Abstract
Introduction:
In addition to changes in seizure frequency, pregnant women with epilepsy (WWE) are at increased risk of complications during pregnancy or delivery. In the absence of a nationwide WWE registry, hospital-based studies may provide important information regarding current management and outcomes in these patients.
Objectives:
The aims of this study were to determine changes in seizure frequency, and pregnancy and birth outcomes among pregnant WWE.
Materials and Methods:
We conducted a retrospective review of medical records of pregnant patients with epilepsy, who obtained medical care (from 2006 to 2011) at one of the general hospitals in the North-Eastern State of Malaysia. Data were collected for seizure frequency before and during the pregnancy, concurrent medications, pregnancy complications, and neonatal outcomes.
Results:
We reviewed records of 25 patients with a total of 33 different pregnancies. All patients were treated with antiepileptic medications during their pregnancies, with 42% monotherapy and 58% polytherapy. Seizure frequency decreased in 5 (15.2%), increased in 18 (54.5%) and unchanged in 10 (30.3%) cases of pregnancies. Pregnancy complications were anemia, gestational diabetes mellitus, gestational hypertension, intrauterine growth retardation, premature rupture of membrane, and vaginal bleeding. Preterm deliveries were recorded in 11 (33.3%) infants.
Conclusion:
In our setting, many patients were being on polytherapy during their pregnancies. This underscores the need for planned pregnancies so that antiepileptic medications can be optimized prior to pregnancy.
Key Words: Epilepsy, outcomes, pregnancy, women
Introduction
In addition to changes in seizure frequency and duration, pregnant women with epilepsy (WWE) are at increased risk of complications during pregnancy or delivery.[1,2,3] They have a higher frequency of pre-eclampsia, vaginal bleeding, and cesarean delivery.[1,3,4] As such, many may need continuous antiepileptic drug (AED) treatment during pregnancy.
The challenge in providing optimum management to these patients is to balance the benefits of AED treatment such as preventing seizures or adverse effects of seizures, with the risks associated with such treatment. Drug treatment during the pregnancy presents a special concern because of potential adverse effects on the fetus. Infants born to WWE are more likely to be premature have low birth weight (LBW), and small head circumference.[3] There is an increase risk in congenital malformation in the offspring of WWE,[1,5,6,7] and the risk is higher in those women taking polytherapy compared to monotherapy.[8] Common major fetal malformations associated with AEDs includes cardiovascular defects, neural tube defects, and cleft/lip palate.[1,5,8]
Unlike other countries which have developed their own AED registries,[9,10,11] there is no systematic registry to capture data on WWE in our local setting. Therefore, we attempted to capture some of this information using a hospital-based study. The aims of this study were to determine the changes in frequency of seizures and to investigate pregnancy and birth outcomes in pregnant WWE.
Materials and Methods
This retrospective study was conducted at the Hospital Raja Perempuan Zainab II (HRPZ II), which is a government-funded hospital located in the North-East of peninsular Malaysia. A list of all pregnant women diagnosed with epilepsy who attended the Obstetrics and Gynecology (O&G) clinic of HRPZ II between 1st October 2006 and 31st March 2011 was obtained from the clinic. Patient medical records were acquired from the hospital's medical record office. This study was approved by the Clinical Research Center and the Director of HRPZ II.
All patients with regular follow-up at O&G clinic and who were pregnant at least once were included. Patients with incomplete antenatal record or those who did not deliver at HRPZ II were excluded.
Data were collected starting from the day patient was referred to the O&G clinic until the patient gave birth at HRPZ II. These included data on demography, AED medications, seizures type and frequency, and pregnancy complications. In our setting, all patients with epilepsy are managed by medical consultants at the Medical Clinic. During the pregnancy, although patients are under the care of the O&G consultants the medical consultants continue to see these patients at the O&G clinic. Data on seizure type was based on what was documented in the O&G medical records. Seizure frequency was determined for 9 months before and during pregnancy.[12] Data for clinical findings of neonates were obtained from individual infant medical record. Neonatal data included gestational age, gender, weight, and Apgar score. Pre-term birth was defined as a birth occurring at gestational age of less than 37 weeks. LBW was defined as weight of less than 2500 g, irrespective of gestational age.[13] Small for gestational age (SGA) refers to birth weight and/or crown-heel length that is less than expected for gestational age and sex.
Descriptive statistics were used to describe demographic profile, pregnancy, related complications, and fetal outcomes.
