Abstract
Aims and Objectives:
To study the change in the incidence and pattern of nevirapine (NVP)-induced adverse cutaneous reactions (ADR) after commencement of revised National AIDS Control Organisation (NACO) guidelines for initiation of antiretroviral therapy (ART) since Nov 2011.
Materials and Methods:
The study was conducted on patients who developed cutaneous reactions after starting NVP based regimen. According to the revised NACO ART initiation guidelines Nov 2011, ART should be started if CD4 count is < 350 cells/mm3 in stages 1, and 2 and irrespective of CD4 count in stages 3, and 4. Patients were divided in groups A and B. Group A consisted of patients enrolled on NVP-based regimen during Jan 2011 to Oct 2011, whereas, in Group B patients from Nov 2011 to Aug 2012 were included. Grading of rash, appropriate investigations and management was done.
Observations:
In Group A, out of 645 patients 30 (4.66%) patients developed cutaneous reactions, where as in Group B out of 720, 65 (9.03%) patients presented with drug reaction. In Group A (n = 30) developed reaction as Grade 1 in 1.55% (n = 10), Grade 2 in 1.86% (n = 12), grades 3 and 4 in 0.76% (n = 5) and 0.47% (n = 3), respectively. In Group B (n = 65) developed reaction, out of which Grade 1 reaction was seen in 1.39% (n = 10), Grade 2 was seen in 2.78% (n = 20), grades 3 and 4 was seen in 3.33% (n = 24) and, 1.53% (n = 11), respectively.
Conclusion:
There is a striking increase in the incidence of NVP-induced cutaneous reactions of all forms and considerable increase in frequency of severe kind of reactions with the revised guidelines.
Keywords: Adverse drug reaction, anti-tubercular treatment, Efavirenz, National AIDS control organisation, NVP- nevirapine
INTRODUCTION
Antiretroviral therapy (ART) plays an important role in the management of HIV-positive patients to prevent the disease progression, further immunosuppression, and the great amount of mortality and morbidity associated with the disease. NVP is most commonly used non-nucleoside reverse transcriptase inhibitor (NNRTI) as part of firstline ART. The drug is often well tolerated, cost effective but it is also, one of the most common drug, which causes varied cutaneous and hepatic side effects.
According to old ART guidelines, ART was started, if CD4 count < 200 cells/mm3 in stages 1 and 2 disease; if CD4 < 350 cells/mm3 in stage 3 and irrespective of CD4 count in stage 4[1] disease. To improve the life quality and expectancy, WHO now recommends earlier initiation of ART for adults and adolescents.[2] On Nov 4, 2011, NACO has revised the ART initiation guideline by increasing the cut off value of CD4 count according to WHO's recommendations. The recent guidelines suggest that, ART should be started if CD4 count is < 350 cells/mm3 in stages 1 and 2 disease and irrespective of CD4 count in stages 3, and 4[3,4] disease. The study was conducted to assess the viewpoint about the changing pattern of NVP-induced adverse cutaneous reactions.
The adverse cutaneous effect caused by NVP are generally seen in first 4-6 weeks of therapy.[5] Depending on the severity of drug reactions caused by NVP, they could be graded as
Grade 1: Mild–localized macular rash
Grade 2: Moderate–diffuse macular, macul opapular, or morbilliform rash or target lesions
Grade 3: Severe–diffuse macular, maculopapular or morbilliform rash or target lesions with vesicles or limited number of bullae or superficial ulceration of mucus membrane limited to one site
Grade 4: Potentially life-threatening-extensive or generalized bullous lesions, or Steven-Johnson syndrome (SJS), or toxic epidermal necrolysis (TEN).[6]
It is also mentioned by NACO that patients who initially were on NVP--based ART and shifted to efavirenz (EFV) due to anti-tubercular treatment (ATT) should again be shifted to NVP without any lead in dose after completion of rifampicin-based ATT.[7]
MATERIALS AND METHODS
An observational study was conducted during Jan 2011 to October 2012 at the Department of Skin and Veneral Disease. This study was carried out to know the incidence of various types of drug reactions caused by NVP, the severity of reactions, treatment options, to study the relationship between CD4 count and occurrence of cutaneous ADRs, effect of restarting of NVP-based ART after completion of ATT before and after the revision of guidelines. A detailed evaluation of these parameters was carried out.
