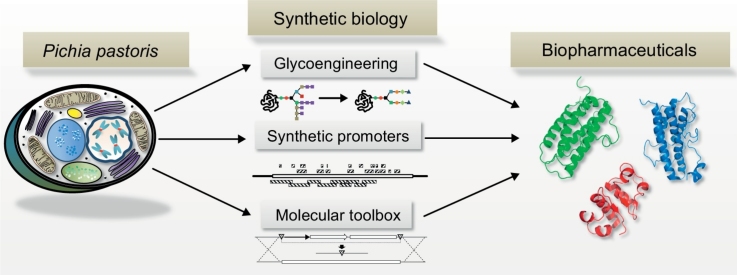
Graphical abstract
Highlights
► The application of Pichia pastoris for biopharmaceutical production is described. ► Synthetic biology approaches and perspectives to improve production are reviewed. ► Glycoengineering efforts to produce humanized, uniform glycoproteins are covered. ► The design and application of synthetic promoter variants are highlighted. ► The molecular toolbox available for synthetic biology in P. pastoris is discussed.
Abstract
Biopharmaceuticals are an integral part of modern medicine and pharmacy. Both, the development and the biotechnological production of biopharmaceuticals are highly cost-intensive and require suitable expression systems. In this review we discuss established and emerging tools for reengineering the methylotrophic yeast Pichia pastoris for biopharmaceutical production. Recent advancements of this industrial expression system through synthetic biology include synthetic promoters to avoid methanol induction and to fine-tune protein production. New platform strains and molecular cloning tools as well as in vivo glycoengineering to produce humanized glycoforms have made P. pastoris an important host for biopharmaceutical production.
Current Opinion in Biotechnology 2013, 24:1094–1101
This review comes from a themed issue on Pharmaceutical biotechnology
Edited by Ajikumar Parayil and Federico Gago
For a complete overview see the Issue and the Editorial
Available online 20th March 2013
0958-1669/$ – see front matter, © 2013 Elsevier Ltd. All rights reserved.
Introduction
Biopharmaceuticals are indispensable in modern medicine. The estimated market value is $70 to 80 billion (depending on the definition) and annual growth rates between 7 and 15% are expected [1–3]. This is another major reason for the worldwide focus of pharmacy and biotechnology on biopharmaceutical development and production. By definition, the term ‘biopharmaceutical’ refers to recombinant therapeutic proteins and nucleic acid based products and in the broader sense also to engineered cell or tissue-based products [2]. Vaccines, interferons and hormones like insulin, human growth hormone (hGH) and erythropoietin (EPO) are examples for protein biopharmaceuticals. Antibodies (including fragments like Fabs, scFvs and nanobodies) represent the biggest group of protein biopharmaceuticals [1–3].
Therapeutic proteins are typically produced in mammalian cell lines and Escherichia coli. While bacterial systems exhibit fast and robust growth in bioreactors using simple media, mammalian cells resemble their human counterparts more closely in terms of typical eukaryotic post translation modifications (PTMs) like glycosylation [2,4–6]. However, mammalian cell culture processes are relatively slow, require complex media, and are susceptible viral contaminations (Table 1).
Table 1.
Comparison of expression systems used for biopharmaceutical production [4,6,7]
| Higher eukaryotes | Yeast | Escherichia coli | |||
|---|---|---|---|---|---|
| Ease of genetic modifications | Moderate | Simple | Simple | ||
| Cultivation | Slow growth rates, expensive complex (or synthetic) media required | Fast and robust growth, defined minimal media | Fastest growth, defined minimal media | ||
| Contaminations | Risk of viral contaminations, viral clearance required | Little risks of endotoxins or viral DNAs | Endotoxins presence requires thorough purification, possible phage infections | ||
| Post translational modifications (PTMs) | Closely resembling human PTMs; usually mixtures of several glycoform variants | Most human PTMs achievable, but natural glycosylation patterns differ from humans, hypermannosylation, engineered strains can achieve human glycoforms and high uniformity | Limited set of PTMs, some human PTMs (e.g. glycosylation) difficult to achieve | ||
| Protein yields and secretory capacities |
High yields, highly efficient secretion, high specific productivity |
High yields, secretory capacities depending on the species |
High expression capacities, secretion mostly inefficient, extensive purification and downstream processing required |
||
| Most commonly used species | Mammalian cells | Insect cells | Pichia pastoris | Saccharomyces cerevisiae | |
| Recently approved biopharmaceuticalsa | 32 | 2 | 2b | 4 | 17 |
| Additional information and specific differences between host species of the same class | Commonly used cell lines: CHO (Chinese Hamster Ovary), BHK (baby hamster kidney), murine-myeloma-derived NS0, SP2/0 cell lines [2] and HEK293 | Baculo virus based systems most commonly used for transfection Easy scale up Contaminations less problematic Mammalianized glycosylation [5] |
Efficient and selective secretion, often higher protein titers than S. cerevisiae, for example, [8••] | Important eukaryotic model organism, high molecular- and cell biological knowledge | Fastest efficient expression system Inexpensive Well established processes suitable for mass production Folding problems may lead to the formation of inclusion bodies and require expensive refolding (yet, inclusion bodies provide a valuable strategy to achieve high protein yields and simple purification) Inefficient acetate metabolism may hamper high cell density cultivation of some strains |
| Crabtree negative, high cell density cultivations | Crabtree positive, leading to ethanol production | ||||
| GRAS status | |||||
| Hypermannosylation is less pronounced in P. pastoris and critical terminal α-1,3-mannose linkages were not observed [19], engineered strains providing fully humanized glycosylation not available for S. cerevisiae | |||||
Using yeasts enables to combine robust growth on simple media (in large scale bioreactors) with easily achievable genetic modifications and the introduction of the desired PTMs [7].
