Abstract
Objectives:
Small dense (sd) low-density lipoprotein (LDL), tumor necrosis factor (TNF) alpha (α), and nitric oxide (NO) have recently emerged as important stroke risk factors. The aim of the study was to investigate the effects of increased levels of small LDL particle size, TNF-α and NO on the developed ischemic stroke and increased carotid artery intima-media thickness (CIMT).
Materials and Methods:
A total of 29 women and 25 men (a total of 54 ischemic stroke patients) and a similar age group of 50 controls (29 females and 21 males) were included in the study. CIMT, C-reactive protein (CRP), TNF-α, NO, and lipid subfraction test of the two groups were measured.
Results:
The mean LDL particle size was smaller in patients with stroke than in the controls (26.8 ± 0.31 nm vs. 27.0 ± 0.31 nm, P = 0.003). sd-LDL, TNF-α, NO, CRP, right CIMT, and left CIMT were higher in patients with stroke than in the controls (respectively; 8.2 ± 7.8 mg/dL vs. 3.3 ± 3.5 mg/dL, P < 0.001;75.6 ± 25.0 pg/mL vs. 65.4 ± 9.1 pg/mL, P = 0.009;76.4 ± 53.3 mmol/L vs. 41.5 ± 27.0 mmol/L, P < 0.001;1.9 ± 2.6 mm vs. 0.4 ± 0.3 mm P < 0.001;0.97 ± 0.38 mm vs. 0.83 ± 0.15 mm, P = 0.007;1.04 ± 0.44 mm vs. 0.87 ± 0.19 mm, P = 0.010).
Conclusion:
These results show that sd-LDL is independently associated with the incidence of stroke and may be a risk factor in the development of stroke. In addition, TNF-α, NO, right CIMT, and left CIMT may be a risk factor in the development of ischemic stroke.
Key Words: Carotid artery intima-media thickness, ischemic stroke, lipid subfraction, small dense-low-density lipoprotein, tumor necrosis factor-alpha
Introduction
Stroke, which is associated with significant mortality and morbidity, is the second leading cause of death worldwide. Even though great effort has been applied to decrease the risk factors leading to stroke, rates of stroke and its mortality continue to increase. Ischemic stroke is the most common type of stroke. It accounts for nearly 80-90% of strokes worldwide.[1] It is a complex multifactorial polygenic disorder modulated by interactions between an individuals’ genetic background and different environmental factors. Although all risks leading to ischemic stroke cannot be documented, many factors have been reported to be related to ischemic stroke including age, hypertension (HT), diabetes, hyperlipidemia, cardiac disease (myocardial infarction, coronary artery plaque, atrial fibrillation, ventricular arrhythmias, etc), smoking, and body mass index (BMI).[2]
The extent of atherosclerosis can be reflected by the carotid artery, as being atherothrombotic stenosis at the bifurcation of the common carotid artery with extension into the external and internal carotid arteries.[3] Since the carotid artery is supplying the cerebrovascular artery system, the risk for developing ischemic cerebrovascular diseases as transient ischemic attack and cerebral infarction can be predicted by evaluating the extent of arteriosclerosis of the carotid artery.[4] The carotid artery is easy to study due to its superficial position and its relative thickness. It is known that carotid artery intima-media thickness (CIMT) measured by ultrasonography is a noninvasive and clinically useful method for evaluating the extent of mature or premature atherosclerosis.[5]
Plasma low-density lipoprotein (LDL) is a powerful risk factor for atherosclerosis. It consists of particles which vary in size, density, lipid, protein composition, and receptor-binding. Recent studies have shown that small or oxidized LDL can be one of the most powerful risk factors for developing atherosclerosis and coronary disease. A heritable subclass profile of LDL is associated with increased risk of coronary disease[6] and stroke[7] in humans and is characterized by a predominance of small dense (sd)-LDL particles. Meanwhile, there are a few likely mechanisms underlying the association of lipids and stroke.[8,9] One of the most significant is presumably the effects of lipids on the formation of carotid artery atherosclerotic plaque.[10] In this study, we have investigated whether there is a difference in CIMT, sd-LDL, tumor necrosis factor-alpha (TNF-α), and nitric oxide (NO) levels between patients with newly diagnosed ischemic stroke and patients without stroke.
