Abstract
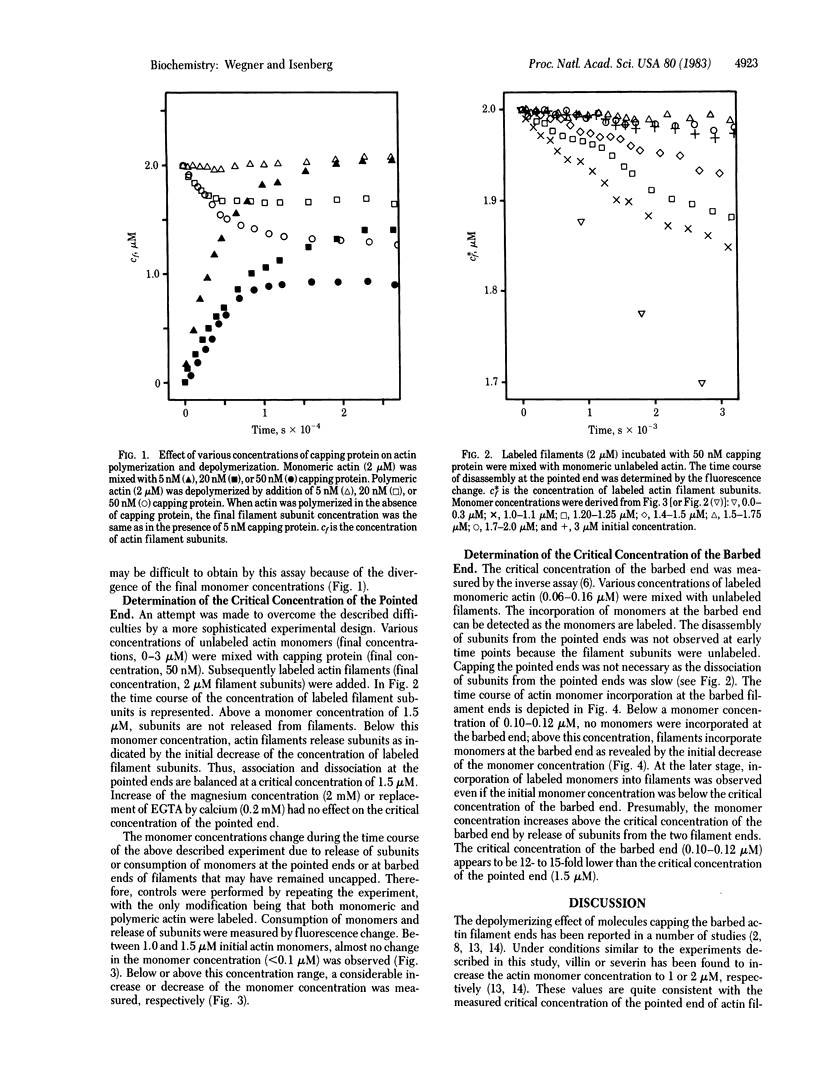
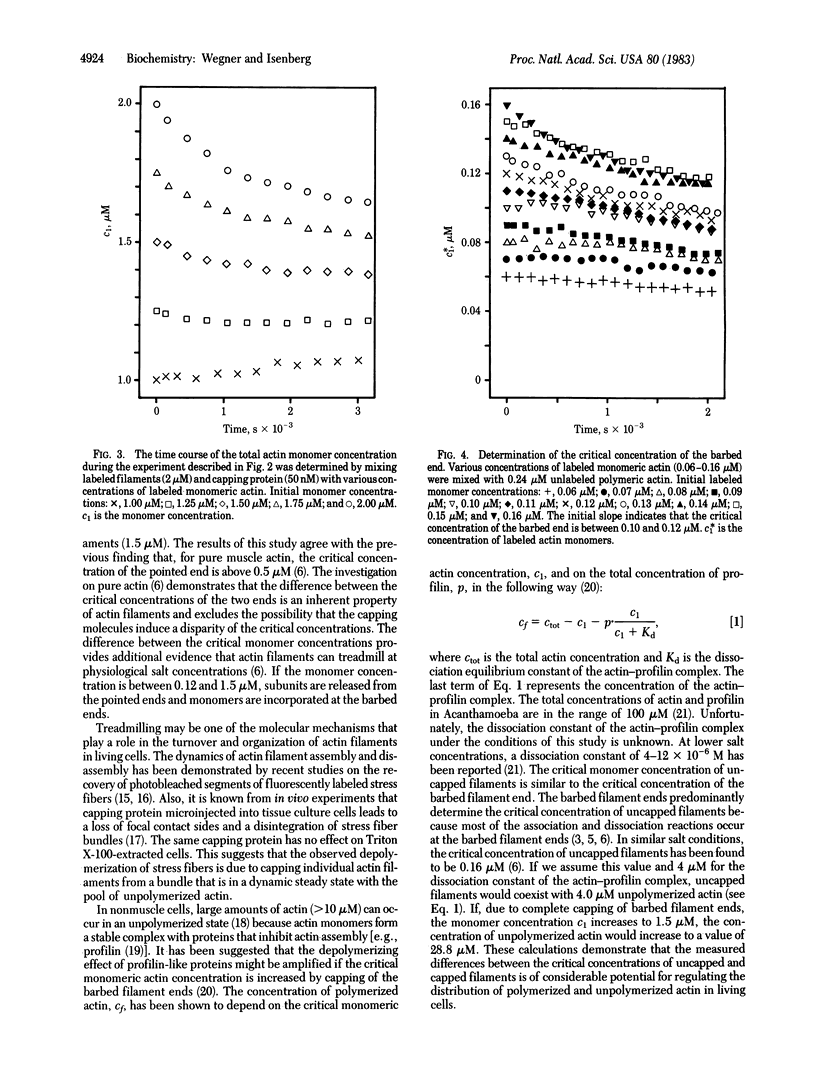
We determined the critical monomer concentrations at which association and dissociation reactions are balanced at the two ends of actin filaments. For measurement of the critical concentration of the pointed end, interference with the high dynamics of the barbed end was excluded by capping the barbed ends with an actin filament capping protein isolated from bovine brain. The critical concentration of the pointed end (1.5 microM) was found to be 12- to 15-fold higher than the critical concentration of the barbed end (0.10-0.12 microM) at a temperature of 37 degrees C and physiological salt concentrations (100 mM KCl/1-2 mM MgCl2/0.3 mM EGTA or 0.2 mM CaCl2, pH 7.5).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bray D., Thomas C. Unpolymerized actin in fibroblasts and brain. J Mol Biol. 1976 Aug 25;105(4):527–544. doi: 10.1016/0022-2836(76)90233-3. [DOI] [PubMed] [Google Scholar]
- Brenner S. L., Korn E. D. Substoichiometric concentrations of cytochalasin D inhibit actin polymerization. Additional evidence for an F-actin treadmill. J Biol Chem. 1979 Oct 25;254(20):9982–9985. [PubMed] [Google Scholar]
- Carlsson L., Nyström L. E., Sundkvist I., Markey F., Lindberg U. Actin polymerizability is influenced by profilin, a low molecular weight protein in non-muscle cells. J Mol Biol. 1977 Sep 25;115(3):465–483. doi: 10.1016/0022-2836(77)90166-8. [DOI] [PubMed] [Google Scholar]
- Detmers P., Weber A., Elzinga M., Stephens R. E. 7-Chloro-4-nitrobenzeno-2-oxa-1,3-diazole actin as a probe for actin polymerization. J Biol Chem. 1981 Jan 10;256(1):99–105. [PubMed] [Google Scholar]
- Hill T. L., Kirschner M. W. Bioenergetics and kinetics of microtubule and actin filament assembly-disassembly. Int Rev Cytol. 1982;78:1–125. [PubMed] [Google Scholar]
- Isenberg G., Aebi U., Pollard T. D. An actin-binding protein from Acanthamoeba regulates actin filament polymerization and interactions. Nature. 1980 Dec 4;288(5790):455–459. doi: 10.1038/288455a0. [DOI] [PubMed] [Google Scholar]