The ‘classic’ yeast Saccharomyces cerevisiae is one of the best studied eukaryotes and has been used as expression host for biopharmaceuticals since the early days of genetic engineering and recombinant protein production [8••]. Recently, the first biopharmaceutical produced in the methylotrophic yeast Pichia pastoris has been approved by the FDA (Kalbitor by Dyax Corp., a Kallikrein inhibitor) [1]. P. pastoris features all favorable traits of yeasts mentioned and has successfully been used to produce high titers of numerous heterologous proteins [7,9,10••]. Additionally, P. pastoris is suitable for high cell density cultivations, reaching more than 150 g dry cell weight per liter [11] and has high secretory capabilities for heterologous proteins, while secreting only low amounts of endogenous proteins (Table 1) [12].
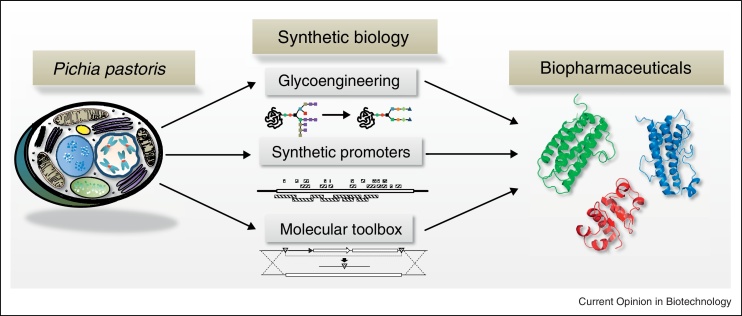
In this review we focus on new opportunities for biopharmaceutical production by reengineered P. pastoris employing new tools, (semi-) synthetic parts and PTM pathways (see Figure 1). We also summarized already published approaches to identify regulatory elements and to reengineer promoters for bottom-up regulatory circuit design.
Figure 1.
Current synthetic biology approaches to improve biopharmaceutical yields and quality in P. pastoris. Glycoengineered strains provide humanized N-glycosylation patterns [14,15,16•], synthetic promoters allow the fine-tuning of expression levels [41,42,43•] and various tools for strain engineering [47–49,50•] and metabolic modeling [55•,56•,57•] are available.
Recent developments in synthetic biology have extended the toolset of classical genetic engineering [13]. Tailor-made expression systems have been created by modifying transcription, translation, PTMs and designing synthetic regulatory networks [14,15••].
Glycoengineering
The majority of therapeutic proteins contain post-translational modifications, with glycosylation being the most common and at the same time the most complex PTM [2].
Yeasts can perform typical eukaryotic PTMs, but final glycosylation patterns of yeasts and humans differ significantly. Hypermannosylation and terminal α-1,3-mannose linkages associated with glycoproteins from S. cerevisiae, can result in poor serum half-life or even immunogenic effects of therapeutic proteins [2,16•]. Thus, there have been efforts to humanize yeast glycosylation which has been accomplished in P. pastoris (see [16•–18•] for reviews). Also hypermannosylation is less pronounced in P. pastoris and terminal α-1,3-mannose linkages are not observed [19].
Here, we focus on recent developments of glycoengineering in P. pastoris and highlight the synthetic biology approaches and the heterologous and chimeric enzymes used for this purpose.
Achieving humanized glycosylation in yeast required on the one hand the elimination of hyperglycosylation by deleting the appropriate yeast genes, but on the other hand also the introduction of additional glycosidases and glycosyltransferases, including missing biosynthetic pathways and transporters for sugars not present in yeast, for example, sialic acid. In the case of galactose, UDP-glucose was converted to UDP-galactose in the Golgi by providing the respective epimerase activity [16•,17•].
In addition to simple expression of these genes, correct spatial positioning along the secretory pathway in the ER and Golgi is essential, as the sequential activity of one enzyme produces the substrate for the next. To achieve the suitable positioning of the required factors along this cellular assembly line in P. pastoris, synthetic glycobiology [20] approaches were used.
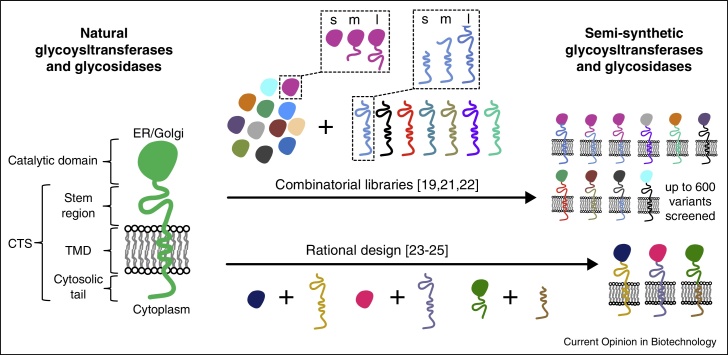
Tailor-made glycosyltransferases and glycosidases with the desired catalytic properties and localization characteristics were created [19,21–25]. The strategy was based on the knowledge, that eukaryotic glycosyltransferases and glycosidases are type II membrane proteins, consisting of an N-terminal cytoplasmic tail, a membrane anchor domain, a stem region and a C-terminal catalytic domain (see Figure 2) [20]. The C-terminal catalytic domain is active independently of the localization conferring N-terminal part, which is also termed ‘CTS’ (cytoplasmic, transmembrane, stem). Fusions of catalytic domains to CTS fragments allowed the creation of semi-synthetic glycosyltransferases and glycosidases. A combinatorial library approach paired with a high-throughput screening was used to create and evaluate these proteins [19,21,22]. Rational design led to similar results [23–25], but eventually input from combinatorial libraries was also used [25].