Materials and Methods
Patients
A total of 54 subjects 29 females and 25 males who were brought into the emergency department of our hospital with neurologic symptoms were admitted to the neurology ward with diagnosed stroke. Subjects of stroke group had patients with diabetes mellitus (N = 18), HT (N = 47), cardiac disease [(N = 17), (previous myocardial infarction, N = 6), (coronary artery plaque without myocardial infarction, N = 5), (heart failure, N = 5), (atrial fibrillation, N = 1)], and hyperlipidemia (N = 31). Additionally, 14 active smokers were included to the stroke group. A control group of 50 persons (29 women, 21 men), who had applied to the neurology and internal medicine outpatient clinic in the hospital, were enrolled in the study. All the patients were included in the control groups since patients without cerebrovascular disease were diagnosed by neurology specialist. The patients were diagnosed low back pain, migraine, headache, and chronic diseases (such as diabetes, hyperlipidemia, HT, cardiac disease, etc).
All the subjects’ age, gender, BMI, blood pressure, chronic diseases, smoking, and consumption of alcohol were recorded. The subjects who were younger than 45 years of age, who had experienced a stroke other than ischemic stroke, or who had a previous history of cerebrovascular disease, were excluded from this study. The study was approved by the local ethics committees and informed consent from each participant was obtained (Approval numbers: 2012/51).
Evaluation of carotid atherosclerosis
For the measurement of CIMT, patients were asked to lie down in the supine position, with heads tilted backward. The right and left arteries were displayed by using a 10 mHz direct probe of the ultrasound (Toshiba Xario, Tokyo, Japan). A region of 1 cm within the initial 2 cm of the proximal common carotid artery bulb was identified, and an optimal longitudinal ultrasound image was obtained. Three different measurements were taken from the posterior wall of the artery in the systolic phase and their mean was calculated as CIMT. The measurement was taken on both common carotid arteries.
Stroke analysis
The patient's brain magnetic resonance was performed by 1.5 tesla by Philips Achieva device (Philips, Holland). Subtypes of index stroke were determined by the consensus of three neurologists (Ahmet Tufekci, Serkan Kirbas, and Sevim Cakmak) as follows: Ischemic stroke of undetermined etiology, as described in the Trial of Org 10172 in Acute Stroke Treatment study. Patients without ischemic stroke were excluded from the study.
Measures of laboratory tests
All blood samples were collected into tubes and centrifuged with 4500 rpm for 10 min, and the serums were stored at −30°C. The biochemical tests were performed with the photometric assays of the Abbott Architect C16000 analyzer (Abbott Diagnostics, USA) and the thyroid stimulating hormone was performed using the chemiluminescent microparticle immunoassay method of the Abbott Architect I 2000 immunology analyzer (Abbott Diagnostics, USA). The C-reactive protein (CRP) test was performed with the nephelometric method of the Coulter Image 800 device (Beckman, USA). Lipid subfraction test was performed with the Quantimetrix lipoprint system LDL subfraction kit (USA). Lipoprint system is a linear, polyacrylamide gel electrophoresis system.
Measures of TNF-α
The concentration of TNF-α was measured using enzyme-linked immunosorbent assay (ELISA) method. We used commercially available human TNF-α ELISA kit (Abcam, United Kingdom). The procedure for the ELISA method was according to the instructions provided by the manufacturer. Absorbance was measured at a wavelength of 450 ηm using ELISA reader. The levels of TNF-α are presented as ng/mL. The intraassay and interassay coefficient of variation were 4.9% and 7.0%, respectively. The limit of detection (LOD) for the TNF-α assay was 15 pg/mL.
Measures of NO
The concentration of NO was measured using colorimetric assay method. We used commercially available NO kit (Cayman Chemical Company, USA). The procedure for the colorimetric method was according to the instructions provided by the manufacturer. Absorbance was measured at a wavelength of 540 ηm using reader. The levels of NO are presented as μM (μmol/L). The intraassay and interassay coefficient of variation were 2.7% and 3.4%, respectively. The LOD for the NO assay was 2.5 μM.
