Abstract
Context:
Visual evoked potentials are useful in investigating the physiology and pathophysiology of the human visual system. Flash visual evoked potential (FVEP), though technically easier, has less clinical utility because it shows great variations in both latency and amplitude for normal subjects.
Aim:
To study the effect of eye closure, low luminance, and monochromatic stimulation on the variability of FVEPs.
Subjects and Methods:
Subjects in self-reported good health in the age group of 18-30 years were divided into three groups. All participants underwent FVEP recording with eyes open and with white light at 0.6 J luminance (standard technique). Next recording was done in group 1 with closed eyes, group 2 with 1.2 and 20 J luminance, and group 3 with red and blue lights, while keeping all the other parameters constant. Two trials were given for each eye, for each technique. The same procedure was repeated at the same clock time on the following day.
Statistical Analysis:
Variation in FVEP latencies between the individuals (interindividual variability) and the variations within the same individual for four trials (intraindividual variability) were assessed using coefficient of variance (COV). The technique with lower COV was considered the better method.
Results:
Recording done with closed eyes, 0.6 J luminance, and monochromatic light (blue > red) showed lower interindividual and intraindividual variability in P2 and N2 as compared to standard techniques.
Conclusions:
Low luminance flash stimulations and monochromatic light will reduce FVEP latency variability and may be clinically useful modifications of FVEP recording technique.
Key Words: Eye closure, flash visual evoked potentials, latency variability, luminance, monochromatic light, visual evoked potentials
Introduction
Visual Evoked Potentials (VEPs) are summated averaged cerebral electrical potentials generated in response to visual stimuli. Visual stimuli of standard intensity, luminance, pattern, frequency, and color are flashed before the subject's eyes and the net potential changes taking place in the visual cortex in response to the stimulus are recorded using the surface electrodes placed over the scalp. VEPs are useful for investigating the physiology and pathophysiology of the human visual system. Flash VEP (FVEP) is a technique in which repeated flashes of light of fixed luminance, frequency, and colors are given as stimuli, using a xenon flash tube. The advantage of the FVEP is that it is less dependent on eye position than pattern reversal-VEPs and hence it can be used to assess visual function in young or uncooperative subjects and in those who are undergoing intracranial surgery.[1,2] Other advantages of FVEP include the brevity of the stimulus and the ease with which it can be synchronized with the recording. FVEP may also provide a superior index of the temporal activation pattern in the visual pathways, as shown by Schroeder and Givre, that diffuse luminance produces robust and reliable activation of structures throughout the visual pathways, on contrary to the widely held view that flash is a poor visual stimulus.[3,4]
Despite these special advantages of FVEP, as flash light stimulates a larger area in the cortex, FVEP obtained show larger interindividual variation in both latency and amplitude and also variation in the same subject when studied at different times.[5] This is as an important limiting factor in its widespread clinical application. Factors such as frequency[6] and luminance[7] of the stimulus, age[8] gender,[9] visual acuity,[10] eye position,[11,12] retinal luminance,[13] serum glucose level,[14] drugs, auditory stimulation,[15] alertness of the subject[16] and duration of recording[17] are known to affect the FVEP.[18] Coburn et al., had concluded recording FVEP with closed eyes yielded most reliable latencies and amplitudes.[18] On the other hand, Pratt et al., had suggested the use of high intensity, goggle mounted light emitting diodes to improve the consistency of FVEP recordings.[19] Shaw and Cant too showed that when the high luminance level flash is used, effect of age on the P100 latency is minimal.[20] Our team had investigated and reported on the impact of monochromatic versus white light on FVEP latency and variance in latency of FVEP.[21] White light is considered as a mixture of colors of different wavelength in specific combinations. Individuals may respond differently to the white light because each of them may respond differently to different wavelengths depending on the type, number, and distribution of cones, which may be one reason for variability. If one particular particular type of cones gets stimulated in all the subjects, then the difference between the individuals may be reduced. This prompted us to investigate by recording the FVEP with white light stimulation followed by recording using stimulation using particular wavelength (colour) and compare the variations between the two methods. Color stimulation is known to produce larger amplitude and longer latency responses than does achromatic stimulation.[22] Our studies had also showed that, the use of color stimuli may help to increase the signal-to-noise ratio (S/N ratio) of FVEP recordings in humans.[21]
We designed this study to minimize the effect of factors that can influence FVEP variability and to study the effect of eye closure, luminance, and monochromatic stimulation on the variation of latency of the standard waveforms seen in FVEP.