Figure 2.
Design strategies to create semi-synthetic glycosyltransferases and glycosidases for glycoengineering. On the left side, the general domain structure of glycosyltransferases and glycosidases is shown. These type II membrane proteins consist of an N-terminal cytosolic tail, a transmembrane domain (TMD), a stem region (these elements are referred to as CTS), and a C-terminal catalytic domain. In the middle and on the right side, design strategies for creating tailor-made enzymes with the desired catalytic activity and the proper localization in the sec pathway are shown. The combinatorial library approach involved the combination of large sets of catalytic domains with CTS fragments to fusion proteins, which were then screened for the desired activity [19,21,22]. Different lengths of the catalytic domains and the CTS fragments were tested (referred to as ‘s’ for short, ‘m’ for medium, ‘l’ for long and shown exemplarily for one catalytic domain and one CTS). Rational approaches were also used to design these chimeric enzymes [23–25]. The schematic for the domain architecture and the combinatorial libraries is based on Czlapinski et al. [20] and Nett et al. [26••].
Notably, the initial publications of the combinatorial libraries [19,21] contained barely any information on their composition and how the chimeric glycosyltransferases were designed. More recently, a comprehensive report about the catalytic domains, the CTS fragments, and how they were fused was published [26••]. The authors had not only started from a large set of 33 catalytic domains from different eukaryotes (e.g. fungi, worm, fruit fly, mouse, rat, human) and 66 fungal leader sequences, but also tested fusions of various lengths of both the catalytic domain and the CTS. Up to 600 variants were screened for optimal desired activity and localization along the generated artificial glycosylation pathways in P. pastoris (see Figure 2).
An essential milestone was achieved in 2006 by introduction of nine synthetic genes and deletion of six endogenous genes enabling the production of complex terminally sialylated glycoproteins in P. pastoris [22]. In the last five years, N-glycosylation site occupancy has been increased from 75–85% to 99% [27] and undesired β-linked mannose residues have been removed by creating a P. pastoris quadruple knock-out devoid of all four endogenous β-mannosyl transferases [28]. Furthermore, the production processes using glycoengineered P. pastoris strains have been optimized [29–31], antibody production in glycoengineered strains reached the g/l scale [32,33] and glycoengineered strains have also been established for surface display applications [34,35].
In addition to human like microbial glycosylation such heterologous synthetic pathways allow direct control of the intricate glycosylation process. Thereby, tailor-made glycoforms of a protein can be produced which can exhibit moderately differing pharmacodynamics. For example an antibody expressed in glycoengineered P. pastoris with a uniform, single glycoform showed improved antibody-mediated effector functions, compared to mammalian cell culture derived glycoforms with variable glycosylation patterns [36]. Therefore, better than nature glycoengineered P. pastoris strains pave the way for the creation of synthetic, supernatural glycoform preparations with altered properties compared to naturally occurring variants.
Synthetic promoters
Efficient transcription is a critical step in gene expression. Therefore strong and controllable promoters are an essential tool for high titers in recombinant protein production [7,37]. In addition to natural promoters there has been a growing interest in synthetic promoters driving enhanced expression, improving folding or showing tailor-made regulatory profiles [37–39]. In P. pastoris, up to 22 g/l intracellular protein and 15 g/l secreted protein have been obtained with the most frequently applied, tightly controlled, strong and methanol inducible AOX1 promoter (PAOX1) [40].
As result, this promoter was the starting point for creating synthetic variants with increased promoter strength and altered, methanol free regulation, as the use of toxic and flammable methanol can cause a considerable safety risk in industrial processes.
One semi-rational approach to create synthetic PAOX1 variants relied on an in silico analysis for putative conserved eukaryotic transcription factor binding sites (TFBS) in PAOX1. Subsequently, the respective short sequence stretches were deleted [41]. These deletion variants showed both increased and decreased reporter gene expression levels spanning 6–160% of wildtype PAOX1 driven expression. Alternative approaches relied on the systematic deletion of larger adjacent fragments of almost the entire promoter [42]. Surprisingly, some small deletions and point mutations resulted in altered regulation as these variants were moderately active when glucose was depleted, without requiring the inductor methanol [41]. This derepression effect was further optimized by combinations of deletions and insertions of important sequence stretches. Such altered induction properties now enable the consecutive induction of coexpressed proteins such as chaperons and the therapeutic protein of interest. Putative TFBS of PAOX1 were also fused to natural core promoter fragments to create short semi-synthetic variants, which again showed altered regulation and surpassed the full-length wildtype promoter in certain applications especially when multiple copies of the expression cassettes were integrated [41,43•]. Also the constitutive promoter of the glyceraldehyde-3-phosphate dehydrogenase gene (PGAP) of P. pastoris has been engineered by a random mutagenesis approach [44] showing the potential of additional promoters for expression fine tuning or the generation of new regulatory circuits. Bio-process strategies for biotechnologically relevant enzymes have been improved by employing these synthetic promoters [41,43•,44,45] and similar effects can be expected for biopharmaceuticals. Furthermore, multiple positive and negative factors involved in PAOX1 regulation have been identified since this initial semi-rational promoter engineering (see [40] for a recent review), opening the way for the design of novel synthetic regulatory circuits for gene expression and pathway design.
