Abstract
Objective:
To evaluate the effectiveness of mirror therapy on lower extremity motor recovery, balance and mobility in patients with acute stroke.
Design:
A randomized, sham-controlled, assessor blinded, pilot trial.
Setting:
Inpatient stroke rehabilitation unit.
Subjects:
First time onset of stroke with mean post-stroke duration of 6.41 days, able to respond to verbal instructions, and Brunnstrom recovery stage 2 and above were enrolled.
Intervention:
Mirror therapy group performed 30 minutes of functional synergy movements of non-paretic lower extremity, whereas control group underwent sham therapy with similar duration. In addition, both groups were administered with conventional stroke rehabilitation regime. Altogether 90 minutes therapy session per day, six days a week, for two weeks duration was administered to both groups.
Outcome Measures:
Lower extremity motor subscale of Fugl Meyer Assessment (FMA), Brunnel Balance Assessment (BBA) and Functional Ambulation Categories (FAC).
Results:
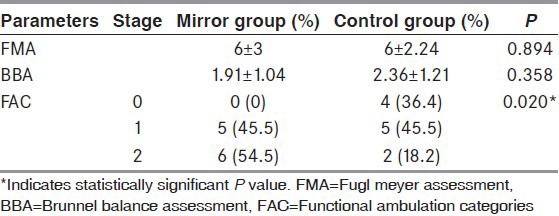
Amongst the 22 patients included, equal number of patients participated in mirror group (N = 11) and control group (N = 11). Baseline variables were similar in both groups, except for Brunnstrom recovery stage. There was no statistical difference between groups, except for FAC. (FMA: P = 0.894; BBA: P = 0.358; FAC: P = 0.02). Significance was set at P < 0.05.
Conclusion:
Administration of mirror therapy early after stroke is not superior to conventional treatment in improving lower limb motor recovery and balance, except for improvement in mobility.
Key Words: Acute stroke, balance, mobility, mirror therapy, motor recovery
Introduction
Stroke is defined as a clinical syndrome characterized by rapidly developing signs of focal or global disturbance of cerebral functions, lasting for more than 24 h or leading to death, with no apparent causes other than vascular origin. It is the leading cause of long term disability in adults.[1] Residual motor weakness, abnormal movement synergies and spasticity often predispose the stroke survivor to a sedentary life-style, which further limits the individual's activities of daily living and reduces cardiovascular reserves. These primary neurological deficits also result in altered gait patterns and contribute to poor balance, risk for falls and increased energy expenditure during walking. Efforts to minimize the impact and to improve functional outcomes after a stroke thus, pose an important challenge for rehabilitation professionals.[2]
The recovery mechanism, after stroke is known to be most prominent within the first three months.[3] Furthermore, the level of recovery achieved in the first month of stroke determines the functional outcome in the chronic phase. Thus, implementation of intensive therapy within the first month of stroke can lead to enhanced and faster improvement in performance of activities.[1] Several promising rehabilitation approaches have been developed addressing the motor recovery and balance in stroke; such as virtual reality, body supported treadmill training, mental imagery, neuromuscular stimulation, and robotic interactive therapy.[4]
In 1992, Ramachandran et al. introduced the concept of mirror visual feedback (MVF) as a simple non-invasive technique for the treatment of phantom pain and hemiparesis following stroke.[5] He first used MVF to induce kinesthetic sensations in the phantom limbs of arm amputees by placing the mirror vertically in front of the subjects, who moved the intact arm while looking at its reflection visually superimposed on the phantom arm.[6] In later studies, Ramachandran et al. demonstrated beneficial effects of this treatment in hemiparesis and hemineglect following stroke.[7,8] Subsequently, successful use of mirror therapy has been reported in patients with complex regional pain syndrome, and in sensory re-education of severe hyperesthesia after hand injuries.[9] Mirror therapy in stroke involves performing the movements of the non-paretic limb while viewing its mirror reflection superimposed over the unseen paretic limb.[10] This visual feedback can substitute for the missing proprioceptive feedback from the paretic limb.[9] Thus, mirror therapy helps to prevent or reduce the learned non-use of the paretic limb and also enhance neuroplasticity.[11] Furthermore, the cross facilitatory drive from the intact hemisphere gives rise to increased excitability in the mirror neurons and the homologous motor pathways of the paretic limb, thus, enhancing the preparedness of these motor pathways and facilitating recovery of function.[9]