Subjects and Methods
This study was carried out in the Electrophysiology laboratory of the Department of Physiology under the guidance of the neurologist at, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER). The approval of the JIPMER Scientific Advisory Committee and Ethics Committee was obtained prior to the commencement of the study.
Participants
Subjects recruited were undergraduate and postgraduate students of JIPMER belonging to the age group 18-30 years (Mean age: 22.06, standard deviation (SD): 4.36). Participants (N-80: Male (M)-40, Female (F)-40) in self-reported good health and with medical history free of neurological problems and currently not on any medication that could directly affect brain activity were considered for the study. They were subjected to ophthalmological examination and those with corrected visual acuity 6/6, normal fundus examination, and normal intraocular pressure without anisocoria were included in the study (N-63: M-37, F-26). Subjects were instructed to wash their hair with shampoo (for oil free scalp) on the day of investigation, to reduce skin impedance for better recording of VEPs. Informed written consent was obtained after explaining the procedures and answering all their queries.
Procedure
Laboratory temperature was maintained at 25-27°C. The laboratory was dimly lit with ambient light. Participants were made to sit in a chair comfortably with headrest to avoid muscle artefacts. Standard silver-silver chloride electrodes of 1 cm diameter were used for recording. The electrodes were applied to the scalp following international 10-20 system, using conduction jelly, after thoroughly cleaning the contact area. International Society for Clinical Electrophysiology of Vision (ISCEV) guidelines was followed to record FVEP.[6] Recording electrode was placed at Oz position, reference electrode at Fz, and ground electrode at M1 position. Subjects were to initially spend 5 minutes in the concerned laboratory to get acquainted with the laboratory environment before the actual procedures were carried out. Skin impendence was maintained below 5 kΩ and only when the difference between two electrodes was below 20%, the procedure was carried out. Flash stimulus was given through a xenon flash tube kept at a distance of 30 cm, at 1 Hz frequency. The stimulus was white light of 0.6 J luminance. The stimulus was initially given for each eye separately with the eye open, while the other eye was being covered with an opaque patch. Recording was done with Neuropack MEB-9200J/K EP/EMG Measuring System, Tokyo. MEB-9200 system software was used. Flash stimulator used was the model number, SLS-3100. Participants were watched for any eye movements or attention lapse during the procedure. This was deemed as the standard technique for the present study. ISCEV has reported that out of all the waveforms seen in FVEP, positive wave around 120 ms called P2, and negative wave just before P2 at around 90 ms called N2 were the most robust of all.[6] Studies have reported that when the VEP is recorded continuously, there is little change in the latency of P100 although its amplitude and morphology may change considerably.[17] Therefore, we too decided to ascertain only latencies of the waveforms P2 and N2 to assess variability.
Groups
Participants were divided into three groups. Standard FVEP was done for all the participants and in addition, all the participants in each group underwent specified altered techniques.
Group 1 (N-33: M-19, F-14): Altered technique was done with eyes closed, while other settings remained the same.
Group 2 (N-30: M-17 F-13): Altered technique was done with flash luminance at 1.2 and 20 J. As the instrument was not able to deliver 20 J flash at 1 Hz, all the recordings were done at 0.5 Hz so that the results are comparable.
Group 3 (N-28: M-16, F-12): Altered technique was done with red and blue lights. Red and blue lights were obtained by placing suitable color filters in front of the xenon flash tube. The peak wavelength for the filters used was 490 nm for blue light and 650 nm for red light. The technique has been described by our team elsewhere.[21]
Four trials were conducted for each eye for both standard and alternate techniques. The sequence of conduct of various techniques was randomized within the groups to avoid carry over effects. Trial 1 and trial 2 were conducted same day at a fixed time between 5:00 and 6:00 pm, 4 h after meal for all the participants, to avoid diurnal variation if any from the effect of varying serum glucose levels. The exact procedure of trial 1 and 2 were then repeated at the same clock time the following day (trial 3 and 4), this spacing deemed necessary to reduce subject discomfort.
Data recording
Brain electrical activity was amplified 50,000 times through an inbuilt amplifier and recorded with a sampling rate of 500 Hz and filtered through a band pass filter of 1-100 Hz. Data were epoched from the flash stimulus synch pulse (0-300 ms) on each trial. Epochs containing > 50 μV amplitude were rejected automatically. Sixty-five epochs were averaged to form a FVEP. The positive wave around 120 ms was designated as P2 and the negative wave before it around 90 ms as N2 and peak latencies of both the waveforms were documented.