Molecular toolbox for synthetic biology in P. pastoris
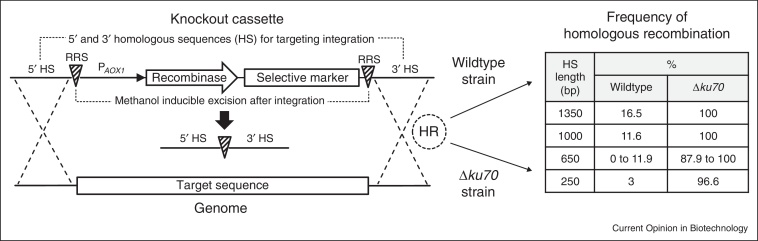
Synthetic biology applications require efficient tools for strain engineering. For example, the creation of P. pastoris strains providing a fully humanized glycosylation pattern necessitated in the first place the development of suitable genetic strategies to knock out and introduce multiple genes [46]. Efficient strategies for gene replacements and marker recycling have now become available for P. pastoris. Namely, systems based on new counter selective markers [47], a Cre/loxP strategy [48,49] and an advanced flipper cassette application [50•] have been reported and applied. The recombinase based strategies [48,49,50•] allow active excision of the marker gene used in a deletion cassette and to thereby recycle markers and perform sequential rounds of deletions. This is achieved by designing a deletion cassette, in which the marker gene and the recombinase are flanked by two recombinase recognition sites and the recombinase is placed under the tight control of the methanol inducible AOX1 promoter (see Figure 3). Näätsaari et al. [50•] applied such a strategy to generate a new platform of P. pastoris expression strains and Marx et al. [49] boosted riboflavin production in P. pastoris by subsequently overexpressing all six genes of the riboflavin biosynthetic pathway by inserting the strong constitutive GAP promoter upstream of these genes. Coupling such approaches with synthetic promoter variants [41,42,43•,44] might support the transcriptional fine tuning of individual enzyme activities of biosynthetic pathways.
Figure 3.
Recombinase based self-excisable knockout cassettes for marker regeneration (left side). Increased rates of homologous recombination in a P. pastoris Δku70 strain (right side). The knockout cassettes consist of a recombinase (Cre or FLP [48,49,50•]) and a marker gene flanked by the respective recombinase recognition sites and are directed to the genome via the 5′ and 3′ homologous sequences to delete the desired target sequence. After integration via a double cross-over event, self-excision of the recombinase and the marker gene can be initiated by the expression of the recombinase from the methanol inducible AOX1 promoter (PAOX1), leaving only the recombinase recognition site in the genome (notably Marx et al. [49] provided the recombinase transiently on a CEN/ARS plasmid). The initial integration in the genome is dependent on homologous recombination (HR). Exemplary frequencies of homologous recombination (in %) of the wildtype compared to the Δku70 strain are shown (right side). The length of the homologous sequence indicates the number of base pairs (bp) added on both sides of the cassette [50•]. For 650 bp two different integration loci were tested, therefore two % values are given.
Site specific integration and knock-out strain generation rely on endogenous homologous recombination. While in S. cerevisiae HR is working highly efficiently, non-homologous end joining is the preferred pathway in most other filamentous fungi and yeasts, including P. pastoris. HR occurs at less than 1% and up to 30% of all integration events, depending on the length of the homologous targeting sequence [50•]. For example during glycoengineering of P. pastoris only 5 out of 460 clones showed the desired gene replacement [46]. Targeted integration and deletion should become more efficient in the future by employing a P. pastoris ku70 deletion strain with increased rates of HR [50•]. By the deletion of a Ku70 homologue, a protein involved in NHEJ, HR rates of up to 100% were achieved (see Figure 3). The Δku70 strain did not show genetic instability, but the growth rates were 10–30% lower than those of the wildtype (depending on the carbon source) and the strain showed a decreased survival rate under UV light. This hints an increased susceptibility to DNA damage and complementing the wildtype KU70 gene after completion of strain engineering was recommended [50•].
In addition to precise deletions, site specific integration, and marker recycling, new cloning techniques facilitate the construction of the respective gene expression and deletion constructs. Efficient in vitro recombination methods such as Gibson assembly [51] enable flexible restriction free cloning and library generation allowing the simple testing of libraries of promoters, artificial or natural expression enhancers and signal-sequences or other targeting sequences. Although, bottom up approaches to design individual parts for P. pastoris strain reengineering and expression cassette constructions are ongoing, there is no systematic synthetic biology parts collection for this yeast so far.
Bioinformatics tools complete the toolbox for synthetic biology applied in P. pastoris. High-quality genome sequences [52–54] and metabolic models [55•,56•,57•] of P. pastoris have recently become available. This comprehensive new background knowledge enables research towards systems wide understanding of the P. pastoris expression system and provides the basis for reengineering this host using synthetic parts and pathways to improve biopharmaceutical production. For example, recent studies in P. pastoris have hinted an interconnection of both the carbon metabolism [58] and the cellular redox state [59] with protein production and secretion. Thus, similar to S. cerevisiae [60,61], a systems biology view on secretion coupled with a synergistic use of metabolic engineering and synthetic biology approaches [62,63] promise coming improvements for biopharmaceutical production by P. pastoris.
Conclusions
Over the last two decades, P. pastoris has been established as one of the most frequently used expression systems in both industry and academia. Beside a large number of various enzymes, many human proteins and biopharmaceuticals were also efficiently produced by P. pastoris. The adaptation of the yeast high-mannose type glycosylation to the complex humanized glycosylation was a major achievement and resulted in uniform glycoforms from microbial production. Synthetic promoter variants with altered regulatory profiles and expression levels surpassed their natural counterparts for enzyme production. Equally, these variants can be used to optimize and fine-tune the expression of therapeutic proteins. Also other new key methodologies for synthetic biology such as efficient gene deletion and assembly strategies, metabolic models and strains with altered recombination properties have become available. Together with milestones such as the FDA approval, these new tools and techniques have a high potential to boost the production of biopharmaceuticals and for efficient metabolic engineering (see Box 1).