Functional brain imaging studies of healthy subjects suggest that excitability of primary motor cortex and somatosensory cortex ipsilateral to a unilateral hand or knee movement is facilitated by viewing the mirror reflection of the moving limb.[10,12] In addition, Luft et al. confirmed the presence of significant recruitment of contralateral primary motor cortex, supplementary motor cortex, and bilateral somatosensory cortex during lower limb movements in chronic stroke.[13] Thus, observing mirrored movements causes additional neural activity in motor areas located in the affected hemisphere, which can facilitate the cortical reorganization and recovery of function.[14]
Neuronal Group Selection theory advocates the application of early intervention after brain lesion, using functional and task-oriented activities to facilitate brain plasticity. These activities enable the recruitment of appropriate functional movement synergies.[15] Thus, activation of functional movement synergy may be more effective in facilitating brain plasticity than practicing isolated joint movements. Hence, we hypothesized that the practice of functional movement synergies of the non-paretic lower extremity with mirror therapy in addition to convention stroke rehabilitation would show more extensive recovery in function compared to the convention stroke rehabilitation alone. We designed this randomized, sham-controlled, assessor blinded, pilot study to evaluate the effects of mirror therapy using functional movement synergies on lower extremity motor recovery, balance and mobility in acute stroke.
Methods
Participants
We identified the potential participants from an inpatient stroke rehabilitation unit. The participants were assessed for their eligibility by a physiotherapist. The study included 22 in-patients (12 males, 10 females) with hemiparesis after stroke (mean age 62.95 years, time since onset of stroke 6.41 days). Patients were required to meet the following criteria for inclusion in the study: (1) First episode of unilateral stroke with hemiparesis (onset ≤ 2 weeks), (2) able to understand and follow simple verbal instructions, (3) Brunnstrom recovery stage 2 and above, (4) no severe cognitive disorders that would interfere with the study's purpose (Mini-Mental State Examination score > 23), (5) stable medical condition to allow participation in the study, (6) ambulatory before stroke. Patients with neglect, pusher syndrome, visual deficits, and history of multiple stroke, or comorbidities that influenced lower extremity usage were excluded. The study was approved by the Manipal University Ethics Committee and all the patients gave written informed consent before participating in the study.
Study design
An assessor blinded, randomized sham-controlled design was used. After obtaining baseline measurements, the patients were assigned to either the mirror group (n = 11) or the control group (n = 11) by block randomization. Post-treatment measurements were performed after the last treatment session, which was at the end of 2 weeks. All assessments were carried out by the same investigator, who was blinded to the group allocation.
Intervention
Both the mirror group and the control group participated in the conventional stroke rehabilitation program for 1 h a day, 6 days a week, for 2 weeks. The conventional program was patient-specific and consisted of neurodevelopmental facilitation techniques, sensory motor re-education, active exercises, mobility training, balance, and gait training.
The mirror group received an additional 30 min of mirror therapy program consisting of performance of functional movement synergies using non-paretic hip, knee and ankle joints. These movements were performed in semi-reclined and sitting positions with the mirror placed between the two lower extremities. The mirror was mounted on a stand with a tilt towards the paretic side to prevent the paretic limb from being viewed by the subject. For the mirror group, the reflective surface was kept facing the non-paretic limb. The exercises performed in half-lying position [Figure 1] were: (1) hip-knee-ankle flexion, (2) with the hip and knee placed in flexion, moving the knee inward and outward, (3) hip abduction with external rotation followed by hip adduction with internal rotation. The exercises performed in sitting position [Figure 2] were: (1) Hip-knee-ankle flexion, (2) knee extension with ankle dorsiflexion, (3) knee flexion beyond 90°. Each of the above 6 exercises were performed in 2 sets of 10 repetitions.
Figure 1.
