure to chlorine gas. Possible beneficial effects of steroid treatment. Chest. 1977;72:247–50. doi: 10.1378/chest.72.2.247. [DOI] [PubMed] [Google Scholar]
- 21.Fleta J, Calvo C, Zuñiga J, Castellano M, Bueno M. Intoxication of 76 children by chlorine gas. Hum Toxicol. 1986;5:99–100. doi: 10.1177/096032718600500205. [DOI] [PubMed] [Google Scholar]
- 22.Schelble DT. Phosgene and phosphine. In: Haddad LM, Shannon MW, Winchester J, editors. Clinical Management of Poisoning and Drug Overdose. 3rd ed. Philadelphia: WB Saunders; 1998. pp. 960–3. [Google Scholar]
- 23.Borak J, Diller WF. Phosgene exposure: Mechanisms of injury and treatment strategies. J Occup Environ Med. 2001;43:110–9. doi: 10.1097/00043764-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 24.CD-ROM; 2000. [Last accessed 2012 Feb 12]. European Chemicals Bureau; IUCLID Dataset, Phosgene (75-55-5) p. 41. Available from: http://www.esis.jrc.ec.europa.eu/ [Google Scholar]
- 25.ATSDR; Medical Management for Phosgene (COCl2) CAS 75-44-5; UN 1076. [Last accessed 2012 Feb 12]. pp. 5–6. Available from: http://www.atsdr.cdc.gov/MHMI/mmg176.pdf .