Box 1. Milestones and recent accomplishments for biopharmaceutical production in P. pastoris.
-
(1)
FDA GRAS (generally regarded as safe) status in 2006 (Phospholipase C by Diversa Corp., for degumming vegetables oils for food use).
-
(2)
FDA approved biopharmaceutical production processes in 2009 (Kalbitor by Dyax Corp., a Kallikrein inhibitor) and 2012 (Jetrea by ThromboGenics NV, for the treatment of vitreomacular traction).
-
(3)
Glycoengineered strains providing humanized, uniform N-glycosylation patterns [22,25].
-
(4)
Synthetic promoters for fine-tuning expression levels [41,42,43•].
-
(5)
Efficient strategies for knockouts of multiple genes and overexpression of entire pathways [48,49,50•].
-
(6)
High quality genome sequences [52–54].
-
(7)
Establishment of in silico metabolic models for strain engineering [55•,56•,57•].
Altered and new biosynthetic pathways for posttranslational modifications such as precise glycosylation are enabling techniques giving access to new therapeutics with uniform and excellent quality.
Synthetic biology will certainly not only further improve industrial enzyme production, but also stimulate and facilitate innovative approaches for biopharmaceutical production in P. pastoris.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
T.V. is financed by theEuropean Union's Seventh Framework Programme FP7/2007-2013 under grant agreement no. 289646 (Kyrobio). We also gratefully acknowledge the Austrian Science Fund (FWF) project number W901 (DK ‘Molecular Enzymology’ Graz) and the Austrian Centre of Industrial Biotechnology (ACIB) contribution was supported by FFG, bmvit, mvwfi, ZIT, Zukunftsstiftung Tirol and Land Steiermark within the Austrian COMET programme (FFG grant 824186). We would also like to thank Andrea Camattari and Claudia Ruth for valuable discussions.
References
- 1.Walsh G. Biopharmaceutical benchmarks 2010. Nat Biotechnol. 2010;28:917–924. doi: 10.1038/nbt0910-917. [DOI] [PubMed] [Google Scholar]
- 2.Walsh G. Post-translational modifications of protein biopharmaceuticals. Drug Discov Today. 2010;15:773–780. doi: 10.1016/j.drudis.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Goodman M. Market watch: sales of biologics to show robust growth through to 2013. Nat Rev Drug Discov. 2009;8:837. doi: 10.1038/nrd3040. [DOI] [PubMed] [Google Scholar]
- 4.Demain A.L., Vaishnav P. Production of recombinant proteins by microbes and higher organisms. Biotechnol Adv. 2009;27:297–306. doi: 10.1016/j.biotechadv.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Palmberger D., Klausberger M., Berger I., Grabherr R. MultiBac turns sweet. Bioengineered. 2012;4:1–6. doi: 10.4161/bioe.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berlec A., Strukelj B. Current state and recent advances in biopharmaceutical production in Escherichia coli, yeasts and mammalian cells. J Ind Microbiol Biotechnol. 2013 doi: 10.1007/s10295-013-1235-0. [DOI] [PubMed] [Google Scholar]
- 7.Mattanovich D., Branduardi P., Dato L., Gasser B., Sauer M., Porro D. Recombinant protein production in yeasts. Methods Mol Biol. 2012;824:329–358. doi: 10.1007/978-1-61779-433-9_17. [DOI] [PubMed] [Google Scholar]
- 8••.Martínez J.L., Liu L., Petranovic D., Nielsen J. Pharmaceutical protein production by yeast: towards production of human blood proteins by microbial fermentation. Curr Opin Biotechnol. 2012;23:965–971. doi: 10.1016/j.copbio.2012.03.011. [DOI] [PubMed] [Google Scholar]; Concise overview of biopharmaceutical production in S. cerevisiae. Covers approaches for strain engineering and also shortly biopharmaceutical production in P. pastoris.
- 9.Daly R., Hearn M.T.W. Expression of heterologous proteins in Pichia pastoris: a useful experimental tool in protein engineering and production. J Mol Recognit. 2005;18:119–138. doi: 10.1002/jmr.687. [DOI] [PubMed] [Google Scholar]
- 10••.Gasser B., Prielhofer R., Marx H., Maurer M., Nocon J., Steiger M., Puxbaum V., Sauer M., Mattanovich D. Pichia pastoris: protein production host and model organism for biomedical research. Future Microbiol. 2013;8:191–208. doi: 10.2217/fmb.12.133. [DOI] [PubMed] [Google Scholar]; Detailed overview on protein production in P. pastoris covering key aspects for high level expression, strain engineering, secretion and novel methods. Protocols are also provided and the use of P. pastoris as a model for human cell biology is summarized.
- 11.Jahic M., Veide A., Charoenrat T., Teeri T., Enfors S.-O. Process technology for production and recovery of heterologous proteins with Pichia pastoris. Biotechnol Prog. 2006;22:1465–1473. doi: 10.1021/bp060171t. [DOI] [PubMed] [Google Scholar]
- 12.Damasceno L.M., Huang C.-J., Batt C.A. Protein secretion in Pichia pastoris and advances in protein production. Appl Microbiol Biotechnol. 2012;93:31–39. doi: 10.1007/s00253-011-3654-z. [DOI] [PubMed] [Google Scholar]
- 13.Liang J., Luo Y., Zhao H. Synthetic biology: putting synthesis into biology. Wiley Interdiscip Rev Syst Biol Med. 2012;3:7–20. doi: 10.1002/wsbm.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch S.A., Gill R.T. Synthetic biology: new strategies for directing design. Metab Eng. 2012;14:205–211. doi: 10.1016/j.ymben.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 15••.Krivoruchko A., Siewers V., Nielsen J. Opportunities for yeast metabolic engineering: lessons from synthetic biology. Biotechnol J. 2011;6:262–276. doi: 10.1002/biot.201000308. [DOI] [PubMed] [Google Scholar]; Excellent, comprehensive review highlighting the use of synthetic biology to engineer S. cerevisiae. Describes approaches ranging from synthetic promoters, pathway engineering, riboswitches, protein engineering to synthetic regulatory networks.