Results
A total of 1,014 pregnant women attended the O&G Clinic during the 5-year period. 44 women were diagnosed with epilepsy. Due to technical problems, 11 patient records were not available at the time of review. Five patients did not give birth at the hospital and three patients did not keep regular clinic appointments. Only records of 25 patients were included for analysis.
From 25 patients, a total of 33 different pregnancies were determined. For the purpose of analysis, each pregnancy was treated separately. All patients were of Malay ethnic origin. The mean age of these patients was 28.18 (±6.2) years old. The distribution of cases (n = 33) by seizure type was as follows: Generalized tonic-clonic, n = 6; complex partial, n = 9; and unspecified seizure type, n = 10.
Before pregnancy, 15 (45.5%) patients received AED monotherapy, 16 (48.5%) were on polytherapy and 2 (6.1%) were not on any AED. During their pregnancies, all patients were treated with AED; 14 monotherapy and 19 polytherapy [Table 1]. 97% were on folate supplement (5 mg daily). During the pregnancy, the most common AEDs used were carbamazepine (CBZ) (n = 17) and phenytoin (PHT) (n = 17), followed by valproic acid (VPA) (n = 13), lamotrigine (LTG) (n = 8) and topiramate (n = 1).
Table 1.
Antiepileptic drug treatment before and during pregnancy
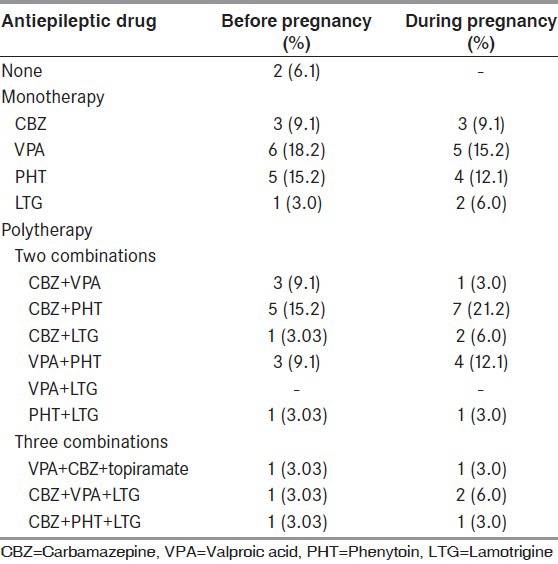
Seizure frequency decreased in 5 (15.2%), increased in 18 (54.5%) and unchanged in 10 (30.3%). Cesarean deliveries were conducted in 6 cases (18.2%). They were performed because of the occurrence of seizure (n = 1), breech presentation (n = 1), prolonged delivery (n = 2), placenta previa major (n = 1) and acute fetal distress (n = 1). Other types of pregnancy-related complications are shown in Table 2.
Table 2.
Pregnancy-related complications

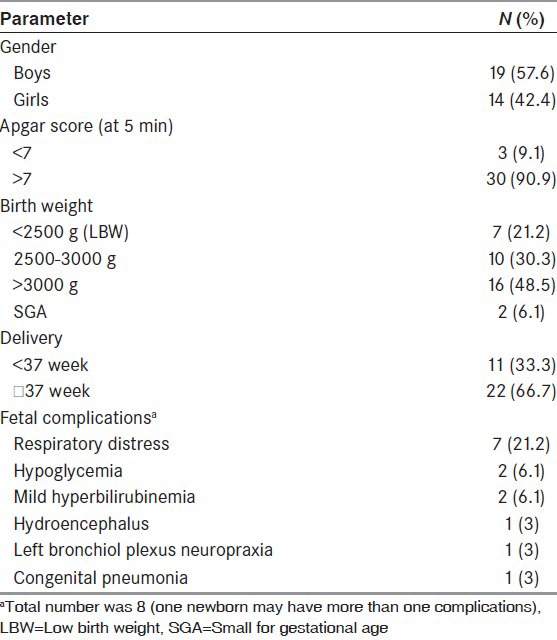
19 (57.6%) boys and 14 (42.4%) girls were born [Table 3]. Preterm delivery (≤37 weeks) was recorded in 11 (33.3%) infants. Seven (21.2%) infants had a birth weight of less than 2500 g LBW. The mean head circumference in boys was 29.6 ± 1.34 cm and in girls 32.1 ± 1.49 cm. Eight infants were referred to the neonatal intensive care unit for ventilator support and close observation. Fetal complications occurred in 8 (30%) newborns, which included hyperglycemia, respiratory distress, congenital pneumonia, bronchiol plexus neuropraxia, hydroenchephalus, and mild hyperbilirubinemia.