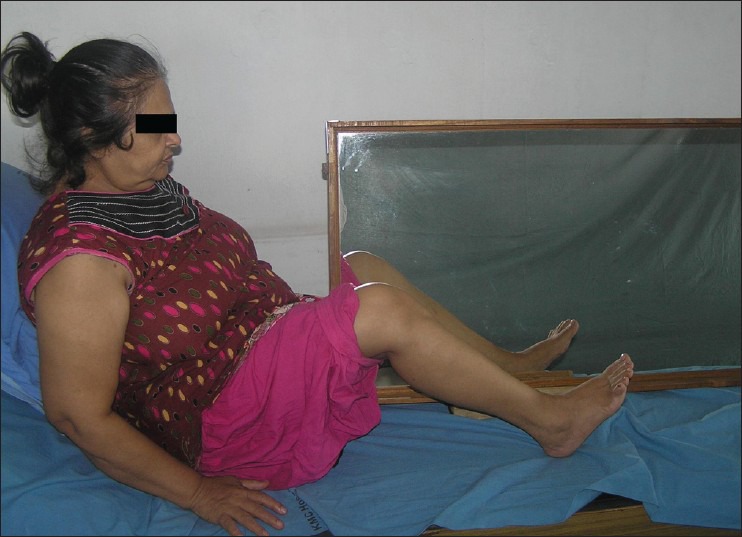
Mirror therapy in half-lying position
Figure 2.
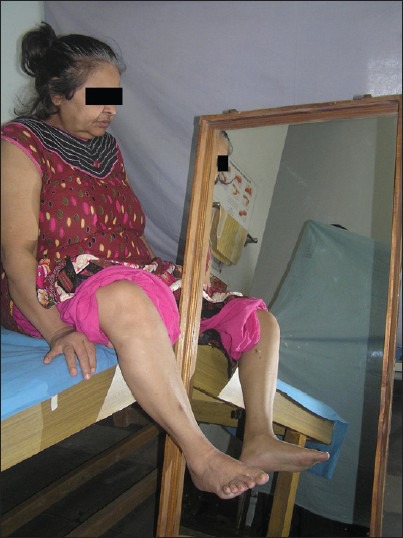
Mirror therapy in sitting position
Both mirror group and control group performed only non-paretic lower limb movements during mirror therapy (sham therapy in case of control group). Subjects did not move their paretic limb. For the control group, the non-reflecting surface of the mirror was kept facing the non-paretic limb. This prevented the control subjects from acquiring the visual feedback of the non-paretic lower extremity movements and performed the same exercises for the same duration. The same physiotherapist delivered the mirror or sham therapy to the patients.
Outcome measures
Outcomes were measured in terms of motor recovery (lower extremity subscale of Fugl Meyer assessment [FMA]), balance (Brunnel Balance assessment [BBA]) and mobility (functional ambulation categories [FAC]). These measures were taken at baseline and end of 2 weeks of treatment.
Two secondary outcome measures were also assessed before treatment for baseline comparison – Brunnstrom stage of recovery and modified composite spasticity index (MCSI).
Lower extremity motor recovery
We assessed lower extremity motor recovery using FMA. It consists of items grouped into six parts, scoring the components on an ordinal scale ranging from 0 to 2, with two representing no deficit. The total score for lower extremity motor components is 34. It shows excellent reliability, construct validity and responsive to change in stroke patients.[16]
Brunnstrom stage of recovery was assessed at baseline. It consists of 6 sequential stages of motor recovery. The six stages of Brunnstrom for lower extremity are (1) flaccidity, (2) minimal voluntary movements, (3) voluntary flexor synergy in sitting and standing, (4) some movements deviating from synergy (knee flexion beyond 90° and ankle dorsiflexion with the heel on the floor in sitting position), (5) independence from basic synergies (isolated knee flexion with the hip extended and isolated ankle dorsiflexion with the knee extended in standing position), (6) isolated joint movements (hip abduction in the standing position and knee rotation with inversion and eversion of the ankle in the sitting position).
Balance
We used BBA to measure the functional balance of the patients. This measure consists of a hierarchical series of 12 functional performance tests ranging from supported sitting balance to advanced stepping tasks. There are three sections to the assessment: Sitting, standing, and stepping. This scale has good reliability, criterion and predictive validity in stroke.[17,18]
Mobility
Mobility was assessed using FAC. It is a reliable and valid measure, with six categories providing information on the level of physical support needed to ambulate safely both indoors and outdoors.[19]
Spasticity
MCSI was used to grade the spasticity of ankle plantarflexors. It consists of two components: Achilles tendon jerk (graded from 0 to 4) and plantarflexor spasticity (graded according to modified Ashworth scale – double weighted). Higher scores indicate worse spasticity.[20]
Statistical analysis
The data was analyzed using SPSS version 16. Statistical analysis was performed on the data obtained from all patients, and there was no missing data. Demographic data of the patients were compared at baseline using unpaired t test for the continuous variables (age, time since the onset of stroke) and Chi-square test for dichotomous variables (gender, dominance, affected side, type of lesion). Chi-square test was also used to compare the Brunnstrom stage of recovery and Mann Whitney test was used for MCSI scores.