- 16•.De Pourcq K., De Schutter K., Callewaert N. Engineering of glycosylation in yeast and other fungi: current state and perspectives. Appl Microbiol Biotechnol. 2010;87:1617–1631. doi: 10.1007/s00253-010-2721-1. [DOI] [PubMed] [Google Scholar]; See annotation to Ref. [18•].
- 17•.Hamilton S.R., Gerngross T.U. Glycosylation engineering in yeast: the advent of fully humanized yeast. Curr Opin Biotechnol. 2007;18:387–392. doi: 10.1016/j.copbio.2007.09.001. [DOI] [PubMed] [Google Scholar]; See annotation to Ref. [18•].
- 18•.Bollok M., Resina D., Valero F., Ferrer P. Recent patents on the Pichia pastoris expression system: expanding the toolbox for recombinant protein production. Recent Pat Biotechnol. 2009;3:192–201. doi: 10.2174/187220809789389126. [DOI] [PubMed] [Google Scholar]; Refs. [16•,17•,18•] provide an in-depth coverage of the glycoengineering efforts in P. pastoris before the period under review. The most recent reviews from the two main groups involved (of Gerngross [17•] and Callewaert [16•]) and a review covering patent issues [18•] are listed.
- 19.Choi B.-K., Bobrowicz P., Davidson R.C., Hamilton S.R., Kung D.H., Li H., Miele R.G., Nett J.H., Wildt S., Gerngross T.U. Use of combinatorial genetic libraries to humanize N-linked glycosylation in the yeast Pichia pastoris. Proc Natl Acad Sci U S A. 2003;100:5022–5027. doi: 10.1073/pnas.0931263100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Czlapinski J.L., Bertozzi C.R. Synthetic glycobiology: exploits in the Golgi compartment. Curr Opin Chem Biol. 2006;10:645–651. doi: 10.1016/j.cbpa.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton S.R., Bobrowicz P., Bobrowicz B., Davidson R.C., Li H., Mitchell T., Nett J.H., Rausch S., Stadheim T.A., Wischnewski H. Production of complex human glycoproteins in yeast. Science. 2003;301:1244–1246. doi: 10.1126/science.1088166. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton S.R., Davidson R.C., Sethuraman N., Nett J.H., Jiang Y., Rios S., Bobrowicz P., Stadheim T.A., Li H., Choi B.-K. Humanization of yeast to produce complex terminally sialylated glycoproteins. Science. 2006;313:1441–1443. doi: 10.1126/science.1130256. [DOI] [PubMed] [Google Scholar]
- 23.Callewaert N., Laroy W., Cadirgi H., Geysens S., Saelens X., Min Jou W., Contreras R. Use of HDEL-tagged Trichoderma reesei mannosyl oligosaccharide 1,2-alpha-d-mannosidase for N-glycan engineering in Pichia pastoris. FEBS Lett. 2001;503:173–178. doi: 10.1016/s0014-5793(01)02676-x. [DOI] [PubMed] [Google Scholar]
- 24.Vervecken W., Kaigorodov V., Callewaert N., Geysens S., De Vusser K., Contreras R. In vivo synthesis of mammalian-like, hybrid-type N-glycans in Pichia pastoris. Appl Environ Microbiol. 2004;70:2639–2646. doi: 10.1128/AEM.70.5.2639-2646.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobs P.P., Geysens S., Vervecken W., Contreras R., Callewaert N. Engineering complex-type N-glycosylation in Pichia pastoris using GlycoSwitch technology. Nat Protoc. 2009;4:58–70. doi: 10.1038/nprot.2008.213. [DOI] [PubMed] [Google Scholar]
- 26••.Nett J.H., Stadheim T.A., Li H., Bobrowicz P., Hamilton S.R., Davidson R.C., Choi B., Mitchell T., Bobrowicz B., Rittenhour A. A combinatorial genetic library approach to target heterologous glycosylation enzymes to the endoplasmic reticulum or the Golgi apparatus of Pichia pastoris. Yeast. 2011;28:237–252. doi: 10.1002/yea.1835. [DOI] [PubMed] [Google Scholar]; Revelation of the exact composition and design of the combinatorial genetic libraries (initially described in [19,21]) for the generation of the synthetic glycosyltransferases and glycosidases used to humanize P. pastoris N-glycosylation.