Statistical analysis
The results were reported as the mean ± standard deviation. The data analysis was performed using the statistical software SPSS for Windows (version 13.1; SPSS, Chicago, IL, USA). All the results were analyzed by applying the Kolmogorov-Smirnov for the determination of both normal and abnormal data distribution. The statistical significance of the differences in all the parameters between the stroke and the control groups was analyzed using the independent sample t-test for normal distribution parameters and Mann-Whitney U tests for creatinin, high-density lipoprotein (HDL), triglyceride (TG), CRP, NO, and sd-LDL. The level of CIMT, sd-LDL, and mean LDL particle size were investigated by multivariate analysis. Subgroup's analyses for gender were done by Nested Anova, followed by Bonferroni analysis. The relationship between the variables was analyzed with the Pearson's correlation. The differences were considered significant at P < 0.05.
Results
The mean age of the stroke group was 71 ± 10 years, the BMI was 30.5 ± 6.7 kg/m2, and the average right CIMT was 0.97 ± 0.38 mm and left CIMT was found to be 1.04 ± 0.44 mm. The mean age of the control group was 69 ± 8 years, the BMI was 29.5 ± 6.8 kg/m2, and the average right CIMT was 0.83 ± 0.15 mm (P = 0.007) and the left CIMT was found to be 0.87 ± 0.19 mm (P = 0.010). The patient's demographic data, blood pressures, and CIMT measurements are shown in Table 1.
Table 1.
Demographic data, blood pressures, chronic diseases and coronary artery intima-media thickness measurements of study populations
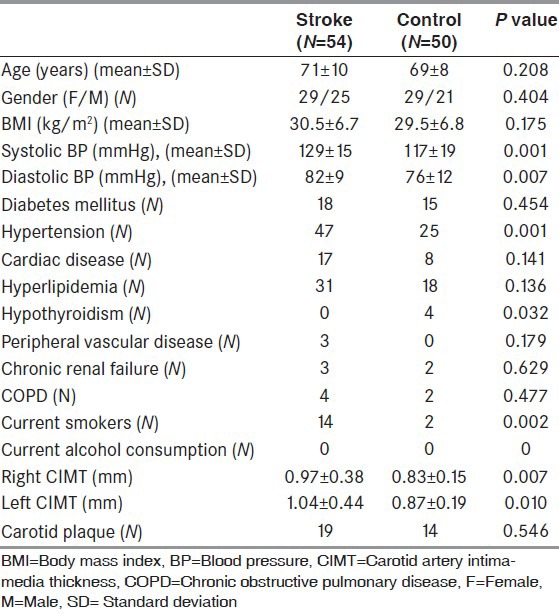
In the stroke group, the level of sd-LDL was 8.2 ± 7.8 mg/dL, the mean LDL was 26.8 ± 0.31 nm, the TNF-α was 75.6 ± 25.0 pg/mL, NO was 76.4 ± 53.3 μmol/L, and the CRP was 1.9 ± 2.6 mg/dL, and in the control group, the level of sd-LDL was 3.3 ± 3.5 mg/dL (P = 0.001), the mean LDL was 27.0 ± 0.31 nm (P = 0.003), the TNF-α was 65.4 ± 9.1 pg/mL (P = 0.009), NO was 41.5 ± 27.0 μmol/L (P = 0.001), and the CRP was 0.4 ± 0.3 mg/dL (P = 0.001). The sd-LDL levels of the stroke patients with carotid plaque subgroups were higher than that of the control group with carotid plaque subgroups (11.5 ± 7.5 mg/dL, 3.5 ± 4.2 mg/dL, P < 0.001, respectively). All the biochemical results of the patients are given in Table 2. The sd-LDL levels of the stroke patients with carotid plaque subgroups were higher than that of the stroke patients without carotid plaque groups (11.5 ± 7.5 mg/dL, 5.4 ± 5.2 mg/dL, P = 0.004, respectively).
Table 2.
All the biochemical result of two groups
Comparison of all subjects (stroke plus control) according to the presence of carotid plaque those with plaque had higher sd-LDL level and lower mean LDL level than patients without plaque (for sd-LDL, 8.1 ± 7.4 mg/dL, 4.6 ± 4.7 mg/dL, P = 0.007; for mean LDL, 26.7 ± 0.2 nm, 26.9 ± 0.3 nm, P = 0.037, respectively).