Data analysis
The data were analyzed for interindividual and intraindividual variability. Coefficient of Variance (COV) = Standard deviation*100/Mean. A useful concept here is that if two groups have significantly different means, their SD cannot be directly compared. To compare them, SDs have to be corrected for the mean, using COV.
Interindividual variability
The average of four trials was calculated for P2 and N2 latencies for each subject for each eye separately. The mean and SD of the averaged latencies of participants were calculated. The coefficient of variance for interindividual variability was determined (COVinter) and compared between different techniques within the group. A lesser COVinter in FVEP latency for a particular technique is reflective of lower variations in interindividual variability of FVEP latency for that technique.
Intraindividual variability
The SD for four trials in each subject was determined (SDintra). To remove the effect of interindividual variability, the COV for each subject was calculated (COVintra). The mean of COVintra was considered as a score of intraindividual variability. The method with lesser mean of COVintra was considered a better method. The construction of the average intraindividual variability and interindividual variability values had been schematized in our earlier paper which may further clarify their nature for the reader.[21]
Results
Comparison between standard (eye open) and eye closure technique
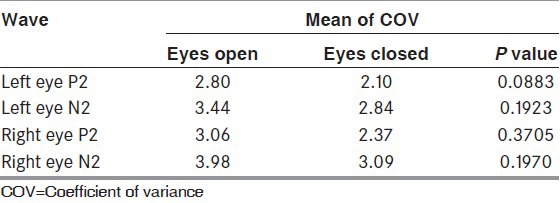
The latencies of P2 and N2 waveforms were significantly delayed in eye closure technique as compared to standard technique in all the trials [Table 1]. While assessing interindividual variability the COVinter showed lower values for the eyes closed technique for both P2 and N2 [Table 2]. When assessing for intraindividual variability, the COVintra was lower in technique with eyes closed as compared to standard technique, for both P2 and N2 waveforms [Table 3].
Table 1.
Comparison of P2 and N2 waveforms latencies between eye open and eye closed techniques (n=33)
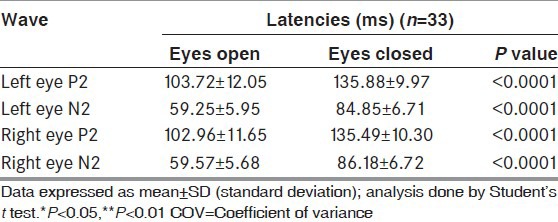
Table 2.
Interindividual variability: Comparison of COV of P2 and N2 waveforms in between eye open and eye closed technique (n=33)
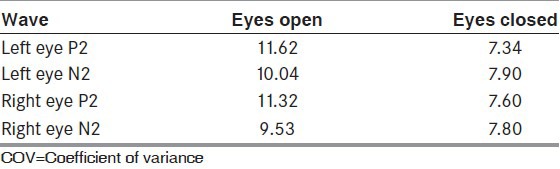
Table 3.
Intraindividual variability: Comparison of mean of COVi of P2 and N2 waveforms between eye open and eye closed techniques (n=33)
Comparison between standard (0.6 J) with 1.2 and 20 J strength luminance
The latencies of P2 and N2 were significantly reduced in techniques with higher luminance [Table 4]. While assessing the interindividual variability, the COV was less for both P2 and N2 waveforms in standard technique as compared to techniques with higher luminance [Table 5]. While assessing the intraindividual variability too, the COV was less in standard technique as compared with higher luminance techniques [Table 6].
Table 4.
Comparison of P2 and N2 waveforms latencies between stimuli of three different luminance in healthy volunteers (n=30)
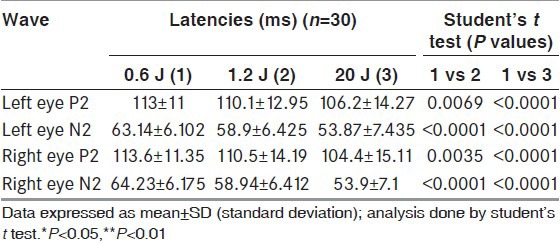
Table 5.
Interindividual variability: Comparison of COV of P2 and N2 waveforms between stimuli of three different luminance in healthy volunteers (n=30)
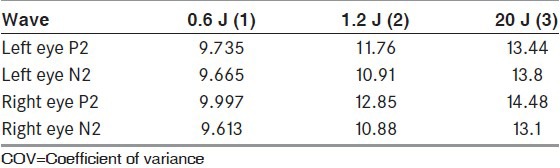
Table 6.