- 27.Choi B.-K., Warburton S., Lin H., Patel R., Boldogh I., Meehl M., D’Anjou M., Pon L., Stadheim T.A., Sethuraman N. Improvement of N-glycan site occupancy of therapeutic glycoproteins produced in Pichia pastoris. Appl Microbiol Biotechnol. 2012;95:671–682. doi: 10.1007/s00253-012-4067-3. [DOI] [PubMed] [Google Scholar]
- 28.Hopkins D., Gomathinayagam S., Rittenhour A.M., Du M., Hoyt E., Karaveg K., Mitchell T., Nett J.H., Sharkey N.J., Stadheim T.A. Elimination of β-mannose glycan structures in Pichia pastoris. Glycobiology. 2011;21:1616–1626. doi: 10.1093/glycob/cwr108. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs P.P., Inan M., Festjens N., Haustraete J., Van Hecke A., Contreras R., Meagher M.M., Callewaert N. Fed-batch fermentation of GM-CSF-producing glycoengineered Pichia pastoris under controlled specific growth rate. Microb Cell Fact. 2010;9:93. doi: 10.1186/1475-2859-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nett J.H., Gomathinayagam S., Hamilton S.R., Gong B., Davidson R.C., Du M., Hopkins D., Mitchell T., Mallem M.R., Nylen A. Optimization of erythropoietin production with controlled glycosylation-PEGylated erythropoietin produced in glycoengineered Pichia pastoris. J Biotechnol. 2012;157:198–206. doi: 10.1016/j.jbiotec.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Berdichevsky M., D’Anjou M., Mallem M.R., Shaikh S.S., Potgieter T.I. Improved production of monoclonal antibodies through oxygen-limited cultivation of glycoengineered yeast. J Biotechnol. 2011;155:217–224. doi: 10.1016/j.jbiotec.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Potgieter T.I., Cukan M., Drummond J.E., Houston-Cummings N.R., Jiang Y., Li F., Lynaugh H., Mallem M., McKelvey T.W., Mitchell T. Production of monoclonal antibodies by glycoengineered Pichia pastoris. J Biotechnol. 2009;139:318–325. doi: 10.1016/j.jbiotec.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Ye J., Ly J., Watts K., Hsu A., Walker A., McLaughlin K., Berdichevsky M., Prinz B., Sean Kersey D., D’Anjou M. Optimization of a glycoengineered Pichia pastoris cultivation process for commercial antibody production. Biotechnol Prog. 2011;27:1744–1750. doi: 10.1002/btpr.695. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs P.P., Ryckaert S., Geysens S., De Vusser K., Callewaert N., Contreras R. Pichia surface display: display of proteins on the surface of glycoengineered Pichia pastoris strains. Biotechnol Lett. 2008;30:2173–2181. doi: 10.1007/s10529-008-9807-1. [DOI] [PubMed] [Google Scholar]
- 35.Lin S., Houston-Cummings N.R., Prinz B., Moore R., Bobrowicz B., Davidson R.C., Wildt S., Stadheim T.A., Zha D. A novel fragment of antigen binding (Fab) surface display platform using glycoengineered Pichia pastoris. J Immunol Methods. 2012;375:159–165. doi: 10.1016/j.jim.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Li H., Sethuraman N., Stadheim T.A., Zha D., Prinz B., Ballew N., Bobrowicz P., Choi B.-K., Cook W.J., Cukan M. Optimization of humanized IgGs in glycoengineered Pichia pastoris. Nat Biotechnol. 2006;24:210–215. doi: 10.1038/nbt1178. [DOI] [PubMed] [Google Scholar]
- 37.Juven-Gershon T., Cheng S., Kadonaga J.T. Rational design of a super core promoter that enhances gene expression. Nat Methods. 2006;3:917–922. doi: 10.1038/nmeth937. [DOI] [PubMed] [Google Scholar]
- 38.Ruth C., Glieder A. Perspectives on synthetic promoters for biocatalysis and biotransformation. Chembiochem. 2010;11:761–765. doi: 10.1002/cbic.200900761. [DOI] [PubMed] [Google Scholar]
- 39.Blount B.A., Weenink T., Vasylechko S., Ellis T. Rational diversification of a promoter providing fine-tuned expression and orthogonal regulation for synthetic biology. PLoS ONE. 2012;7:e33279. doi: 10.1371/journal.pone.0033279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vogl T., Glieder A. Regulation of Pichia pastoris promoters and its consequences for protein production. New Biotechnol. 2012 doi: 10.1016/j.nbt.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Hartner F.S., Ruth C., Langenegger D., Johnson S.N., Hyka P., Lin-Cereghino G.P., Lin-Cereghino J., Kovar K., Cregg J.M., Glieder A. Promoter library designed for fine-tuned gene expression in Pichia pastoris. Nucleic Acids Res. 2008;36:e76. doi: 10.1093/nar/gkn369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xuan Y., Zhou X., Zhang W., Zhang X., Song Z., Zhang Y. An upstream activation sequence controls the expression of AOX1 gene in Pichia pastoris. FEMS Yeast Res. 2009;9:1271–1282. doi: 10.1111/j.1567-1364.2009.00571.x. [DOI] [PubMed] [Google Scholar]
- 43•.Ruth C., Zuellig T., Mellitzer A., Weis R., Looser V., Kovar K., Glieder A. Variable production windows for porcine trypsinogen employing synthetic inducible promoter variants in Pichia pastoris. Syst Synth Biol. 2010;4:181–191. doi: 10.1007/s11693-010-9057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes the use of PAOX1 deletion variants and the design of small synthetic promoters (by the fusion of cis-acting elements to core promoter fragments) to improve protein production.