By Pearson's correlation analysis, there was positive correlation among right CIMT (r = 0.353, P < 0.001), left CIMT (r = 0.312, P = 0.001), TNF-α (r = 0.250, P < 0.011), compared with sd-LDL. There was negative correlation among right CIMT (r2 = 0.564, P < 0.001), left CIMT (r2 = 0.361, P < 0.001), TNF-α (r2 = 0.046, P < 0.028), NO (r2 = 0.184, P < 0.001), sd-LDL (r2 = 0.067, P = 0.008) compared with mean size LDL particle. There was positive correlation among right CIMT (r = 0.296, P < 0.002), left CIMT (r = 0.288, P = 0.003) compared with TNF-α. There was positive correlation among right CIMT (r = 0.540, P < 0.001), left CIMT (r = 0.557, P < 0.001) compared with NO.
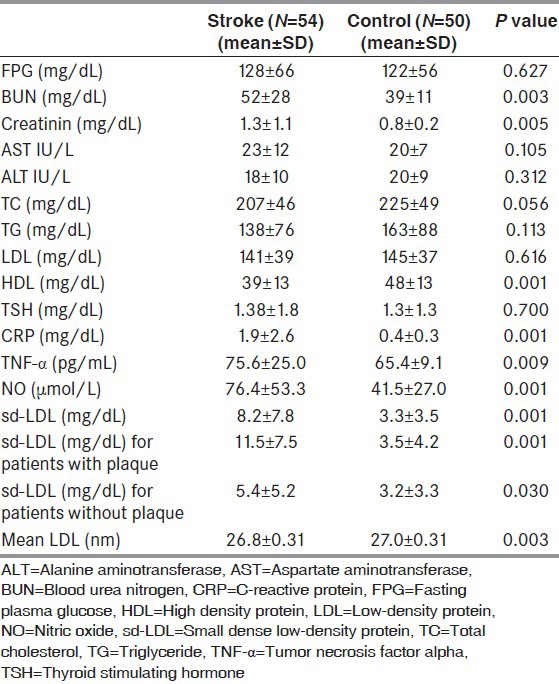
A multiple logistic regression analysis showed that sd-LDL [odds ratio (OR): 0.4, 95% confidence interval (CI): 0.237-0.837, P = 0.012) and mean size LDL particle (OR: 8.5, 95% CI: 2.6-9.4, P = 0.027) are an independent risk factor for stroke. All the results of logistic regression analysis are shown Table 3.
Table 3.
Multiple logistic regression analysis for stroke
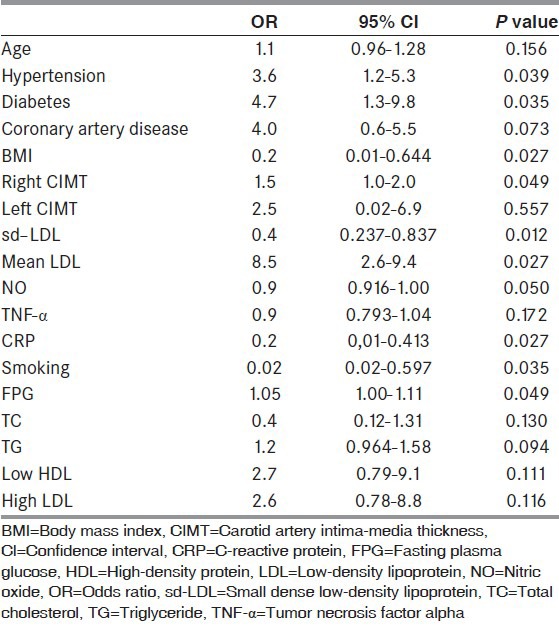
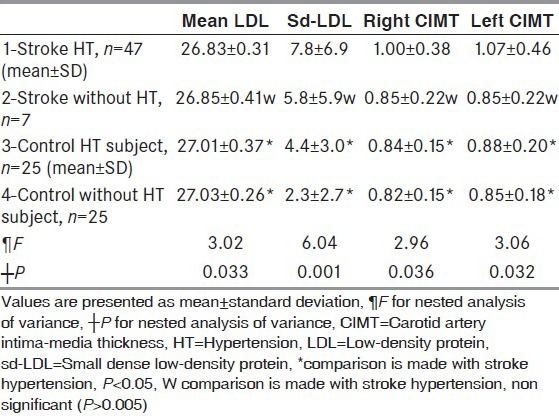
When the test population were divided into the subgroups stroke males, control males, stroke females, and control females; sd-LDL, right and left CIMT in female and male of stroke groups were higher than female and male of control groups. All result of subgroups are shown in Table 4. When the test population were divided into the subgroups stroke HT, control HT, stroke free of HT, and control free of HT; sd-LDL, right and left CIMT in both HT groups were higher than both free of HT groups. All results of subgroups are shown in Table 5.
