Abstract
Context:
The diagnostic accuracy of the currently available tools carries poor sensitivity resulting in significant delay in specific diagnosis of cortical dementias. Considering the properties of default mode networking of the brain it is highly probable that specific changes may be seen in frontotemporal dementias (FTDs) and Alzheimer's disease sufficiently early.
Aim:
The aim of this study is to look for changes in Transcranial Magnetic Stimulation (TMS) in cortical dementia.
Materials and Methods:
Evaluated with a single pulse TMS with the figure of eight coil and recorded from right first dorsal interossei (FDI). Resting Motor Threshold (RMT) was estimated on the opposite motor cortex (T1). Second site of stimulation was cervical spine at C7-T2. Central motor conduction time (CMCT) is equal toT1-T2. Silent Period (SP) identified by applying TMS pulse to contracting FDI.
Conclusions:
RMT was reduced in seven out of eight Alzheimer's dementias. CMCT was in the upper limit of normal in both patients with FTD. The most consistent observation was that SP was reduced and there were escape discharges noticed during the SP suggesting increased cortical excitability and decreased cortical inhibition. This suggests probable early asymptomatic changes in the gamma-aminobutyric acid (GABA) nergic and cholinergic system is taking place. This if confirmed may give some insight into early diagnosis and therapeutic role of GABA agonists in these disorders.
Key Words: Alzheimer's dementia, central motor conduction time, cortical inhibition, fronto-temporal dementias, transcranial magnetic stimulation
Introduction
Degenerative cortical dementias are the most common form of debilitating dementias affecting 36 million people world-wide.[1] Dementia makes the largest independent contributor of any chronic disease to dependence (needs for care). Caring for older people with dementia is almost bereavement as the loved personality almost does not exist.[2] Alzheimer's dementia (AD) forms 40%, fronto-temporal dementia (FTD) includes (18.7%) of patients with Dementia.[3] Some studies have also pointed out that early onset AD and FTD have similar prevalence in the presenium(<65 years).[4] It is estimated that the global dementia burden will double by 2020. Currently, there is no tool for early definitive ante mortem diagnosis of cortical dementias. Most of the available therapeutic agents work only if used in the very early stages and postpones deterioration to dependency by about 2 years. Available methods are time consuming and less sensitive for early diagnosis.[5] In the default mode networking of the brain, motor pathways are harbored in the frontal circuits and parietal lobes are concerned with inhibition. Transcranial Magnetic Stimulation (TMS) being a non-invasive test the presence of specific features if found will improve the anti-mortem diagnostic accuracy greatly without the use of invasive tools. Studies in TMS in dementia are not there from India to our knowledge and the aim of this study was to find out whether considerable differences in TMS parameters are there between FTD and AD in early stages. If present it will serve as a diagnostic bio-marker. Paired pulse was not used as the aim of this pilot study was not to analyze the pathophysiological mechanisms of alteration in these parameters. There is conflicting reports regarding the basis of alteration in the TMS parameters in patients with AD and FTD when studied using single pulse and paired pulse paradigms.
TMS serves as a simple method of evaluating the excitatory and inhibitory properties of the motor cortex.[6] The threshold of motor response is an index of excitability of the corticospinal motor neurons and the amplitude of the motor response and duration of silent period (SP) is related to cortical inhibition mediated through gamma-aminobutyric acid (GABA)-B receptors. Using a study performed in fifteen patients with a single pulse stimulation the authors found the following changes in Alzheimer's disease using the short-latency afferent inhibition (SAI), which is produced by inhibitory interactions within the cerebral cortex.[7,8] SAI begins about 1 millisecond after latency of the N20 component of the somatosensory evoked potential obtained from median nerve stimulation and lasts for about 7-8 milliseconds. This was decreased in patients with Alzheimer's disease and improved after a single dose of rivastigmine. Same observation was found in dementia with Lewy bodies by Nardone et al.[9] However, it was normal in FTD and thus reflecting a correlation with cholinergic deficiency. Another group of authors found similar changes with reference to SAI along with lowered cortical threshold and decreased cortical inhibition in 12 patients.[10] Using paired conditioning test in 17 patients another group of authors found that the modification of the excitability of motor cortex in patients with Alzheimer's disease does not result from impaired intracortical inhibition as proposed by other authors.[11] Paired stimuli was studied in patients with Alzheimer's disease and Frontotemporal Dementia patients by Alberici et al., and concluded that there is no change in the intracortical excitatory circuits in these patients but the observations made in single pulse studies reflected cholinergic deficiency and abnormal N-methyl D-aspartate (NMDA) transmission.[12]
However, the aim of the authors was not to determine the physiological basis of the changes in TMS but to know whether the observations can serve as an early biomarker.
