Abstract
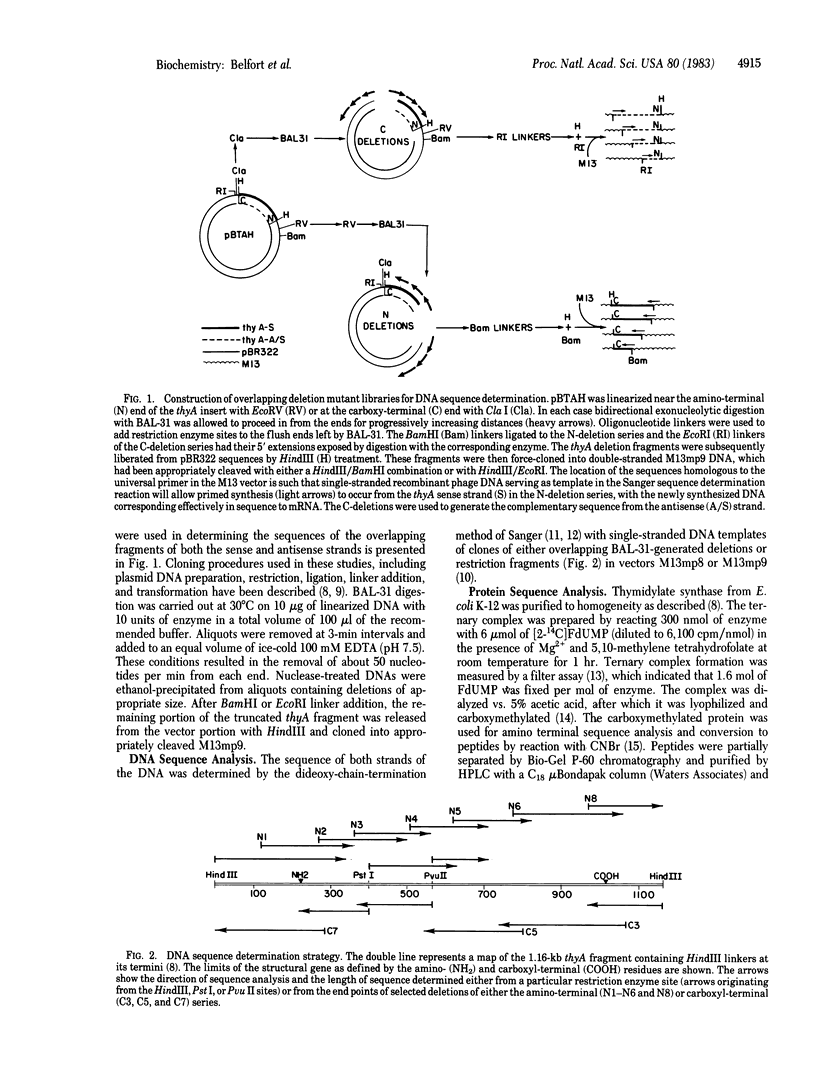
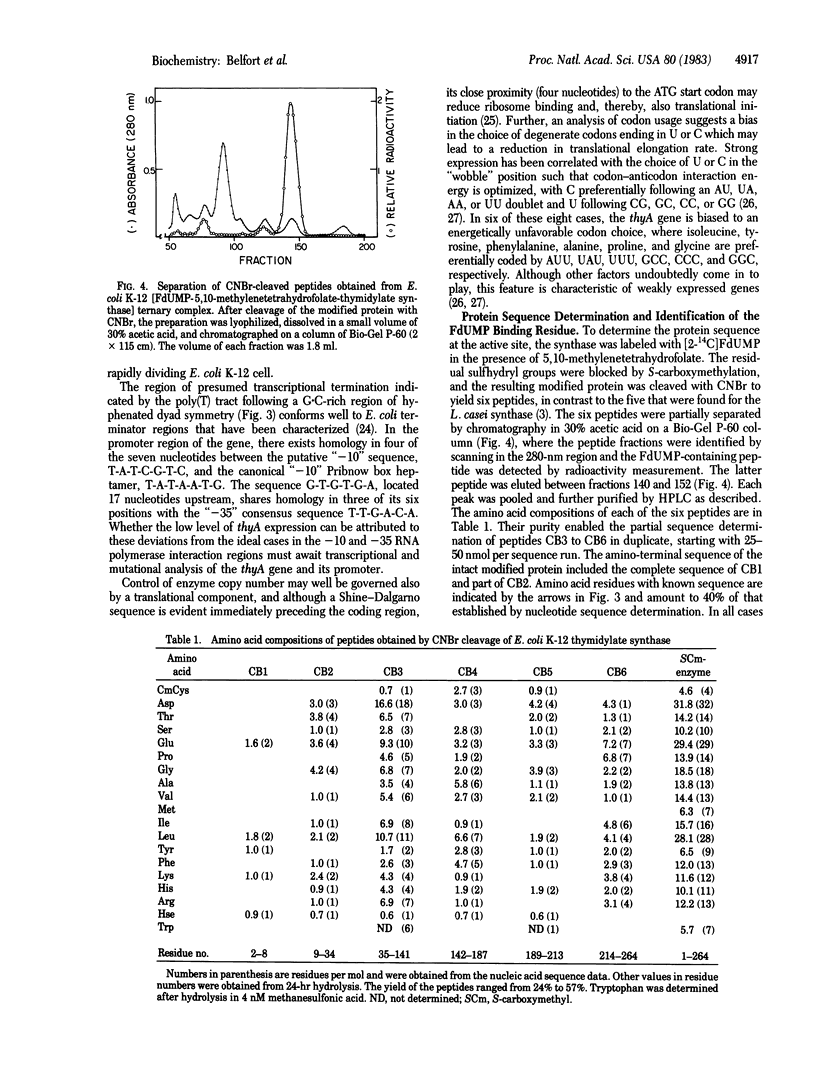
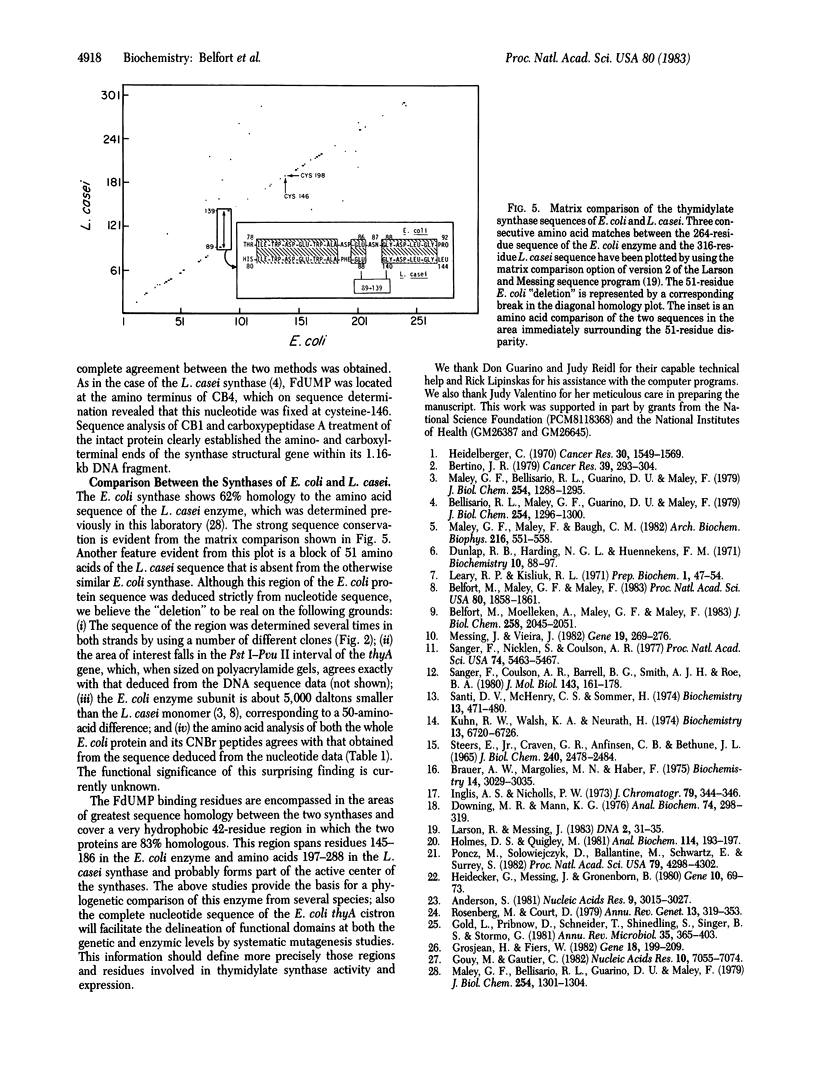
The nucleotide sequence of a 1,163-base-pair fragment that encodes the entire thyA gene of Escherichia coli K-12 was determined. The strategy involved sequence determination of both DNA strands by using overlapping deletions that had been generated in vitro from the two ends of the fragment with BAL-31 nuclease. The amino-terminal sequence of thymidylate synthase (5,10-methylenetetrahydrofolate:dUMP C-methyltransferase, EC 2.1.1.45), the product of the thyA gene, located the 792-base-pair open reading frame, which codes for the 264 amino acid residues of this enzyme. The amino acid sequence deduced from the nucleotide data was confirmed to the extent of 40% by partial sequence analysis of the enzyme purified from extracts of the amplified cloned gene. Transcriptional and translational control areas were apparent in the regions flanking the structural gene. The 5-fluorodeoxyuridylate-binding residue of the active site was identified as cysteine-146. Comparison of the E. coli and Lactobacillus casei synthase sequences reveals consistent homology (62%) over extensive regions. This homology is particularly striking in a very hydrophobic region bordering cysteine-146. In the two enzymes, this region, which probably defines the active site, is 82% homologous. However, a dramatic difference between the two sequences is reflected by the surprising finding that a 51-amino-acid stretch, present midway through the L. casei sequence, is completely absent from the E. coli enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. Shotgun DNA sequencing using cloned DNase I-generated fragments. Nucleic Acids Res. 1981 Jul 10;9(13):3015–3027. doi: 10.1093/nar/9.13.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort M., Maley G. F., Maley F. Characterization of the Escherichia coli thyA gene and its amplified thymidylate synthetase product. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1858–1861. doi: 10.1073/pnas.80.7.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort M., Moelleken A., Maley G. F., Maley F. Purification and properties of T4 phage thymidylate synthetase produced by the cloned gene in an amplification vector. J Biol Chem. 1983 Feb 10;258(3):2045–2051. [PubMed] [Google Scholar]