Intraindividual variability: Comparison of mean of COVi of P2 and N2 waveforms in between stimuli of three different luminance in healthy volunteers (n=30)
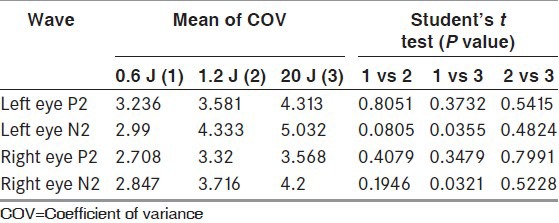
Comparison between white light and monochromatic light
Our team has earlier shown that mean latencies of both P2 and N2 waveforms were significantly higher with monochromatic stimulation, but the COVinter was less for both red and blue FVEPs. Between red and blue, COVinter was lesser for blue. Further, the mean COVintra was also consistently lower in blue and red, as compared to white and latency variation lesser in blue for N2 and lesser in red for P2.
Discussion
Comparison between standard and eye closure technique
We observed a significant difference between open and closed eye recordings. FVEP was recorded for short intervals with subjects under the careful observation of the examiner and they were encouraged to talk in between the recordings. Therefore, it is more plausible that rather than a decrease in vigilance, it is the decrease in luminance that could have caused the delay in latency associated with the closed eye recordings. This observation goes hand in hand with the results in group 2 where the mean latencies of FVEP increased with decrease in flash luminance [Table 3]. Variability was less with closed eye recordings. Subjects who participated in the study felt more comfortable as well with eye closure technique. This was felt to be helpful in reducing the eye movements, which might have contributed towards reducing the variability. However, a reduction in eye movements was not confirmed with any electro-oculogram (EOG). However, FVEP is considered to be least affected by eye movements when compared to pattern VEP[23] and eye movements are expected to affect VEP at the early components.[5] Further studies are needed to test this postulations.
As light crosses the capillary meshwork of the eyelid, white light may be perceived as red. Reduced variability could have also been contributed by this change in chrominance of the incident light to red. Red light is shown to produce FVEP with larger amplitude.[24] Increased amplitude increases S/N ratio and reduces variability. This may be another possible explanation for decreased variability in FVEP latency in eye closure technique.
Comparison between standard (0.6 J) and 1.2 and 20 J strength flash luminance
We observed that the latencies of N2 and P2 components were less in 1.2 J flash strength as compared to 0.6 J flash strength and latencies of 20 J flash strength were less as compared to 1.2 J flash strength. As the luminance increases, the mean latency decreases. This observation goes hand in hand with that of Vaughan et al., who concluded that as the strength of flash luminance increases, the latency of FVEP components decreases.[7] Therefore, the difference in the luminance of the flash produced by the xenon flash tube, the distance between the flash tube and the participant's eyes, pigmentation and thickness of the eyelid, all have to be considered as the factors affecting the retinal luminance, while determining the latency of waveforms.
We observed a decrease in variation of latencies as the flash luminance decreases (COV for 20 J > 1.2 J > 0.6 J). However, one is not sure whether this trend for decrease in variation of latencies with a decrease in luminance has any threshold value, beyond which further reductions in luminance may increase rather than decrease the variance, owing to insufficient stimulation of the retina. If so, there should be an optimal level of luminance for a fixed distance between the eye and the flash tube at which the variance is lowest. This of course could be the recommended luminance for FVEP stimulation. We suggest that further research should therefore focus on finding out this ideal luminance level for FVEP.