Within group comparison for baseline and post-treatment scores was done using Wilcoxon test for FMA and BBA, and Chi-square test for FAC.
To investigate whether the mirror group improved more than the control group, we calculated change score of the primary outcome measures and compared them by using Mann Whitney test for FMA and BBA, and Chi-square test for FAC. Significance was set at P < 0.05.
Results
All patients in the mirror group as well as the control group participated in all treatment sessions. We did not observe any adverse events during treatment. Demographic and clinical characteristics of the 22 patients are presented in Table 1. Baseline comparison revealed that gender, age, dominance, time since stroke, paretic side, type of lesion, MCSI, BBA, and FAC did not differ between the groups (P > 0.05). However, significant differences were found in BBA and FMA scores.
Table 1.
Demographic and clinical characteristics of mirror and control groups
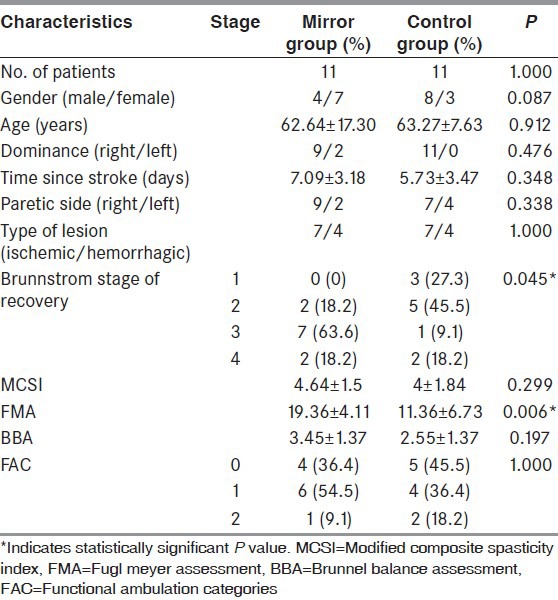
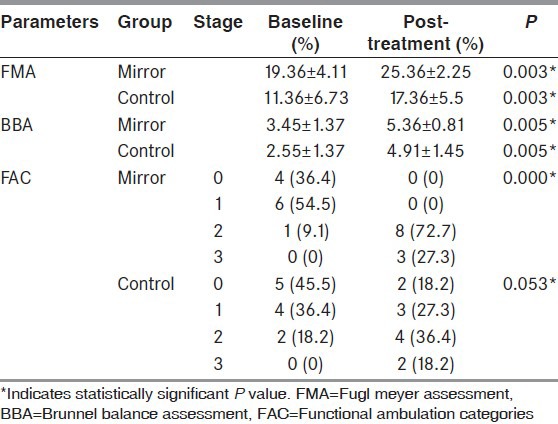
Table 2 shows the within group comparison of FMA, BBA, and FAC scores. All assessed outcome parameters improved significantly in both the groups after treatment.
Table 2.
Within group comparison of scores
Between group comparisons of change score from pre-treatment to post-treatment for FMA, BBA and FAC are detailed in Table 3. The change score of FAC showed more improvement in the mirror group than the control group, whereas the change score of other outcome parameters did not show significant differences between the groups.
Table 3.
Between group comparison of change scores
Discussion
The aim of this study was to assess the efficacy of mirror therapy using functional movement synergies on lower extremity motor recovery, balance, and mobility in acute stroke patients. Compared to Sutheyaz et al.[10] study the mirror therapy program for lower extremity motor recovery which included isolated non-paretic ankle dorsiflexion movements for chronic stroke subjects, our study mirror therapy program consisted of functional movement synergies as voluntary movement patterns are functionally specific units of muscles and joints that are constrained by the central nervous system to produce an action with precise spatial and temporal organization. These synergistic organizations of movements are disturbed in cases of Upper Motor Neuron syndrome so we incorporated functional movement synergies using non-paretic hip, knee, and ankle movements for acute stroke subjects.