Total 1365 patients who were on NVP-based ART regimen were enrolled in the study. Patients were divided into two groups A and B for comparison convenience. Group A included, 645 (M = 438, F = 207) patients who were on NVP-based ART from Jan 2011 to Oct 2011 that is the prechange era of ART guideline. In Group B, 720 (M = 478, F = 242) patients were included from Nov 2011 to Oct 2012 who were on NVP-based ART after change of guideline. A detailed clinical history of all cutaneous events with respect to origin, duration, and progress; past history of any ART; change of any drug regimens; history of ATT, any history of any form of drug reactions, and drugs taken other than ART were taken. All baseline investigations such as hemogram, liver function test, renal function test, urine routine, and microscopy were carried out in each patient. Patients were also clinically evaluated and treated for any opportunistic infections. X-ray chest and ultrasonography of abdomen were done if required and to rule out any tubercular focus in the body. CD4 counts of all patients were noted at each point. They were generally done every 6 months or more frequently if clinically indicated.
While starting with NVP-based regimen, the drug is given in a lead in dose for first 14 days, that is, 200 mg once daily, if no reaction is observed during this period then the dosage of NVP is increased to 200 mg twice daily.[8] Adverse cutaneous events developed after the introduction of drug was graded as per the above-mentioned criteria. The final grade of the rash experienced by the patient after which further progression of rash was halted, was considered as the final grade of the rash. Patients were treated accordingly for symptomatic relief and if required the change was made in ART regimen under strict observation. Clinical evaluation for general condition, disease progression, and opportunistic infections were done at each visit along with appropriate referrals and treatment.
RESULTS AND OBSERVATIONS
NVP-induced rash was observed in total 30 (4.64%) patients in Group A and 65 (9.03%) patients in Group B [Table 1]. On further grading of the rash, Grade 1 rash [Figure 1] was present in 1.39%, Grade 2 [Figure 2] in 2.78%, Grade 3 [Figure 3] in 3.33%, and Grade 4 [Figures 4 and 5] was seen in 1.53% of patients.
Table 1.
Grading of NVP-induced rash
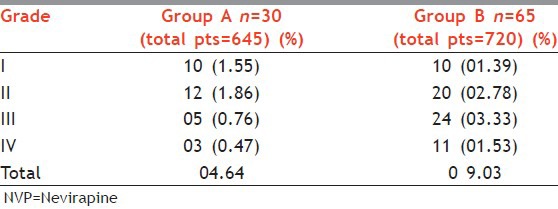
Figure 1.
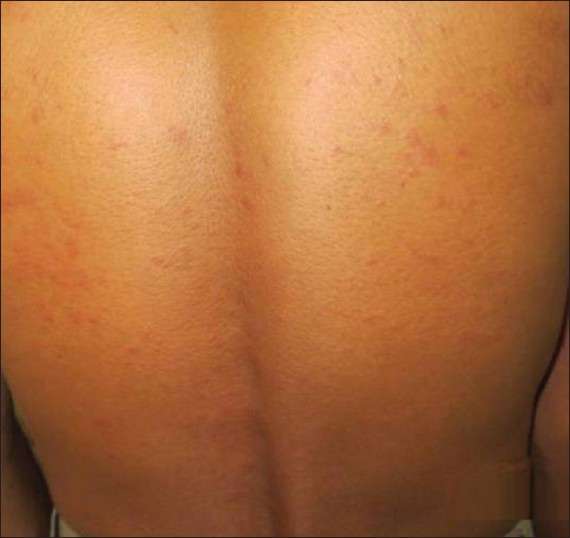
Grade 1 rash involving trunk in a female patient
Figure 2.
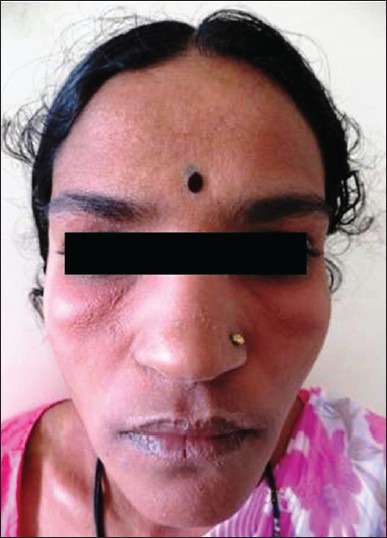
Grade 2 diffuse maculopapular rash over face in a female patient
Figure 3.