Table 4.
Subgroup analysis of stroke and the control groups for gender by nested analysis of variance
Table 5.
Subgroup analysis of stroke and the control groups for hypertension by nested analysis of variance
Discussion
In the current study, the control group was selected to have similar age, gender, and BMI with the stroke group. Diameters of the right and left CIMT, sd-LDL, CRP, and TNF-α levels of stroke group was found to be strongly higher than the control group. The mean size LDL particle of patients with stroke was lower than control groups. Our results indicate that the sd-LDL fraction of total LDL is significantly associated with stroke and mean size LDL is significantly inverse associated with stroke. Besides traditional risk factors for stroke such as age, obesity, smoking, CIMT, hypertension, diabetes mellitus, low HDL, high LDL, our multiple logistic regression analysis showed a significant correlation between sd-LDL and stroke. The analysis showed that a significant inverse correlation between mean size LDL particle and stroke.
Both groups in the study include subjects mostly with chronic diseases such as HT, coronary artery disease, diabetes mellitus, hyperlipidemia, hypothyroidism, peripheral vascular disease, and chronic obstructive pulmonary disease. It is well-known that thickness of CIMT and the risk of stroke are increased with the existence of these diseases.[11,12] The carotid artery atherosclerosis also may result in cerebral embolization and symptoms of cerebral ischemia, which may lead to stroke.[13] These findings may indicate that CIMT, TNF-α, NO and sd-LDL, are factors in the development of stroke. The inflammation processes within the wall of the artery were found to play a critical role in the atherogenesis, and the specific immune responses may affect these processes. Progressive atherothrombotic processes, leading to ischemic stroke, cardiovascular events, or renal failure are responsible for many premature deaths in developed countries.
CIMT indicates the atherosclerotic process and many prospective studies have shown that CIMT is an independent predictor for the risk of incident stroke and myocardial infarction.[14] In the previous study, the proinflammatory cytokines TNF-α and CRP were found to have a part in the process of plaque destabilization and plaque rupture.[15] The relationship between these cytokines and CIMT is well-known.[16] In this study, the levels of TNF-α and CRP were found to be high in the stroke patients. The high levels of these markers in patients with stroke indicate the fast atherosclerotic process in these patients. The diameter of CIMT was found correspondingly to be higher patients with stroke than control groups. In addition, previous study reported that sd-LDL was strongly associated with CIMT.[17] Our study showed that patients with carotid plaque had higher sd-LDL level and lower mean LDL than patients without carotid plaque. Current study showed that sd-LDL was strongly associated with CIMT diameter sd-LDL may contribute to the formation of carotid plaque. As a result of increased CIMT and sd-LDL, the risk of stroke may be raised.
NO is an important biomarker of inflammation and oxidative stress. NO is a major vasodilator released by the endothelium and is generated as a result of the enzymatic activity of endothelial NO synthase (eNOS), which is continuously expressed.[18] Many studies conducted on both humans and animals have shown a relationship between elevated eNOS and atherosclerosis. Since eNOS plays an important role in the function of veins and excessive NO production may be a factor in the development of atherosclerosis.[19] It has been suggested that CRP may affect the NO pathway. Previous studies have shown that there is an association with NO with inflammation, endothelial dysfunction, and oxidative stress that can lead to atherosclerosis.[20] Bacci et al.,[21] have shown that TNF-α and NO may lead to atherosclerotic plaques in the arterial wall. Rajeshwar et al.,[22] have shown that increased levels of CRP and NO to be associated with ischemic stroke. In our study also, TNF-α, NO, and CRP were found to be higher in the stroke group, and these findings are in parallel with previous studies.