Couburn et al., suggested using lower luminance flash to generate a P2 component of FVEP, without the EOG contamination, caused by higher luminance flashes.[18] The flash evoked retinal responses depend upon the amount of light reaching the retina, which in turn depends upon the position of the eye with respect to the flash tube. The decrease in latency of FVEP with an increase in luminance of flash has been attributed to an increase in retinal response.[4] Jeffrey Froehlich showed that most of the alteration in VEP latency can be accounted for, by a nearly equal alteration in the B-wave latency of simultaneously recorded pattern electroretinogram (PERG).[25] Increase in variance of the latency of FVEP in response to an increase in luminance can be explained as follows: Increase in the luminance of the flash increases eye movements. Increase in eye movements increases the variance in retinal illumination, which in turn increases the variance in retinal response. Increased variance in retinal response is the cause of increased variance seen in the latencies of FVEP. Givre et al., concluded that, reducing the flash intensity does not affect the response morphology, the sequence of intracortical events, and the pattern of the cortical contribution to the generation of surface VEP.[22] The results of the present study suggest that the FVEP with lower interindividual variability and without any change in morphology of VEP may be recorded with a lower luminance of 0.6 J strength
Monochromatic light
Our own studies earlier on monochromatic stimulation had shown that interindividual variability and intraindividual variability in FVEP latency is significantly less when monochromatic stimulation is used (Subramanian SK et al., 2012).[21] The amplitudes of the FVEP are maximum for red color compared to other colors.[24] Monochromatic stimulation with both red and blue light had increased the latency of the standard waveforms. Our explanation for this was that red and blue light evoked a reduced perception of brightness/luminance as compared to equiluminant white light, resulting in prolonged latency. The reduced luminance induced by monochromatic stimulation also produces reduced interindividual variability seen in FVEP. We postulated that that reduction in eye movements, which in turn reduces the variation in retinal luminance, is the most probable explanation for the reduced variation seen in monochromatic stimulation.[21]
Limitations
In this study, it was observed that eye closure reduced variability leading to the assumption that eye movements and resultant variations in retinal luminance were the primary cause of variations seen in latency. Nevertheless, the eye movements could not be directly correlated with the increased variation, as EOG was not done as a part of this study. One way to address this would be to perform FVEP before and after paralyzing eye movements, immediately prior to an ophthalmic surgery, where transient induction of ophthalmoparesis is a common preoperative procedure. Another opportunity is in the brainstem-dead subjects with intact hemispherical functions and recordable VEPs. Simultaneous recording of PERG, EOG, and FVEP may also be useful to sort out the influence of eye movements. Such studies may form a logical extension of the current study.
Conclusion
Stimulus with decreased strength of luminance up to 0.6 J may reduce intraindividual and interindividual variability seen in peak latencies of P2 and N2 waveforms of FVEP. Recording with closed eyes also reduces peak latency variability of FVEP. Hence, we recommend that the FVEP be performed with eyes closed, wherever possible. Use of monochromatic light especially blue can also lessen changes in variability of FVEP. These appear promising modifications in techniques of how best we do FVEP. However, further studies are needed to sort out whether these results are indirect effects of decreased eye movements and resultant reduced alterations in retinal luminance.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Harding GF, Bland JD, Smith VH. Visual evoked potential monitoring of optic nerve function during surgery. J Neurol Neurosurg Psychiatry. 1990;53:890–5. doi: 10.1136/jnnp.53.10.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kodama K, Goto T, Sato A, Sakai K, Tanaka Y, Hongo K. Standard and limitation of intraoperative monitoring of the visual evoked potential. Acta Neurochir. 2010;152:643–8. doi: 10.1007/s00701-010-0600-2. [DOI] [PubMed] [Google Scholar]
- 3.Givre SJ, Arezzo JC, Schroeder CE. Effects of wavelength on the timing and laminar distribution of illuminance-evoked activity in macaque V1. Vis Neurosci. 1995;12:229–39. doi: 10.1017/s0952523800007914. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder CE, Tenke CE, Givre SJ. Subcortical contributions to the surface-recorded flash-VEP in the awake macaque. Electroencephalogr Clin Neurophysiol. 1992;84:219–31. doi: 10.1016/0168-5597(92)90003-t. [DOI] [PubMed] [Google Scholar]