Materials and Methods
Subjects
Patients were chosen from neurology out-patient and in-patient department of our institution. Patients were evaluated with Hindi Mental Status Examination (HMSE), Clinical Dementia Rating scale (CDR) and DSM IV. Those with HMSE score more than 20 and CDR: 0.5-1.5 was included. There were eight AD and two FTD patients [Figure 1] with a mean age of 62.5 ± 9.7 years [Table 1]. Their duration of disease was less than 24 months. All of them underwent MRI magnetic resonance imaging with 1.5 tesla machine. Those with mixed features, pyramidal, extrapyramidal or cerebellar signs, advanced dementia and those with seizures, aneurysm clips were excluded. The normal values were differentiated from abnormal values based on the institute normative data for age and gender matched persons. These normative data were generated as controls from healthy bystanders for previous studies in TMS for other disorders assessed in our institute with proper ethical guidelines. Our patient number is small but the differences in the parameters between the two groups of patients studied was consistent and therefore we decided to consider publication of this pilot work in India so that other institutions can parallelly initiate their data in this aspect.
Figure 1.
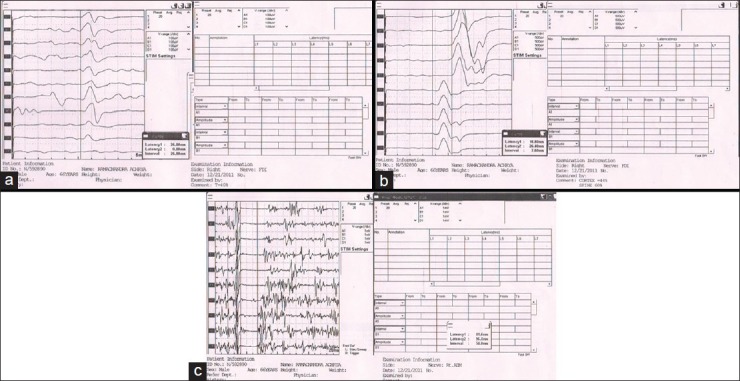
Transcranial magnetic stimulation changes in Alzheimer's dementia. (a) Resting motor threshold. (b) Central motor conduction time. (c) Silent period
Table 1.
Demographic details

TMS procedure
All participants were seated comfortably in a chair. TMS was performed using a figure of eight magstim 200 stimulator discharging a maximum output of 2.2 Tesla. A single pulse stimulation of the left motor cortex was done at optimum scalp position. Surface muscle response was recorded using belly tendon method. Active electrode was placed over the first dorsal interossei (FDI) belly and reference electrode over the metacarpophalangeal joint of the right index finger. Figure of eight coil handle was positioned at an angle 45°pointing backwards. The stimulus intensity was gradually increased in 5% increments until a satisfactory motor evoked potential (MEP) of at least 50μV was obtained. The stimulation was repeated at least 10 times at intervals of 3s. The resting motor threshold (RMT) was calculated as follows. The minimum stimulus intensity that evokes at least five MEP of a minimum of 50 μVin the relaxed FDI.[13] Central motor conduction time (CMCT) was calculated as follows from the relaxed FDI. MEP elicited with supramaximal stimuli that is 150% of RMT from the motor cortex (T1). Second MEP was elicited from C7 spinous process (T2) and CMCT is equal to T1-T2. Silent period was studied in the partially contracted FDI on the right side. About 150% intensity stimulus was delivered. SP was identified as follows; which is the period of electro - (EMG) arrest to the appearance of EMG.
Results
Demographic details
There were eight patients with AD and two patients with FTD. Their mean age was 62.5 ± 9.7 years. The male: female ratio was 4:1 [Table 1].
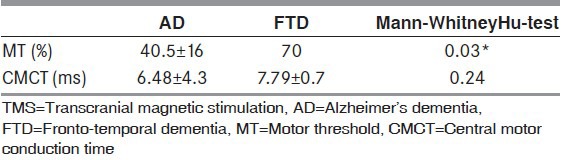
Mean HMSE score for patients with AD and FTD were 23 ± 1.07 and 26.5 ± 0.71 respectively. Since the number of samples was less, Mann-Whitney Hu-test was used for comparison between the groups. Both the FTD cases had CMCT values on a higher range as compared to the AD cases even though it was not statistically significant. Silent Period was also reduced in both groups, but more so in the FTD group. Motor threshold values were significantly reduced in the AD group as compared with the FTD group as shown in Table 2.
Table 2.
TMS variables
Discussion
This pilot study reveals the following. RMT is reduced in Alzheimer's disease and normal in FTD, central motor conduction is slightly increased in FTD and normal in Alzheimer's disease, Silent period is reduced in both groups. Reduced RMT and silent period in AD suggest increased cortical excitability and reduced inhibition [Figures 1 and 2]. This might suggest a role for asymptomatic changes in GABAnergic, cholinergic system. In FTD, patient's central motor conduction is prolonged and Silent Period is reduced suggesting early sub clinical involvement of motor pathways as well as reduced inhibition. The common TMS parameter between FTD and AD seems to be the reduced Silent Period. This might indicate a common chemical factor existing between these two diseases may be the underlying mechanism for the reduced SP, which can be postulated as NMDA transmission. This study is perhaps the first of its kind in India utilizing the value of TMS as a tool for studying cortical dementias. Our findings suggest that TMS can be considered as a complementary and useful tool in detecting and differentially diagnosing cortical dementias in the early stage itself. There is a possibility that early asymptomatic changes in the GABAnergic, Cholinergic systems are taking place in AD. These changes are absent in FTD. There may be comparable changes in NMDA mediated excitotoxicity in both groups. This explains the usefulness of cholinesterase inhibitors in Alzheimer's disease and its ineffectiveness in FTD as well as the utility of NMDA blockers in both groups. Possible role of GABA agonists in the management of Alzheimer's disease in early stages deserves to be evaluated. We are continuing to study and the data on a larger number of patients will be generated within the next 2 years. We did not include diffuse Lewy body disease cases as it was not part of our objective.