Table 3.
Neonatal outcomes
Discussion
Increased in seizure frequency occurred in more than half of pregnancies in our patients. Reported rates of patients with changes in seizure frequency during pregnancy are quite variable, probably due to sample size and methodological differences. The percentages of patients with an increase in seizure frequency range from 14% to 32%.[7,14,15] Factors that have been suggested to influence changes in seizure during pregnancy in an individual patient include altered disposition of AEDs, poor compliance with treatment, psychological stressors, and physiological and hormonal changes.[6,16,17,18] In addition, there is about 25% risk of getting a seizure during the pregnancy if the seizure-free period before pregnancy is 1 year and the risk decreases with the length of seizure freedom.[19]
In the present study, the increase in the number of patients on polytherapy is consistent with the higher percentage of patients with increased seizure frequency during their pregnancies. This may imply that seizures were not fully controlled in many patients, even before pregnancy. Doctors might have instituted polytherapy in an attempt to control seizures in these patients. Further analysis shows that among patients with increased seizure frequency during pregnancies, more than 60% were on polytherapy. Our small sample size might have overestimated the percentage of patients on polytherapy in this study. A recent study conducted in India based on a large registry of epilepsy and pregnancy shows only 30% of WWE were on more than one AED.[20]
Although ideally, conception should be delayed until seizure control is established, unplanned pregnancy is still common.[21,22] Faced with such circumstances, it may be difficult for doctors to provide effective counseling to potential mothers. Nevertheless, findings from other studies in developed countries have shown that quite a number of WWE (10% to 66%) did not use AEDs during pregnancy, which may indicate better pre-pregnancy management (e g., seizure control) and planning in these patients compared to our local setting.[2,3,14,23] In addition, there is a higher degree of awareness and knowledge among patients in developed countries. For example, in Norway more than 60% WWE of childbearing age were aware of the need to consult with their Neurologist when planning a pregnancy.[24] Therefore, raising awareness among our patients regarding the importance of pre-pregnancy planning should be given a priority and needs to be examined in the future in our setting.
Our result shows that the AED most prescribed was CBZ and PHT. In India, with the exception of phenobarbitone, a similar pattern of older AED use has been reported.[20] The use of VPA has been associated with increased risk of malformation compared to use of CBZ, PHT or LTG.[8,20,25] A recent report from Australia suggests a decreasing trend of VPA being prescribed in WWE, which was reflected in a similar downward trend in fetal malformation.[26] Likewise, an analysis of several specialty centers in the USA and UK shows a decline in WWE being treated with VPA.[23] VPA is still commonly prescribed among our patients and it would be interesting to look at the trend in prescribing in such patients in the future in response to current awareness of the effects of VPA.
Cesarean deliveries were performed in about 18% of pregnancies in this cohort of patients. In Malaysia, the rates of cesarean deliveries have been increasing steadily over the years, and in 2006 the average rate in public hospitals was about 16%.[27] Findings from studies evaluating cesarean rates differ[3,15] although in general, WWE taking AED may not have substantial increased risk in cesarean delivery.[7] Other pregnancy related-complications among our patients include anemia, gestational diabetes mellitus, intrauterine growth retardation, gestational hypertension, vaginal bleeding, and pre-term premature rupture of membrane. These are complications also commonly reported in several other studies.[2,3,15] Anemia was found to be a common medical problem among our patients during the pregnancy. It is a standard practice to prescribe folate and iron supplements to all pregnant women in our setting.[28] Such supplements are usually initiated at the beginning of pregnancy and continued until 1 month after delivery. Despite this effort, only 50% of pregnant women had improved hemoglobin levels.[29] Poor compliance to folate therapy has been reported.[30] Postpartum bleeding is a common cause of maternal mortality and anemia has been identified as one of the risk factors.[31] AED use during pregnancy has been associated with an increased tendency for postpartum bleeding;[4] and therefore, it is critical that anemia is monitored and treated adequately during pregnancy.
In our study, about one-third of babies were delivered preterm and 21.2% of babies were born with LBW. Even though, the sample size is small, the spectrum of reported fetal complications in our patients is similar to those reported elsewhere.[3,15] Although drug treatment presents a risk of such neonatal outcomes,[3,15] recent report by Chen et al. 2009,[32] showed that the occurrence of seizures itself is associated with the risk of having preterm delivery, LBW and SGA neonates.