Grade 3 rash, atypical Erythema Multiforme and purpuric lesions involving trunk in a male patient
Figure 4.
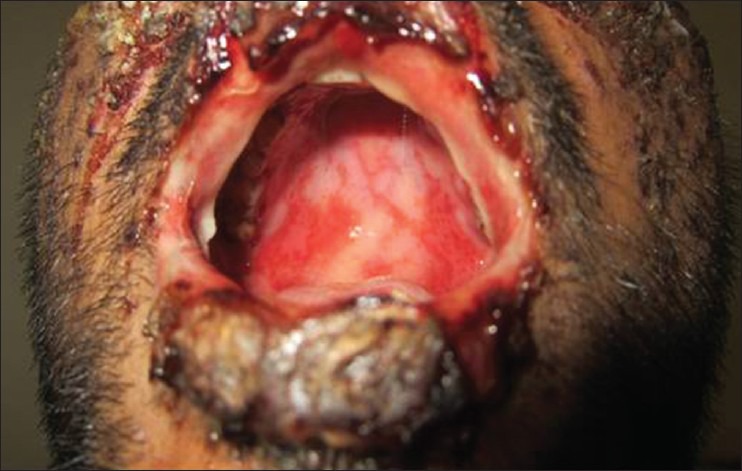
Grade 4 rash–oral mucosal ulceration and lip crusting in a male patient of Steven–Johnson syndrome
Figure 5.
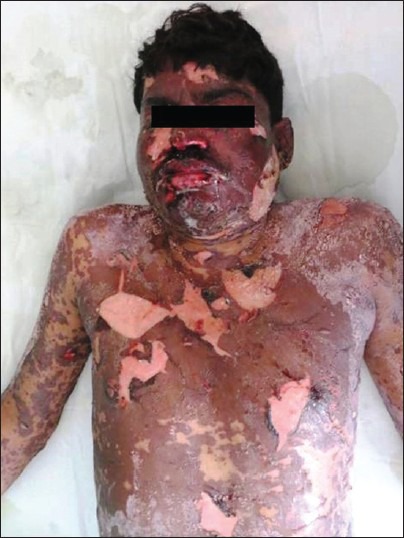
Grade 4 rash–toxic epidermal necrolysis a male patient
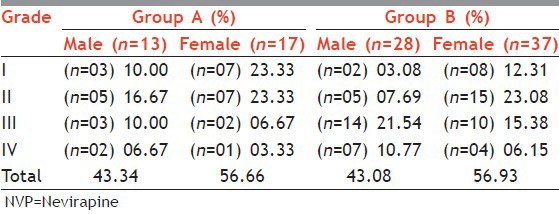
Out of the total males in Group A, 3% developed rash and in Group B 5.9% experienced the same, whereas in Group A 8% of females and in Group B 15.3% of females showed the drug rash. In both the groups, overall incidence of drug reactions were more common in female patients (M:F,~ 43:57) [Table 2], but male patients showed increased propensity towards higher grades of reactions.
Table 2.
Sex distribution and NVP rash
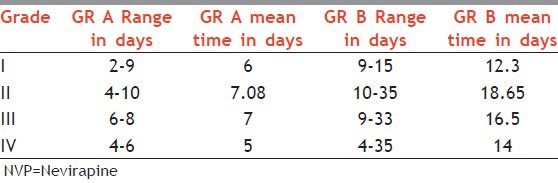
In Group A the mean time for development of cutaneous ADR ranged from 2 to 10 days, whereas in Group B the same is in the range of 4-35 days, with a mean duration of approximately 12-19 days [Table 3]. The patients showed great variation in time period from the onset of treatment and development to the ultimate progression of grade of rash.
Table 3.