The mirror and control group showed significant difference in baseline scores for FMA and Brunnstrom stage of recovery. Participants from both the groups showed mild spasticity at baseline, as measured by MCSI. At the end of 2 weeks of intervention; there was significant improvement in the mirror group as well as control group in motor recovery, balance and mobility. The mirror group exhibited better performance in ambulation categories than the control group, whereas lower extremity motor recovery and balance did not show significant difference when compared between the groups.
It is a general consensus that early implementation of intensive stroke rehabilitation is associated with enhanced and faster improvement in the performance of activities after stroke.[1] Based on the evidence that maximum improvement in lower extremity motor recovery is achieved from 1 week to 1 month after stroke,[21] we decided to administer mirror therapy within the acute phase of stroke. On account of early discharge of patients encountered in our acute inpatient rehabilitation setup, the intervention was administered for 2 weeks. We sought to determine, if addition of mirror therapy program to conventional stroke rehabilitation within the acute phase of stroke would produce additional benefits in term of lower extremity motor recovery and function.
As the recovery of motor function in stroke shows a gradual progression from synergistic movement patterns towards voluntary movements outside of stereotypical synergy,[22] the incorporation of functional movement synergies in the mirror therapy exercise protocol was considered for this study. We hypothesized that incorporation of functional movement synergies of non-paretic lower extremity would produce better recovery in function than performance of isolated joint movements. FMA was considered as a reliable tool to assess the motor recovery of the lower extremity, since it quantifies the extent of utilization of synergistic movements in the stroke subjects.[22] Furthermore, since 95% of the patients achieve independence in walking during the early phase of stroke,[23] we included BBA and FAC to assess for the task specific and carryover effect on balance and gait.
The mirror group and control group both showed improvement in FMA, BBA, and FAC after undergoing 2 weeks of intervention. Recent functional MRI studies have shown that mirror therapy increases the functional coupling between each premotor region and the left supplementary motor area, which in turn showed an increased functional interaction with the ipsilesional sensorimotor cortex, which enhances neural plasticity and these changes are specific to observation and imitation of tasks that are trained in the therapy.[24] This improvement could also be attributed to the comprehensive impairment-based training followed in the conventional stroke rehabilitation and also to the spontaneous recovery occurring in these patients.
At the end of intervention, there was no significant difference observed between the groups in the change score for FMA. Both the mirror and control group achieved equal mean change scores for this outcome measure. This could probably be due to the short duration of administration of mirror therapy compared to the previous studies. Previous studies on mirror therapy have shown significant improvement in motor function when administered for durations ranging from 3 weeks to 6 weeks.[9,10,11,14,25,26] Since, we administered the intervention for 2 weeks, it may not have been adequate to produce significant change in motor recovery. Thus we can hypothesize that longer duration of intervention would probably result in a better and clinically significant motor recovery. Furthermore, since mirror therapy is a form of visually guided motor imagery, lack of attention towards the mirror during the intervention could confound the outcome, thus reducing its effectiveness.[1]
Change in BBA score also did not show significant difference between the groups. A significant positive correlation exists between lower limb motor recovery and balance disability in stroke patients.[22] Thus, lack of significant improvement in FMA scores can be correlated with the non-significant change in BBA scores.
The mirror group showed significant change in FAC at the end of treatment, despite absence of significant improvement in motor recovery and balance. At baseline, the mirror group presented a higher score for FMA and Brunnstrom stage of recovery. This resulted in achievement of a greater FMA score at the end of intervention. As established in an earlier study, a positive correlation exists between the Fugl Meyer lower extremity assessment score and change in FAC.[27] Thus, achievement of substantial improvement in motor recovery could have led to enhanced ambulation skills in the mirror group subjects.
Limitations
The limitations of this study are the difference observed in baseline measures between both the groups, and the fact that we did not use imaging techniques to detect cortical reorganization after therapy. Future studies may investigate the effectiveness of prolonged duration of mirror therapy using functional movement synergies and also the use of this treatment protocol in subacute and chronic stroke. Studies also need to focus on identifying the optimal treatment duration and subpopulations for the effects of functional movement synergies with mirror therapy to translate into improvements in motor recovery and function.