- Bellisario R. L., Maley G. F., Guarino D. U., Maley F. The primary structure of Lactobacillus casei thymidylate synthetase. II. The complete amino acid sequence of the active site peptide, CNBr 4. J Biol Chem. 1979 Feb 25;254(4):1296–1300. [PubMed] [Google Scholar]
- Bertino J. R. Toward improved selectivity in cancer chemotherapy: the Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res. 1979 Feb;39(2 Pt 1):293–304. [PubMed] [Google Scholar]
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Downing M. R., Mann K. G. High-pressure liquid chromatographic analysis of amino acid phenylthiohydantoins: comparison with other techniques. Anal Biochem. 1976 Aug;74(2):298–319. doi: 10.1016/0003-2697(76)90211-6. [DOI] [PubMed] [Google Scholar]
- Dunlap R. B., Harding N. G., Huennekens F. M. Thymidylate synthetase from amethopterin-resistant Lactobacillus casei. Biochemistry. 1971 Jan 5;10(1):88–97. doi: 10.1021/bi00777a014. [DOI] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Gouy M., Gautier C. Codon usage in bacteria: correlation with gene expressivity. Nucleic Acids Res. 1982 Nov 25;10(22):7055–7074. doi: 10.1093/nar/10.22.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Messing J., Gronenborn B. A versatile primer for DNA sequencing in the M13mp2 cloning system. Gene. 1980 Jun;10(1):69–73. doi: 10.1016/0378-1119(80)90145-6. [DOI] [PubMed] [Google Scholar]
- Heidelberger C. Chemical carcinogenesis, chemotherapy: cancer's continuing core challenges--G. H. A. Clowes Memorial Lecture. Cancer Res. 1970 Jun;30(6):1549–1569. [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Inglis A. S., Nicholls P. W. Identification of phenylthiohydantoins of amino acids by thin-layer chromatography. J Chromatogr. 1973 May 16;79:344–346. doi: 10.1016/s0021-9673(01)85310-3. [DOI] [PubMed] [Google Scholar]