- 44.Qin X., Qian J., Yao G., Zhuang Y., Zhang S., Chu J. GAP promoter library for fine-tuning of gene expression in Pichia pastoris. Appl Environ Microbiol. 2011;77:3600–3608. doi: 10.1128/AEM.02843-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mellitzer A., Weis R., Glieder A., Flicker K. Expression of lignocellulolytic enzymes in Pichia pastoris. Microb Cell Fact. 2012;11:61. doi: 10.1186/1475-2859-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nett J.H., Gerngross T.U. Cloning and disruption of the PpURA5 gene and construction of a set of integration vectors for the stable genetic modification of Pichia pastoris. Yeast. 2003;20:1279–1290. doi: 10.1002/yea.1049. [DOI] [PubMed] [Google Scholar]
- 47.Yang J., Jiang W., Yang S. mazF as a counter-selectable marker for unmarked genetic modification of Pichia pastoris. FEMS Yeast Res. 2009;9:600–609. doi: 10.1111/j.1567-1364.2009.00503.x. [DOI] [PubMed] [Google Scholar]
- 48.Pan R., Zhang J., Shen W.-L., Tao Z.-Q., Li S.-P., Yan X. Sequential deletion of Pichia pastoris genes by a self-excisable cassette. FEMS Yeast Res. 2011;11:292–298. doi: 10.1111/j.1567-1364.2011.00716.x. [DOI] [PubMed] [Google Scholar]
- 49.Marx H., Mattanovich D., Sauer M. Overexpression of the riboflavin biosynthetic pathway in Pichia pastoris. Microb Cell Fact. 2008;7:23. doi: 10.1186/1475-2859-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Näätsaari L., Mistlberger B., Ruth C., Hajek T., Hartner F.S., Glieder A. Deletion of the Pichia pastoris KU70 homologue facilitates platform strain generation for gene expression and synthetic biology. PLoS ONE. 2012;7:e39720. doi: 10.1371/journal.pone.0039720. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report on a P. pastoris Δku70 strain with increased rates of homologous recombination, achieved by deleting a gene (KU70) coding for a protein involved in non-homologous end joining. Thereby access to deletions, which were otherwise difficult to obtain for P. pastoris, was facilitated. Contains also the description of self-excising knock out cassettes (similar to [47–49]) enabling subsequent rounds of strain engineering with only one selective marker.
- 51.Gibson D.G., Young L., Chuang R., Venter J.C., Hutchison C.A., Smith H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 52.De Schutter K., Lin Y.-C., Tiels P., Van Hecke A., Glinka S., Weber-Lehmann J., Rouzé P., Van de Peer Y., Callewaert N. Genome sequence of the recombinant protein production host Pichia pastoris. Nat Biotechnol. 2009;27:561–566. doi: 10.1038/nbt.1544. [DOI] [PubMed] [Google Scholar]
- 53.Mattanovich D., Graf A., Stadlmann J., Dragosits M., Redl A., Maurer M., Kleinheinz M., Sauer M., Altmann F., Gasser B. Genome, secretome and glucose transport highlight unique features of the protein production host Pichia pastoris. Microb Cell Fact. 2009;8:29. doi: 10.1186/1475-2859-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Küberl A., Schneider J., Thallinger G.G., Anderl I., Wibberg D., Hajek T., Jaenicke S., Brinkrolf K., Goesmann A., Szczepanowski R. High-quality genome sequence of Pichia pastoris CBS7435. J Biotechnol. 2011;154:312–320. doi: 10.1016/j.jbiotec.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 55•.Chung B.K., Selvarasu S., Andrea C., Ryu J., Lee H., Ahn J., Lee H., Lee D. Genome-scale metabolic reconstruction and in silico analysis of methylotrophic yeast Pichia pastoris for strain improvement. Microb Cell Fact. 2010;9:50. doi: 10.1186/1475-2859-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]; See annotation to Ref. [57•].
- 56•.Sohn S.B., Graf A.B., Kim T.Y., Gasser B., Maurer M., Ferrer P., Mattanovich D., Lee S.Y. Genome-scale metabolic model of methylotrophic yeast Pichia pastoris and its use for in silico analysis of heterologous protein production. Biotechnol J. 2010;5:705–715. doi: 10.1002/biot.201000078. [DOI] [PubMed] [Google Scholar]; See annotation to Ref. [57•].
- 57•.Caspeta L., Shoaie S., Agren R., Nookaew I., Nielsen J. Genome-scale metabolic reconstructions of Pichia stipitis and Pichia pastoris and in silico evaluation of their potentials. BMC Syst Biol. 2012;6:24. doi: 10.1186/1752-0509-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]; Refs. [55•,56•,57•] describe metabolic models for two different P. pastoris strains GS115 [55•,57•] and DSMZ 70382 [56•] that can be used for in silico predictions as a basis for engineering approaches.
- 58.Heyland J., Fu J., Blank L.M., Schmid A. Carbon metabolism limits recombinant protein production in Pichia pastoris. Biotechnol Bioeng. 2011;108:1942–1953. doi: 10.1002/bit.23114. [DOI] [PubMed] [Google Scholar]
- 59.Delic M., Rebnegger C., Wanka F., Puxbaum V., Haberhauer-Troyer C., Hann S., Köllensperger G., Mattanovich D., Gasser B. Oxidative protein folding and unfolded protein response elicit differing redox regulation in endoplasmic reticulum and cytosol of yeast. Free Radic Biol Med. 2012;52:2000–2012. doi: 10.1016/j.freeradbiomed.2012.02.048. [DOI] [PubMed] [Google Scholar]
- 60.Hou J., Tyo K., Liu Z., Petranovic D., Nielsen J. Engineering of vesicle trafficking improves heterologous protein secretion in Saccharomyces cerevisiae. Metab Eng. 2012;14:120–127. doi: 10.1016/j.ymben.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Hou J., Tyo K.E.J., Liu Z., Petranovic D., Nielsen J. Metabolic engineering of recombinant protein secretion by Saccharomyces cerevisiae. FEMS Yeast Res. 2012;12:491–510. doi: 10.1111/j.1567-1364.2012.00810.x. [DOI] [PubMed] [Google Scholar]
- 62.Nielsen J., Keasling J.D. Synergies between synthetic biology and metabolic engineering. Nat Biotechnol. 2011;29:693–695. doi: 10.1038/nbt.1937. [DOI] [PubMed] [Google Scholar]
- 63.Yadav V.G., De Mey M., Lim C.G., Ajikumar P.K., Stephanopoulos G. The future of metabolic engineering and synthetic biology: towards a systematic practice. Metab Eng. 2012;14:233–241. doi: 10.1016/j.ymben.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]