Conclusion
This pilot study shows that administration of 2 weeks of mirror therapy with functional movement synergies in addition to convention stroke rehabilitation did not produce significant recovery in function compared to convention stroke rehabilitation alone. We believe that applying this intervention for a longer period of time (perhaps continuing the therapy at home after discharge), may be beneficial in improving the effects and outcome. Since the power of this study was low, it cannot be concluded that mirror therapy with functional movement synergies is not beneficial. Another trial with a larger sample size should be conducted to generate enough power to show statistically significant differences. Stratification of participants according to their initial Fugl Meyer scores is recommended, in order to avoid variability at baseline.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Kwakkel G, van Peppen R, Wagenaar RC, Wood Dauphinee S, Richards C, Ashburn A, et al. Effects of augmented exercise therapy time after stroke: A meta-analysis. Stroke. 2004;35:2529–39. doi: 10.1161/01.STR.0000143153.76460.7d. [DOI] [PubMed] [Google Scholar]
- 2.da Cunha IT, Jr, Lim PA, Qureshy H, Henson H, Monga T, Protas EJ. Gait outcomes after acute stroke rehabilitation with supported treadmill ambulation training: A randomized controlled pilot study. Arch Phys Med Rehabil. 2002;83:1258–65. doi: 10.1053/apmr.2002.34267. [DOI] [PubMed] [Google Scholar]
- 3.Quinn TJ, Paolucci S, Sunnerhagen KS, Sivenius J, Walker MF, Toni D, et al. Evidence-based stroke r-ehabilitation: An expanded guidance document from the european stroke organisation (ESO) guidelines for management of ischaemic stroke and transient ischaemic attack 2008. J Rehabil Med. 2009;41:99–111. doi: 10.2340/16501977-0301. [DOI] [PubMed] [Google Scholar]
- 4.Skvortsova VI, Kovrazhkina EA. Recent advances in rehabilitation of stroke survivors. F1000 Med Rep. 2009;1:23–5. doi: 10.3410/M1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramachandran VS, Altschuler EL. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain. 2009;132:1693–710. doi: 10.1093/brain/awp135. [DOI] [PubMed] [Google Scholar]
- 6.Sathian K, Greenspan AI, Wolf SL. Doing it with mirrors: A case study of a novel approach to neurorehabilitation. Neurorehabil Neural Repair. 2000;14:73–6. doi: 10.1177/154596830001400109. [DOI] [PubMed] [Google Scholar]
- 7.Altschuler EL, Wisdom SB, Stone L, Foster C, Galasko D, Llewellyn DM, et al. Rehabilitation of hemiparesis after stroke with a mirror. Lancet. 1999;353:2035–6. doi: 10.1016/s0140-6736(99)00920-4. [DOI] [PubMed] [Google Scholar]
- 8.Ramachandran VS, Altschuler EL, Stone L, Al-Aboudi M, Schwartz E, Siva N. Can mirrors alleviate visual hemineglect? Med Hypotheses. 1999;52:303–5. doi: 10.1054/mehy.1997.0651. [DOI] [PubMed] [Google Scholar]
- 9.Yavuzer G, Selles R, Sezer N, Sütbeyaz S, Bussmann JB, Köseoğlu F, et al. Mirror therapy improves hand function in subacute stroke: A randomized controlled trial. Arch Phys Med Rehabil. 2008;89:393–8. doi: 10.1016/j.apmr.2007.08.162. [DOI] [PubMed] [Google Scholar]
- 10.Sütbeyaz S, Yavuzer G, Sezer N, Koseoglu BF. Mirror therapy enhances lower-extremity motor recovery and motor functioning after stroke: A randomized controlled trial. Arch Phys Med Rehabil. 2007;88:555–9. doi: 10.1016/j.apmr.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 11.Dohle C, Püllen J, Nakaten A, Küst J, Rietz C, Karbe H. Mirror therapy promotes recovery from severe hemiparesis: A randomized controlled trial. Neurorehabil Neural Repair. 2009;23:209–17. doi: 10.1177/1545968308324786. [DOI] [PubMed] [Google Scholar]