- Kilimann M. W., Isenberg G. Actin filament capping protein from bovine brain. EMBO J. 1982;1(7):889–894. doi: 10.1002/j.1460-2075.1982.tb01265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis T. E., Geiger B., Schlessinger J. Mobility of microinjected rhodamine actin within living chicken gizzard cells determined by fluorescence photobleaching recovery. Cell. 1982 Jul;29(3):835–845. doi: 10.1016/0092-8674(82)90445-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pollard T. D., Mooseker M. S. Direct measurement of actin polymerization rate constants by electron microscopy of actin filaments nucleated by isolated microvillus cores. J Cell Biol. 1981 Mar;88(3):654–659. doi: 10.1083/jcb.88.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees M. K., Young M. Studies on the isolation and molecular properties of homogeneous globular actin. Evidence for a single polypeptide chain structure. J Biol Chem. 1967 Oct 10;242(19):4449–4458. [PubMed] [Google Scholar]
- Reichstein E., Korn E. D. Acanthamoeba profilin. A protein of low molecular weight from Acanpthamoeba castellanii that inhibits actin nucleation. J Biol Chem. 1979 Jul 10;254(13):6174–6179. [PubMed] [Google Scholar]
- Tobacman L. S., Korn E. D. The regulation of actin polymerization and the inhibition of monomeric actin ATPase activity by Acanthamoeba profilin. J Biol Chem. 1982 Apr 25;257(8):4166–4170. [PubMed] [Google Scholar]
- Wang Y. L., Lanni F., McNeil P. L., Ware B. R., Taylor D. L. Mobility of cytoplasmic and membrane-associated actin in living cells. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4660–4664. doi: 10.1073/pnas.79.15.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner A. Head to tail polymerization of actin. J Mol Biol. 1976 Nov;108(1):139–150. doi: 10.1016/s0022-2836(76)80100-3. [DOI] [PubMed] [Google Scholar]
- Wegner A. Treadmilling of actin at physiological salt concentrations. An analysis of the critical concentrations of actin filaments. J Mol Biol. 1982 Nov 15;161(4):607–615. doi: 10.1016/0022-2836(82)90411-9. [DOI] [PubMed] [Google Scholar]
- Woodrum D. T., Rich S. A., Pollard T. D. Evidence for biased bidirectional polymerization of actin filaments using heavy meromyosin prepared by an improved method. J Cell Biol. 1975 Oct;67(1):231–237. doi: 10.1083/jcb.67.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Pardee J. D., Reidler J., Stryer L., Spudich J. A. Mechanism of interaction of Dictyostelium severin with actin filaments. J Cell Biol. 1982 Dec;95(3):711–719. doi: 10.1083/jcb.95.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]