Dyslipidemia contributes to the development of atherosclerosis.[23] The levels of blood lipids, especially LDL and decreased HDL, have been significantly associated with an increased risk of cardiovascular disease and stroke related.[24,25] Among several possible mechanisms underlying the association of lipids and stroke[8,9] one of the most important may be the effects of lipids on the formation of the carotid artery atherosclerosis.[10] Patients with higher sd-LDL are highly vulnerable to the development of accelerated atherosclerosis as well its clinical sequelae, including coronary artery disease and myocardial infarction, carotid artery disease and ischemic stroke. sd-LDL and mean size LDL particle may some correlate to increased risk of stroke development. It is inexplicit, however, whether sd-LDL's influence on stroke development is dependent on any other factors, including changes in lipoproteins and lipid parameters. In the current study, while the LDL level was similar in both groups, the sd-LDL level was higher and mean size LDL lower in the stroke group. This may be one factor that may contribute to the stroke process. In summary, LDL particle size was smaller among stroke patients and correlated with the proinflammatory cytokines and CIMT. The present study demonstrates that sd-LDL levels are strongly associated with stroke, are independent of traditional stroke risk factors, and are related to the CIMT. Sd-LDL and mean size LDL are strongly risk factors for stroke.
A relationship has been found in previous studies between lipoprotein remnants such as chylomicron and very LDL and stroke.[26] In another study, a relationship was discovered between lipoprotein and carotid atherosclerosis in young stroke patients.[27] Since this study was conducted on young stroke patients, it strongly reflects the relationship between the stroke and lipid subfraction. In addition, Hoshide and Kario[7] reported that lipid subfraction to be a risk factor for silent cerebral infarction. LDL is composed of heterogeneous particles that differ in size, composition, and electrical charge. sd-LDL, oxidative modified LDL, glycated LDL, and diasylated LDL are qualitatively modified forms of LDL that have been shown to exist in human plasma and to be all atherogenic.[28] In the current study, sd-LDL (subtype 3–7) was found to be high in stroke patients. sd-LDL was found to be strongly associated with CIMT diameter and the risk of stroke. While LDL level was similar in both groups, sd-LDL was found to be higher in the stroke group, which indicates that the LDL subfraction may be a predictor for the risk of stroke in patients. In addition, CIMT, TNF-α, NO, and CRP levels are strongly correlated sd-LDL levels in patients with stroke. In addition, according to subgroups analysis shown that both female and male of stroke groups were higher sd-LDL levels than female and male of control groups. This finding shows that sd-LDL is a risk factor for the development of stroke.
In addition, in the stroke group, there were 14 active smokers (13 male 1 female). As this may accelerate the atherosclerotic process, it can also explain the increase in CIMT. Inflammatory markers and LDL oxidations are increased in smokers. In previous studies CIMT and proinflammatory cytokines have been found to be associated with smoking.[29] On the contrary, the number of hypertensive patients in the stroke group was significantly high. The association of HT with CIMT and stroke is well-known. According to subgroups analysis for HT shown that both stroke HT and control HT groups were higher sd-LDL levels, right and left CIMT than free of HT of stroke and control groups. This finding shows that sd-LDL may be a risk factor for the development of HT and stroke.
Limitation of study
The limited number of subjects in our study may not reflect the population. However, it is in parallel with the previous studies. Both groups had a low number of patients without chronic diseases; this may be due to the increased incidence of chronic diseases with increasing age. The probability of a patient in a selected age group to be without chronic disease is relatively low. Other factors that may lead to stroke have not been investigated such as physical activities, eating habits, hyperhomocysteinemia, and hypercoagulability. Excluding all other strokes in which, LDL subtypes was not investigated, only patients with ischemic stroke were included in the study. Elderly patients with stroke were included in the study; the effect of LDL subtypes on the etiology of stroke in younger ones was not investigated. There is a need for studies of a large group on this subject.
Conclusion
We demonstrated that patients with stroke have markedly increased CIMT compared with healthy participants. This observation can be explained by the increased sd-LDL concentration. In spite of normal LDL in stroke patients, sd-LDL was higher when compared to controls. Patients with stroke had higher levels of proinflammatory cytokines such as CRP and TNF-α, NO than in the control group. Besides other risk factors, sd-LDL may be an important factor in the development of stroke.