- 12.Luft AR, Smith GV, Forrester L, Whitall J, Macko RF, Hauser TK, et al. Comparing brain activation associated with isolated upper and lower limb movement across corresponding joints. Hum Brain Mapp. 2002;17:131–40. doi: 10.1002/hbm.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luft AR, Forrester L, Macko RF, McCombe-Waller S, Whitall J, Villagra F, et al. Brain activation of lower extremity movement in chronically impaired stroke survivors. Neuroimage. 2005;26:184–94. doi: 10.1016/j.neuroimage.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Michielsen ME, Selles RW, van der Geest JN, Eckhardt M, Yavuzer G, Stam HJ, et al. Motor recovery and cortical reorganization after mirror therapy in chronic stroke patients: A phase II randomized controlled trial. Neurorehabil Neural Repair. 2011;25:223–33. doi: 10.1177/1545968310385127. [DOI] [PubMed] [Google Scholar]
- 15.Hadders-Algra M. The neuronal group selection theory: Promising principles for understanding and treating developmental motor disorders. Dev Med Child Neurol. 2000;42:707–15. doi: 10.1017/s0012162200001316. [DOI] [PubMed] [Google Scholar]
- 16.Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232–40. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- 17.Tyson SF, DeSouza LH. Development of the Brunel Balance Assessment: A new measure of balance disability post stroke. Clin Rehabil. 2004;18:801–10. doi: 10.1191/0269215504cr744oa. [DOI] [PubMed] [Google Scholar]
- 18.Tyson SF, Hanley M, Chillala J, Selley AB, Tallis RC. The relationship between balance, disability, and recovery after stroke: Predictive validity of the Brunel Balance Assessment. Neurorehabil Neural Repair. 2007;21:341–6. doi: 10.1177/1545968306296966. [DOI] [PubMed] [Google Scholar]
- 19.Mehrholz J, Wagner K, Rutte K, Meissner D, Pohl M. Predictive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Arch Phys Med Rehabil. 2007;88:1314–9. doi: 10.1016/j.apmr.2007.06.764. [DOI] [PubMed] [Google Scholar]
- 20.Scholtes VA, Becher JG, Beelen A, Lankhorst GJ. Clinical assessment of spasticity in children with cerebral palsy: A critical review of available instruments. Dev Med Child Neurol. 2006;48:64–73. doi: 10.1017/S0012162206000132. [DOI] [PubMed] [Google Scholar]
- 21.Verheyden G, Nieuwboer A, De Wit L, Thijs V, Dobbelaere J, Devos H, et al. Time course of trunk, arm, leg, and functional recovery after ischemic stroke. Neurorehabil Neural Repair. 2008;22:173–9. doi: 10.1177/1545968307305456. [DOI] [PubMed] [Google Scholar]
- 22.Bowden MG, Clark DJ, Kautz SA. Evaluation of abnormal synergy patterns poststroke: Relationship of the Fugl-Meyer Assessment to hemiparetic locomotion. Neurorehabil Neural Repair. 2010;24:328–37. doi: 10.1177/1545968309343215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilsson L, Carlsson J, Danielsson A, Fugl-Meyer A, Hellström K, Kristensen L, et al. Walking training of patients with hemiparesis at an early stage after stroke: A comparison of walking training on a treadmill with body weight support and walking training on the ground. Clin Rehabil. 2001;15:515–27. doi: 10.1191/026921501680425234. [DOI] [PubMed] [Google Scholar]
- 24.Hamzei F, Läppchen CH, Glauche V, Mader I, Rijntjes M, Weiller C. Functional plasticity induced by mirror training: The mirror as the element connecting both hands to one hemisphere. Neurorehabil Neural Repair. 2012;26:484–96. doi: 10.1177/1545968311427917. [DOI] [PubMed] [Google Scholar]
- 25.Stevens JA, Stoykov ME. Simulation of bilateral movement training through mirror reflection: A case report demonstrating an occupational therapy technique for hemiparesis. Top Stroke Rehabil. 2004;11:59–66. doi: 10.1310/GCFE-QA7A-2D24-KHRU. [DOI] [PubMed] [Google Scholar]
- 26.Sciusco A, Ditrenta G, Rahino A, Damiani S, Megna M, Raieri M, et al. Mirror therapy in the motor recovery of upper extremity. Eura Medicophys. 2008;44:1–5. [Google Scholar]
- 27.Kollen B, van de Port I, Lindeman E, Twisk J, Kwakkel G. Predicting improvement in gait after stroke: A longitudinal prospective study. Stroke. 2005;36:2676–80. doi: 10.1161/01.STR.0000190839.29234.50. [DOI] [PubMed] [Google Scholar]