Figure 2.
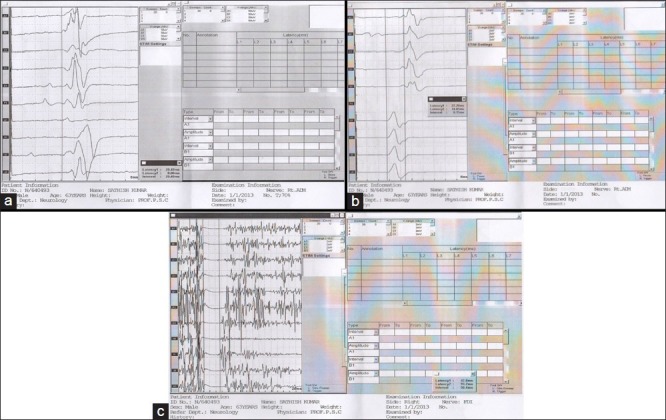
Transcranial magnetic stimulation changes in fronto-temporal dementia. (a) Resting motor threshold. (b) Central motor conduction time. (c) Silent period
Conclusion
The above pilot study suggests that distinct patterns of TMS changes occur in FTD and AD. This finding is largely in agreement with previous papers focusing on TMS features using single pulse stimulation on AD and FTD. Our study used only single pulse TMS on a relatively very small population. When applied to larger number of patients our observations are likely to serve as an early non-invasive biomarker for diagnosis and also probably will broaden therapeutic options. However, this hypothesis needs study in larger populations.
Acknowledgment
The authors would like to thank Dr. Mariamma Philip (Department of Biostatistics; NIMHANS) for help with statistics.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Batsch NL, Mittelman MS. Overcoming the stigma of dementia; Alzheimer's Disease International; World Dementia Report. 2012 [Google Scholar]
- 2.Shaji KS, Jotheeswaran AT, Girish N, Srikala B, Dias A, Pattabiraman M, et al. Prevalence, impact, costs and services for dementia. THE DEMENTIA INDIA REPORT. 2010 [Google Scholar]
- 3.Alladi S, Mekala S, Chadalawada SK, Jala S, Mridula R, Kaul S. Subtypes of dementia: A study from a memory clinic in India. Dement Geriatr Cogn Disord. 2011;32:32–8. doi: 10.1159/000329862. [DOI] [PubMed] [Google Scholar]
- 4.Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–21. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- 5.Panella M, Palmieri MG, Koch G, Giordano A, Marciani MG, et al. Different TMS patterns of intracortical inhibition in early onset Alzheimer dementia and frontotemporal dementia. Clin Neurophysiol. 2004;115:2410–8. doi: 10.1016/j.clinph.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Nardone R, Golaszewski S, Ladurner G, Tezzon F, Trinka E. A review of transcranial magnetic stimulation in the in vivo functional evaluation of central cholinergic circuits indementia. Dement Geriatr Cogn Disord. 2011;32:18–25. doi: 10.1159/000330016. [DOI] [PubMed] [Google Scholar]
- 7.Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, et al. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523(Pt2):503–13. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, et al. The physiological basis of transcranial motor cortex stimulation inconscious humans. Clin Neurophysiol. 2004;115:255–66. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Nardone R, Bratti A, Tezzon F. Motor cortex inhibitory circuits in dementia with Lewy bodies and in Alzheimer's disease. J Neural Transm. 2006;113:1679–84. doi: 10.1007/s00702-006-0551-1. [DOI] [PubMed] [Google Scholar]
- 10.Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, et al. Motor cortex hyper excitability to transcranial magnetic stimulation in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2004;75:555–9. doi: 10.1136/jnnp.2003.018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pepin JL, Bogacz D, dePasqua V, Delwaide PJ. Motor cortex inhibition is not impaired in patients with Alzheimer's disease: Evidence from paired transcranial magnetic stimulation. J Neurol Sci. 1999;170:119–23. doi: 10.1016/s0022-510x(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 12.Alberici A, Bonato C, Calabria M, Agosti C, Zanetti O, Miniussi C, et al. The contribution of TMS to frontotemporal dementia variants. Acta Neurol Scand. 2008;118:275–80. doi: 10.1111/j.1600-0404.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- 13.Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]