- 5.Farrell DF, Leeman S, Ojemann GA. Study of human visual cortex: Direct cortical evoked potentials and stimulation. J Clin Neurophysiol. 2007;24:1–10. doi: 10.1097/WNP.0b013e31802fb614. [DOI] [PubMed] [Google Scholar]
- 6.Odom JV, Bach M, Brigell M, Holder GE, McCulloch DL, Tormene AP, et al. ISCEV standard for clinical visual evoked potentials (2009 update) Doc Ophthalmol. 2010;120:111–9. doi: 10.1007/s10633-009-9195-4. [DOI] [PubMed] [Google Scholar]
- 7.Vaughan HG, Jr, Hull RC. Functional relation between stimulus intensity and photically evoked cerebral responses in man. Nature. 1965;206:720–2. doi: 10.1038/206720a0. [DOI] [PubMed] [Google Scholar]
- 8.Tobimatsu S, Kurita-Tashima S, Nakayama-Hiromatsu M, Akazawa K, Kato M. Age-related changes in pattern visual evoked potentials: Differential effects of luminance, contrast and check size. Electroencephalogr Clin Neurophysiol. 1993;88:12–9. doi: 10.1016/0168-5597(93)90023-i. [DOI] [PubMed] [Google Scholar]
- 9.Celesia GG, Kaufman D, Cone S. Effects of age and sex on pattern electroretinograms and visual evoked potentials. Electroencephalogr Clin Neurophysiol. 1987;68:161–71. doi: 10.1016/0168-5597(87)90023-2. [DOI] [PubMed] [Google Scholar]
- 10.Collins DW, Carroll WM, Black JL, Walsh M. Effect of refractive error on the visual evoked response. Br Med J. 1979;1:231–2. doi: 10.1136/bmj.1.6158.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson F, Etard O, Denise P, Petit L. Early visual evoked potentials are modulated by eye position in humans induced by whole body rotations. BMC. Neurosci. 2004;5:35. doi: 10.1186/1471-2202-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeSouza JF, Dukelow SP, Vilis T. Eye position signals modulate early dorsal and ventral visual areas. Cereb Cortex. 2002;12:991–7. doi: 10.1093/cercor/12.9.991. [DOI] [PubMed] [Google Scholar]
- 13.Sokol S, Moskowitz A, Towle VL. Age-related changes in the latency of the visual evoked potential: Influence of check size. Electroencephalogr Clin Neurophysiol. 1981;51:559–62. doi: 10.1016/0013-4694(81)90232-7. [DOI] [PubMed] [Google Scholar]
- 14.Sannita WG, Fatone M, Garbarino S, Giglioli D, Massimillao S, Riela S. Effects of physiological changes of serum glucose on the pattern-VEP of healthy volunteers. Physiol Behav. 1995;58:1021–6. doi: 10.1016/0031-9384(95)00139-a. [DOI] [PubMed] [Google Scholar]
- 15.Shams L, Kamitani Y, Thompson S, Shimojo S. Sound alters visual evoked potentials in humans. Neuroreport. 2001;12:3849–52. doi: 10.1097/00001756-200112040-00049. [DOI] [PubMed] [Google Scholar]
- 16.Skuse NF, Burke D. Sequence-dependent deterioration in the visual evoked potential in the absence of drowsiness. Electroencephalogr Clin Neurophysiol. 1992;84:20–5. doi: 10.1016/0168-5597(92)90064-i. [DOI] [PubMed] [Google Scholar]
- 17.Meinberg O, Kutak L, Smolenski C, Ludi HP. Pattern reversal evoked cortical responses in normal; study of different methods of stimulation and potential reproducibility. J Neurol. 1979;222:81–93. doi: 10.1007/BF00313002. [DOI] [PubMed] [Google Scholar]
- 18.Coburn KL, Amoss RT, Arruda JE, Kizer LD, Marshall YS. Effects of flash mode and intensity on P2 component latency and amplitude. Int J Psychophysiol. 2005;55:323–31. doi: 10.1016/j.ijpsycho.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Pratt H, Martin WH, Bleich N, Zaaroor M, Schacham SE. A high-intensity, goggle-mounted flash stimulator for short-latency visual evoked potentials. Electroencephalogr Clin Neurophysiol. 1994;92:469–72. doi: 10.1016/0168-5597(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 20.Shaw NA, Cant BR. Age-dependent changes in the latency of the pattern visual evoked potential. Electroencephalogr Clin Neurophysiol. 1980;48:237–41. doi: 10.1016/0013-4694(80)90310-7. [DOI] [PubMed] [Google Scholar]
- 21.Subramanian SK, Gaur GS, Narayan SK. Effect of color of flash stimulus on variability of flash visual evoked potential latencies. Indian J Physiol Pharmacol. 2012;56:322–9. [PubMed] [Google Scholar]
- 22.Givre SJ, Schroeder CE, Arezzo JC. Contribution of extrastriate area V4 to the surface-recorded flash VEP in the awake macaque. Vision Res. 1994;34:415–28. doi: 10.1016/0042-6989(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 23.Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968;195:215–43. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halliday AM, McDonald WI, Mushin J. Visual evoked response in diagnosis of multiple sclerosis. Br Med J. 1973;4:661–4. doi: 10.1136/bmj.4.5893.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Froehlich J, Kaufman DI. Effect of decreased retinal illumination on simultaneously recorded pattern electroretinograms and visual-evoked potentials. Invest Ophthalmol Vis Sci. 1991;32:310–8. [PubMed] [Google Scholar]