- Larson R., Messing J. Apple II computer software for DNA and protein sequence data. DNA. 1983;2(1):31–35. doi: 10.1089/dna.1.1983.2.31. [DOI] [PubMed] [Google Scholar]
- Leary R. P., Kisliuk R. L. Crystalline thymidylate synthetase from dichloromethotrexate resistant Lactobacillus casei. Prep Biochem. 1971 Jan;1(1):47–54. doi: 10.1080/00327487108081929. [DOI] [PubMed] [Google Scholar]
- Maley G. F., Bellisario R. L., Guarino D. U., Maley F. The primary structure of Lactobacillus casei thymidylate synthetase. I. The isolation of cyanogen bromide peptides 1 through 5 and the complete amino acid sequence of CNBr 1, 2, 3, and 5. J Biol Chem. 1979 Feb 25;254(4):1288–1295. [PubMed] [Google Scholar]
- Maley G. F., Bellisario R. L., Guarino D. U., Maley F. The primary structure of Lactobacillus casei thymidylate synthetase. III. The use of 2-(2-nitrophenylsulfenyl)-3-methyl-3-bromoindolenine and limited tryptic peptides to establish the complete amino acid sequence of the enzyme. J Biol Chem. 1979 Feb 25;254(4):1301–1304. [PubMed] [Google Scholar]
- Maley G. F., Maley F., Baugh C. M. Studies on identifying the folylpolyglutamate binding sites of Lactobacillus casei thymidylate synthetase. Arch Biochem Biophys. 1982 Jul;216(2):551–558. doi: 10.1016/0003-9861(82)90244-2. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Poncz M., Solowiejczyk D., Ballantine M., Schwartz E., Surrey S. "Nonrandom" DNA sequence analysis in bacteriophage M13 by the dideoxy chain-termination method. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4298–4302. doi: 10.1073/pnas.79.14.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- STEERS E., Jr, CRAVEN G. R., ANFINSEN C. B., BETHUNE J. L. EVIDENCE FOR NONIDENTICAL CHAINS IN THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2478–2484. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi D. V., McHenry C. S., Sommer H. Mechanism of interaction of thymidylate synthetase with 5-fluorodeoxyuridylate. Biochemistry. 1974 Jan 29;13(3):471–481. doi: 10.1021/bi00700a012. [DOI] [PubMed] [Google Scholar]