Conclusion
Despite being on treatment, many of our patients still had seizures during their pregnancies and many required polytherapy. Because of the adverse effects of seizures and drug treatment on pregnancy outcomes, it is imperative that they are provided with appropriate counseling for planned pregnancies.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Olafsson E, Hallgrimsson JT, Hauser WA, Ludvigsson P, Gudmundsson G. Pregnancies of women with epilepsy: A population-based study in Iceland. Epilepsia. 1998;39:887–92. doi: 10.1111/j.1528-1157.1998.tb01186.x. [DOI] [PubMed] [Google Scholar]
- 2.Borthen I, Eide MG, Veiby G, Daltveit AK, Gilhus NE. Complications during pregnancy in women with epilepsy: Population-based cohort study. BJOG. 2009;116:1736–42. doi: 10.1111/j.1471-0528.2009.02354.x. [DOI] [PubMed] [Google Scholar]
- 3.Veiby G, Daltveit AK, Engelsen BA, Gilhus NE. Pregnancy, delivery, and outcome for the child in maternal epilepsy. Epilepsia. 2009;50:2130–9. doi: 10.1111/j.1528-1167.2009.02147.x. [DOI] [PubMed] [Google Scholar]
- 4.Borthen I, Eide MG, Daltveit AK, Gilhus NE. Delivery outcome of women with epilepsy: A population-based cohort study. BJOG. 2010;117:1537–43. doi: 10.1111/j.1471-0528.2010.02694.x. [DOI] [PubMed] [Google Scholar]
- 5.Samrén EB, van Duijn CM, Christiaens GC, Hofman A, Lindhout D. Antiepileptic drug regimens and major congenital abnormalities in the offspring. Ann Neurol. 1999;46:739–46. [PubMed] [Google Scholar]
- 6.Walker SP, Permezel M, Berkovic SF. The management of epilepsy in pregnancy. BJOG. 2009;116:758–67. doi: 10.1111/j.1471-0528.2009.02141.x. [DOI] [PubMed] [Google Scholar]
- 7.Harden CL, Hopp J, Ting TY, Pennell PB, French JA, Hauser WA, et al. Practice parameter update: Management issues for women with epilepsy – Focus on pregnancy (an evidence-based review): Obstetrical complications and change in seizure frequency: Report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:126–32. doi: 10.1212/WNL.0b013e3181a6b2f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meador K, Reynolds MW, Crean S, Fahrbach K, Probst C. Pregnancy outcomes in women with epilepsy: A systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008;81:1–13. doi: 10.1016/j.eplepsyres.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes LB, Wyszynski DF, Lieberman E. The AED (antiepileptic drug) pregnancy registry: A 6-year experience. Arch Neurol. 2004;61:673–8. doi: 10.1001/archneur.61.5.673. [DOI] [PubMed] [Google Scholar]
- 10.Russell AJ, Craig JJ, Morrison P, Irwin B, Waddell R, Parsons L, et al. U.K. epilepsy and pregnancy group. Epilepsia. 2004;45:1467. doi: 10.1111/j.0013-9580.2004.451104.x. [DOI] [PubMed] [Google Scholar]
- 11.Vajda F, Lander C, O’brien T, Hitchcock A, Graham J, Solinas C, et al. Australian pregnancy registry of women taking antiepileptic drugs. Epilepsia. 2004;45:1466. doi: 10.1111/j.0013-9580.2004.451103.x. [DOI] [PubMed] [Google Scholar]
- 12.Tomson T, Lindbom U, Ekqvist B, Sundqvist A. Epilepsy and pregnancy: A prospective study of seizure control in relation to free and total plasma concentrations of carbamazepine and phenytoin. Epilepsia. 1994;35:122–30. doi: 10.1111/j.1528-1157.1994.tb02921.x. [DOI] [PubMed] [Google Scholar]
- 13.Ismail HI, Ng HP, Thomas T. 2nd ed. Malaysia: Ministry of Health; 2005. Paediatric Protocols for Malaysian Hospitals. [Google Scholar]
- 14.Sabers A, aRogvi-Hansen B, Dam M, Fischer-Rasmussen W, Gram L, Hansen M, et al. Pregnancy and epilepsy: A retrospective study of 151 pregnancies. Acta Neurol Scand. 1998;97:164–70. doi: 10.1111/j.1600-0404.1998.tb00631.x. [DOI] [PubMed] [Google Scholar]