Relationship between NVP rash and time duration for development
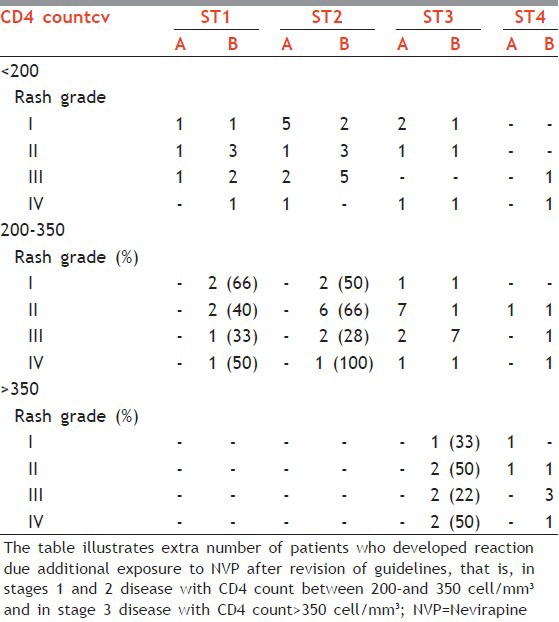
To know the relationship between CD4 count and NVP rash, all patients in the study who developed reaction due to NVP, that is, 30 patients in Group A and 65 patients in Group B were divided into three main categories depending on CD4 count as (1) CD4 < 200, (2) 200-350, and (3) >350 cell/mm3. The patients in each category were further subdivided according to the stage of the disease at which they developed rash and the ultimate grade of the rash seen in each patient. Patients in Group B with disease stages 1 and 2, with CD4 count of 200-350 showed approximately 28-100% increase in all grades of reactions. In stage 3 disease with CD4 count >350, there was 20%-50% increase in the incidence of all grades of reaction [Table 4].
Table 4.
CD4 count and NVP rash
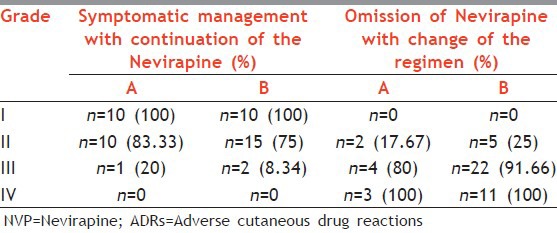
Patients were managed symptomatically with antihistamines in grades 1 and 2. The dose of NVP was not escalated from once daily to twice daily till the rash subsided. If such patients did not show further progression of rash, then the dose of NVP was escalated, however, such dose modification of NVP was not possible with higher grades of rash or patients who developed Grade 3 or Grade 4 rash within less time span, or in whom symptomatic treatment was insufficient to control the rash or patients who moved rapidly along the grading spectrum of rash, that is, from Grade 1 to Grade 4. In grades 3 and 4 rash ART was with held temporarily and the patients were admitted to the skin ward. After improvement of the general condition of the patient, the ART was restarted with EFV-based regimen. SJS and TEN were treated as routine dermatological emergencies. Overall conservative management was preferred and the use of corticosteroid was cautiously avoided. Patients with lower grades of reactions [Grades 1 and 2], symptomatic management (in the form of antihistamines and supportive care) was possible in 100-75% within both the groups [Table 5]. But for higher grades [Grades 3 and 4], change of regimen from NVP to EFV was done in almost 90-100% of cases. Importantly in all these patients NVP was never rechallenged. In Group B with increase in frequency and severity of reactions change of regimen was more often necessary and even life saving.
Table 5.
Management options for NVP induced cutaneous ADRs
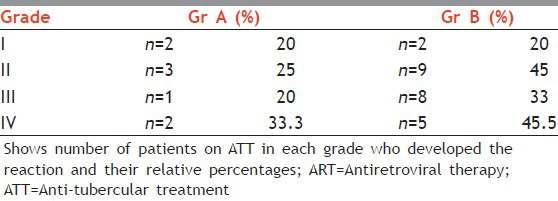
In Group A out of 645 patients 69 patients were on ATT and in Group B out of total 720 patients 90 patients were on ATT during total 10 months duration of the study. In Group A 8 out of 30 patients of NVP rash and in Group B 24 out of 65 patients of NVP rash had a history of ATT. Patients in both the groups showed increased incidence of drug reaction to NVP after completion of ATT [Table 6]. In Group B out of total patients who developed the rash almost 30-40% of the patients had experienced the event after completion of ATT, on re-switching to NVP-based ART. Surprisingly, most of these patients did not show any such reactions on first exposure to NVP, but the incidence and severity of the same, was considerably increased after reintroduction of drug without leading dose after ATT completion.