Footnotes
Source of Support: RTEU Bilimsel Arastirmalar Projeleri birimi (BAP), Project number: 2012.106.01
Conflict of Interest: Nil
References
- 1.Reed DM. The paradox of high risk of stroke in populations with low risk of coronary heart disease. Am J Epidemiol. 1990;131:579–88. doi: 10.1093/oxfordjournals.aje.a115542. [DOI] [PubMed] [Google Scholar]
- 2.Rohr J, Kittner S, Feeser B, Hebel JR, Whyte MG, Weinstein A, et al. Traditional risk factors and ischemic stroke in young adults: The Baltimore–Washington Cooperative Young Stroke Study. Arch Neurol. 1996;53:603–7. doi: 10.1001/archneur.1996.00550070041010. [DOI] [PubMed] [Google Scholar]
- 3.Rothwell PM, Warlow CP. Low risk of ischemic stroke in patients with reduced internal carotid artery lumen diameter distal to severe symptomatic carotid stenosis: Cerebral protection due to low poststenotic flow? On behalf of the European Carotid Surgery Trialists’ Collaborative Group Stroke. 2000;31:622–30. doi: 10.1161/01.str.31.3.622. [DOI] [PubMed] [Google Scholar]
- 4.Sahoo R, Krishna MV, Subrahmaniyan DK, Dutta TK, Elangovan S. Common carotid intima-media thickness in acute ischemic stroke: A case control study. Neurol India. 2009;57:627–30. doi: 10.4103/0028-3886.57822. [DOI] [PubMed] [Google Scholar]
- 5.Lavrencic A, Kosmina B, Keber I, Videcnik V, Keber D. Carotid intima-media thickness in young patients with familiar hypercholesterolaemia. Heart. 1996;76:321–5. doi: 10.1136/hrt.76.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon SW, Yoon SJ, Kang TS, Kwon HM, Kim JH, Rhee J, et al. Significance of small dense low-density lipoprotein as a risk factor for coronary artery disease and acute coronary syndrome. Yonsei Med J. 2006;47:405–14. doi: 10.3349/ymj.2006.47.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoshide S, Kario K. Low-density lipoprotein subfraction as a new risk factor for silent cerebral infarction in hypertensive patients. Hypertens Res. 2006;29:297–8. doi: 10.1291/hypres.29.297. [DOI] [PubMed] [Google Scholar]
- 8.Subramanian R, Ramaswamy M, Wasan KM. Role of lipid and lipoprotein metabolizing enzymes in the development of atherosclerosis. Indian J Exp Biol. 2003;41:14–25. [PubMed] [Google Scholar]
- 9.Amarenco P, Labreuche J, Touboul PJ. High-density lipoprotein-cholesterol and risk of stroke and carotid atherosclerosis: A systematic review. Atherosclerosis. 2008;196:489–96. doi: 10.1016/j.atherosclerosis.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 10.Morrisett JD. The role of lipoprotein[a] in atherosclerosis. Curr Atheroscler Rep. 2000;2:243–50. doi: 10.1007/s11883-000-0026-z. [DOI] [PubMed] [Google Scholar]
- 11.Herder M, Johnsen SH, Arntzen KA, Mathiesen EB. Risk factors for progression of carotid intima-media thickness and total plaque area: A 13-year follow-up study: The Tromsø Study. Stroke. 2012;43:1818–23. doi: 10.1161/STROKEAHA.111.646596. [DOI] [PubMed] [Google Scholar]
- 12.Nomura K, Hamamoto Y, Takahara S, Kikuchi O, Honjo S, Ikeda H, et al. Relationship between carotid intima-media thickness and silent cerebral infarction in Japanese subjects with type 2 diabetes. Diabetes Care. 2010;33:168–70. doi: 10.2337/dc09-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leng XY, Chen XY, Chook P, Xiong L, Lin WH, Liu JY, et al. Correlation of large artery intracranial occlusive disease with carotid intima-media thickness and presence of carotid plaque. Stroke. 2013;44:68–72. doi: 10.1161/STROKEAHA.112.675652. [DOI] [PubMed] [Google Scholar]
- 14.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006 - 2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290–6. doi: 10.1159/000343145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kablak-Ziembicka A, Przewlocki T, Sokołowski A, Tracz W, Podolec P. Carotid intima-media thickness, hs-CRP and TNF-α are independently associated with cardiovascular event risk in patients with atherosclerotic occlusive disease. Atherosclerosis. 2011;214:185–90. doi: 10.1016/j.atherosclerosis.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 16.van Exel E, Gussekloo J, de Craen AJ, Bootsma-van der Wiel A, Frölich M, Westendorp RG. Inflammation and stroke: The Leiden 85-Plus Study. Stroke. 2002;33:1135–8. doi: 10.1161/01.str.0000014206.05597.9e. [DOI] [PubMed] [Google Scholar]