- 15.Viinikainen K, Heinonen S, Eriksson K, Kälviäinen R. Community-based, prospective, controlled study of obstetric and neonatal outcome of 179 pregnancies in women with epilepsy. Epilepsia. 2006;47:186–92. doi: 10.1111/j.1528-1167.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 16.Zahn CA, Morrell MJ, Collins SD, Labiner DM, Yerby MS. Management issues for women with epilepsy: A review of the literature. Neurology. 1998;51:949–56. doi: 10.1212/wnl.51.4.949. [DOI] [PubMed] [Google Scholar]
- 17.Jeha LE, Morris HH. Optimizing outcomes in pregnant women with epilepsy. (942-5).Cleve Clin J Med. 2005;72:938–40. doi: 10.3949/ccjm.72.10.938. [DOI] [PubMed] [Google Scholar]
- 18.Brodtkorb E, Reimers A. Seizure control and pharmacokinetics of antiepileptic drugs in pregnant women with epilepsy. Seizure. 2008;17:160–5. doi: 10.1016/j.seizure.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Vajda FJ, Hitchcock A, Graham J, O’Brien T, Lander C, Eadie M. Seizure control in antiepileptic drug-treated pregnancy. Epilepsia. 2008;49:172–6. doi: 10.1111/j.1528-1167.2007.01412.x. [DOI] [PubMed] [Google Scholar]
- 20.Thomas SV, Sindhu K, Ajaykumar B, Sulekha Devi PB, Sujamol J. Maternal and obstetric outcome of women with epilepsy. Seizure. 2009;18:163–6. doi: 10.1016/j.seizure.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspect. 1998;30:24–9. 46. [PubMed] [Google Scholar]
- 22.Carson C, Kelly Y, Kurinczuk JJ, Sacker A, Redshaw M, Quigley MA. Effect of pregnancy planning and fertility treatment on cognitive outcomes in children at ages 3 and 5: Longitudinal cohort study. BMJ. 2011;343:d4473. doi: 10.1136/bmj.d4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meador KJ, Penovich P, Baker GA, Pennell PB, Bromfield E, Pack A, et al. Antiepileptic drug use in women of childbearing age. Epilepsy Behav. 2009;15:339–43. doi: 10.1016/j.yebeh.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kampman MT, Johansen SV, Stenvold H, Acharya G. Management of women with epilepsy: Are guidelines being followed? Results from case-note reviews and a patient questionnaire. Epilepsia. 2005;46:1286–92. doi: 10.1111/j.1528-1167.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- 25.Vajda FJ, Eadie MJ. Maternal valproate dosage and foetal malformations. Acta Neurol Scand. 2005;112:137–43. doi: 10.1111/j.1600-0404.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- 26.Vajda FJ, Hollingworth S, Graham J, Hitchcock AA, O’Brien TJ, Lander CM, et al. Changing patterns of antiepileptic drug use in pregnant Australian women. Acta Neurol Scand. 2010;121:89–93. doi: 10.1111/j.1600-0404.2009.01260.x. [DOI] [PubMed] [Google Scholar]
- 27.Ravindran J. Rising caesarean section rates in public hospitals in Malaysia 2006. Med J Malaysia. 2008;63:434–5. [PubMed] [Google Scholar]
- 28.Haniff J, Das A, Onn LT, Sun CW, Nordin NM, Rampal S, et al. Anemia in pregnancy in Malaysia: A cross-sectional survey. Asia Pac J Clin Nutr. 2007;16:527–36. [PubMed] [Google Scholar]
- 29.Ahmad Z, Jr, Jaafar R, Mohd Hassan M, Othman M, Hashim A. Anaemia during pregnancy in rural Kelantan. Malays J Nutr. 1997;3:83–90. [PubMed] [Google Scholar]
- 30.Thomas SV, Indrani L, Devi GC, Jacob S, Beegum J, Jacob PP, et al. Pregnancy in women with epilepsy: Preliminary results of Kerala registry of epilepsy and pregnancy. Neurol India. 2001;49:60–6. [PubMed] [Google Scholar]
- 31.Brabin B, Prinsen-Geerligs P, Verhoeff F, Kazembe P. Anaemia prevention for reduction of mortality in mothers and children. Trans R Soc Trop Med Hyg. 2003;97:36–8. doi: 10.1016/s0035-9203(03)90014-9. [DOI] [PubMed] [Google Scholar]
- 32.Chen YH, Chiou HY, Lin HC, Lin HL. Affect of seizures during gestation on pregnancy outcomes in women with epilepsy. Arch Neurol. 2009;66:979–84. doi: 10.1001/archneurol.2009.142. [DOI] [PubMed] [Google Scholar]