Table 6.
Effect of restarting of Nevirapine based ART after completion of ATT
Apart from the dermatological side effects, patients in both the groups independently showed hepatic events such as symptomatic side effects (anorexia, jaundice, vomiting) and elevated liver enzymes (Serum glutamic –pyruvic transaminase SGPT, Serum glutamic oxaloacetic transaminase SGOT, Alkaline phosphatase ALP), prolonged partial thromboplastin time, hyperbilirubinemia. In Group A 8.5% (n = 55) patients showed hepatic side effects, of whom only 9% (n = 5) showed concomitant skin rash, whereas in Group B 11.6% (n = 84) patients showed hepatic side effects, out of which only 13% (n = 11) presented with concomitant skin events. Risk of all these hepatic events (regardless of severity) was found more in the first 6-18 weeks of therapy, few patients even developed it after 4 months. Neutropenia was observed in some of the patients of both the groups.
DISCUSSION
NVP-induced rash was observed in 9.03% of patients after revision of guidelines as compared with the 4.64% with the previous guidelines. This was higher as compared with the earlier study by Sharma et al.,[9] whereas it was lower as compared with the study by Dey et al.[10]
In our study the rash due to NVP was seen in 15.3% of females and 5.9% of males, which is similar for females but lesser for males as compared with the study by Sharma et al.,[9] that is, 14.7% and 10.5%, respectively. Females showed higher propensity for cutaneous reaction to NVP.[11] The reason for such differential behaviour is not well understood. We also observed in our study that NVP rash was more frequent at higher CD4 counts, which was true for all grades of reactions. This finding is consistent with the results of an earlier study by Dong et al.[12] and other studies.[10]
The mean time between the start of regimen and onset of reaction for was 14-19 days, with the range being 4-35 days. The earlier studies by Dey et al.[10] and Sharma et al.[9] had shown it as 8 and 13 days, respectively.
Symptomatic management of NVP rash was possible only in the initial grades of the reactions, that is, in grades 1 and 2. Change of regimen was mandatory in severe grades of reaction [Grades 3 and 4]. Without active intervention initially benign-looking reactions may progress to higher grades.
As understood from the previous studies that severe rash and SJS are recognized complications of nevirapine, they are the most common and first to occur side effects of this drug. In the great majority of patients rashes occur within the first 4-6 weeks of treatment. There are data to support the importance of using a 2-week lead-in dose (200 mg once daily) to reduce the risk of rash of any severity. Starting with full dose of NVP without lead in dose results in higher serum concentration of the drug, which increases the risk of hepatotoxicity and rash.[13]
Due to improved immunity after completion of ATT, the patients showed higher chances of reactions to reintroduction of the same drug. If NVP is interrupted due to any reason for more than 14 days then the drug should be restarted with lead in doses.[8] It can provide the body with a time gap to build the serum concentration of the drug gradually. So patient could be more benefited if the NVP-based ART is reintroduced in lead in dose even after ATT completion under strict observation.
The risk of symptomatic hepatic events (regardless of severity) is greatest in the first 6-18 weeks and occurs next in the frequency and independent of dermatological events.[14] Elevated hepatic enzymes act as guide for hepatotoxicity. A study by Bruck et al. shows that elevated, asymptomatic liver enzymes could be found in equal frequency in patients with NVP as well as EFV.[14]
The first 18 weeks of therapy after starting NVP-based ART are very crucial to avoid the serious skin as well as hepatic side effects of the drug.[15] On the part of the dermatologist it is of paramount importance to recognize and effectively treat the NVP rashes so as to curb the progression of grades. Additional studies are required to help the clinicians to make recommendations about NVP toxicity based on the detailed pharmacokinetic profile of the drug; regional, racial differences; and the individual patient's profile.[11]
CONCLUSION
With revision of ART initiation guidelines the incidence of NVP induced cutaneous ADRs has considerably increased, especially in patients with higher CD4 counts. After completion of anti-tubercular treatment (ATT) patients tends to show higher propensity towards development of drug reactions against reintroduction to NVP as compared to ATT naïve patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.NACO: Ministry of Health and Family Welfare. New Delhi, India: Government of India; 2007. ART in Adults and Adolescents. In: Antiretroviral Therapy Guidelines for HIV Infected Adults and Adolescents including post exposure prophylaxis; p. 19. [Google Scholar]
- 2.WHO's Certified. New HIV recommendations to improve health, reduce infections and save lives World. Media centre. AIDS Day 2009 News release. [Last accessed on 2009 Nov 30]. Available from: http://www.who.int/mediacentre/news/releases/2009/world_aids_20091130/en/index .