- 17.Inukai T, Yamamoto R, Suetsugu M, Matsumoto S, Wakabayashi S, Inukai Y, et al. Small low-density lipoprotein and small low-density lipoprotein/total low-density lipoprotein are closely associated with intima-media thickness of the carotid artery in Type 2 diabetic patients. J Diabetes Complications. 2005;19:269–75. doi: 10.1016/j.jdiacomp.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Libby P, Aikawa M. Effects of statins in reducing thrombotic risk and modulating plaque vulnerability. Clin Cardiol. 2003;26:I11–4. doi: 10.1002/clc.4960261305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu VW, Huang LP. Cardiovascular roles of nitric oxide: A review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc Res. 2008;77:19–29. doi: 10.1016/j.cardiores.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clapp BR, Hirchfield GM, Storry C, Galimore JR, Stidwilal RP, Singer M, et al. Inflamation and endothelial function: Direct vascular effects of human C-reactive protein on nitric oxide bio-availability. Circulation. 2005;111:1530–6. doi: 10.1161/01.CIR.0000159336.31613.31. [DOI] [PubMed] [Google Scholar]
- 21.Bacci S, Pieri L, Buccoliero AM, Bonelli A, Taddei G, Romagnoli P. Smooth muscle cells, dendritic cells and mast cells are sources of TNFalpha and nitric oxide in human carotid artery atherosclerosis. Thromb Res. 2008;122:657–67. doi: 10.1016/j.thromres.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Rajeshwar K, Kaul S, Al-Hazzani A, Babu MS, Balakrishna N, Sharma V, et al. C-reactive protein and nitric oxide levels in ischemic stroke and its subtypes: Correlation with clinical outcome. Inflammation. 2012;35:978–84. doi: 10.1007/s10753-011-9401-x. [DOI] [PubMed] [Google Scholar]
- 23.Gotto AM., Jr Evolving concepts of dyslipidemia, atherosclerosis, and cardiovascular disease: The Louis F. Bishop Lecture. J Am Coll Cardiol. 2005;46:1219–24. doi: 10.1016/j.jacc.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 24.Sacco RL, Benson RT, Kargman DE, Boden-Albala B, Tuck C, Lin IF, et al. High-density lipoprotein cholesterol and ischemic stroke in the elderly: The Northern Manhattan Stroke Study. JAMA. 2001;285:2729–35. doi: 10.1001/jama.285.21.2729. [DOI] [PubMed] [Google Scholar]
- 25.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: Systematic review and meta-analysis. BMJ. 2003;326:1423–7. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JY, Park JH, Jeong SW, Schellingerhout D, Park JE, Lee DK, et al. High levels of remnant lipoprotein cholesterol is a risk factor for large artery atherosclerotic stroke. J Clin Neurol. 2011;7:203–9. doi: 10.3988/jcn.2011.7.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasr N, Ruidavets JB, Farghali A, Guidolin B, Perret B, Larrue V. Lipoprotein (a) and carotid atherosclerosis in young patients with stroke. Stroke. 2011;42:3616–8. doi: 10.1161/STROKEAHA.111.624684. [DOI] [PubMed] [Google Scholar]
- 28.Zhang B, Maeda N, Okada K, Tatsukawa M, Sawayama Y, Matsunaga A, et al. Association between fast-migrating low-density lipoprotein subfraction as characterized by capillary isotachophoresis and intima-media thickness of carotid artery. Atherosclerosis. 2006;187:205–12. doi: 10.1016/j.atherosclerosis.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Kweon SS, Lee YH, Shin MH, Choi JS, Rhee JA, Choi SW, et al. Effects of cumulative smoking exposure and duration of smoking cessation on carotid artery structure. Circ J. 2012;76:2041–7. doi: 10.1253/circj.cj-11-1353. [DOI] [PubMed] [Google Scholar]