- 3.WHO Library Cataloguing-in-Publication Data. Switzerland: 2009. [Last accessed on 2009 Nov 30]. WHO's Certified. Rapid advice: Antiretroviral therapy for HIV infection in adults and adolescents. HIV/AIDS. Key Recommendations; p. 10. Available from: http://www.who.int/hiv/pub/arv/rapid_advice_art.pdf . [Google Scholar]
- 4.New Delhi, India: National AIDS Control Organisation; 2011. T-11020/36/2005-NACO (ART). Revised guidelines on initiation ART in Adults and Adolescents. Department of AIDS Control. Government of India. [Google Scholar]
- 5.Ward HA, Russo GG, Shrum J. Cutaneous manifestations of antiretroviral therapy. J Am Acad Dermatol. 2002;46:284–93. doi: 10.1067/mjd.2002.119105. [DOI] [PubMed] [Google Scholar]
- 6.Division of AIDS Table for Grading the Severity of Adult and Paediatric Adverse events. Version 1. 28 Dec 04/Clarification. 2004 Aug 09;:5. [Google Scholar]
- 7.NACO: Ministry of Health and Family Welfare. New Delhi, India: Government of India; 2007. ART in Adults and Adolescents. In: Antiretroviral Therapy Guidelines for HIV Infected Adults and Adolescents Including Post Exposure Prophylaxis; p. 31. [Google Scholar]
- 8.NACO: Ministry of Health and Family Welfare. New Delhi India: Government of India; 2007. ART in Adults and Adolescents. In: Antiretroviral Therapy Guidelines for HIV Infected Adults and Adolescents Including Post Exposure Prophylaxis; p. 23. [Google Scholar]
- 9.Sharma A, Modi M, Sharma A, Marfatia YS. Cutaneous eruptions associated with nevirapine therapy in AIDS cases. Indian J Sex Transm Dis. 2007;28:94–6. [Google Scholar]
- 10.Dey SK, Pal NKI. Adverse reaction of Nevirapine in antiretroviral naïve HIV seropositive subjects. Antivir Ther. 2004;9:L4o. [Google Scholar]
- 11.NAM AIDS map. Severe nevirapine rash linked to slow clearance of drug. Carole Leach-Lemens. [Last accessed on 2012 Feb 20]. Available from: http://www.aidsmap.com/Severe-nevirapine-rash-linked-to-slow-clearance-of-drug/page/2255527/
- 12.Dong BJ, Zheng Y, Hughes MD, Frymoyer A, Verotta D, Lizak P, et al. Nevirapine (NVP) pharmacokinetics (PK) and risk of rash and hepatitis among HIV-infected sub-Saharan African women. AIDS. 2012;26:833–41. doi: 10.1097/QAD.0b013e328351a521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreas B, Maureen M. Nevirapine and rashes. [Last accessed on 1998 Apr 11];Lancet. 1998 351:1133–4. Available from: http://www.nursingworld.org/AJN/2002/june/Wawatch.htmArticle . [Google Scholar]
- 14.Brück S, Witte S, Brust J, Schuster D, Mosthaf F, Procaccianti M, et al. Hepatotoxicity in patients prescribed efavirenz or nevirapine. [Last accessed on 2008 Jul 28];Eur J Med Res. 2008 13:343–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18700192 . [PubMed] [Google Scholar]
- 15.Drug Information Online-Drugs.com. HIV Infection → Viramune Side Effects. [Last accessed on 2013 Mar 12]. Available from: http://www.drugs.com/druginteractions/nevirapine, viramune.html .


