Abstract
Background:
India is one of the high tuberculosis (TB) burden countries in the world. India ranks second in harboring multi drug resistant (MDR)-TB cases. About 50,000 of MDR cases are recorded in retreatment pulmonary TB cases. This study was conducted in a tertiary care facility (Government General and Chest Hospital) in Hyderabad, India.
Objectives:
Toassess: Proportion of the TB patients having MDR-TB at the initiation of retreatment regimen; the prevalence of isoniazid (INH) resistance in this geographical area.
Materials and Methods:
An analytical, observational, prospective cohort study of patients attending the out-patient department from December 2010 to March 2011.
Results:
Sputum samples from 100 patients were subjected to acid fast bacilli (AFB) culture and drug sensitivity testing. Of these, 28 (28%) were MDR-TB, 42 (42%) were non-MDR-TB and 39% being INH resistance.
Conclusions:
In conclusion, one third of the retreatment pulmonary TB cases attending a tertiary care institute for TB will be MDR-TB at the initiation of treatment and there is a need to include ethambutol in the continuation phase of new TB case treatment in view of high INH resistance.
KEY WORDS: Category IV (category four), directly observed treatment, short-course plus (DOTS plus), India, multidrug resistant tuberculosis, retreatment cases
INTRODUCTION
India is one of the high tuberculosis (TB) burden countries in the world accounting for nearly 20% of the global incidence constituting 9.4 million TB cases.[1] India ranks second in harboring multi drug resistant (MDR)-TB cases, i.e., about 99,000 cases. Among these, 50,000 cases are recorded in retreatment pulmonary TB cases.[2]
To control the TB burden in the country, the Government of India started Revised National Tuberculosis Control Program (RNTCP) in 1997.[3] The RNTCP is implementing directly observed treatment, short-course (DOTS) plus facility in the country, to control the MDR-TB burden, since October 2007.[4]
The Government General and Chest Hospital (GG and CH) is a tertiary care institute, implementing the RNTCP for the management of drug sensitive and drug resistant TB.
There are anecdotal publications in the literature about the prevalence of MDR, among TB patients who are started on retreatment category. In this study, we assessed (a) Proportion of the patients harboring MDR-TB strains, who are considered for retreatment regimen. (b) The prevalence of isoniazid (INH) resistance in this geographical area.
MATERIALS AND METHODS
Design
A prospective, analytical, observational, cohort study.
Study period
From December 2010 to March 2011.
Study setting and population
When our study was initiated, the DOTS plus programme considered a patient whose sputum Acid Fast Bacilli (AFB) smear was positive at the end of 4th month of the retreatment regimen as an MDR-TB suspect and eligible for culture and drug susceptibility testing (DST) [Table 1]. Patients, who were found to be resistant to Rifampicin (R) and INH or resistant to R, were initiated on category IV (category four).
Table 1.
Treatment categories and regimens for TB patients in India
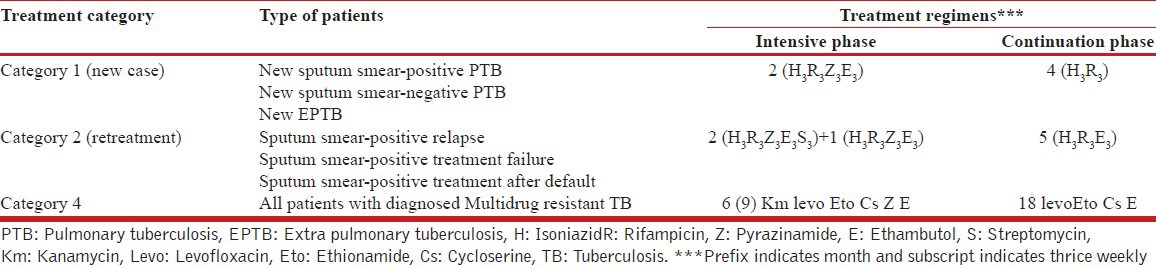
In this study, all TB patients attending the out-patient department of the GG and CH, were registered and categorized, those who are fulfilling the criteria of relapse, treatment after default [Table 2] and who have taken the anti-TB treatment irregularly outside the RNTCP, were admitted.
Table 2.
World Health Organization recommended case definitions

Patients were instructed to collect two sputum samples in the falcon tubes, under the supervision of ward nurse or in-charge doctor. The collected samples are immediately transported to the state accredited intermediate reference laboratory (IRL), which was sharing a common wall with GG and CH. In this laboratory, the samples were subjected to the culture in Lowenstein Jensen media followed by the DST to the four first line anti-TB drugs, i.e., R, INH, ethambutol and streptomycin (SM). The information regarding those patients who are found to be resistant to the R and INH or only R was intimated at the earliest possible time either by telephonic or electronic message and they were initiated on DOTS plus category IV (category four) regimens after pre-treatment evaluation as per the program guidelines.
Data collection and analysis
Data was entered in case records, culture forms and later into a pre-structured Microsoft Excel sheet. The data was analyzed using Epi-Info (version 6.0 CDC, Atlanta, USA).
Ethical approval
The study was approved by Institutional Ethical Committee of GG and CH. As this study was a prospective study, the individual written patient consent was taken. Electronic databases created for analysis were stripped off personal health identifiers and maintained securely.
RESULTS
Study population
A total of 100 patients samples were subjected to the culture and DST, of whom 69 were males. Of the 100 patients, 36 were relapse cases and 64 were defaulters, including patients with irregular treatment outside RNTCP.
Prevalence of drug resistance
Of the 100 patients, 16(16%) showed no growth, 3(3%) patient's samples was contaminated, which are recollected within 1 week of the result and 84(84%) were culture positive.
Nearly, 42(42%) were sensitive to all the four drugs. 28(28%) samples were declared as MDR positive. 14(14%) were declared as drug resistant TB other than MDR. The pattern of drug resistance is shown in Figure 1. About 39 (39%) cases were resistant to INH.
Figure 1.
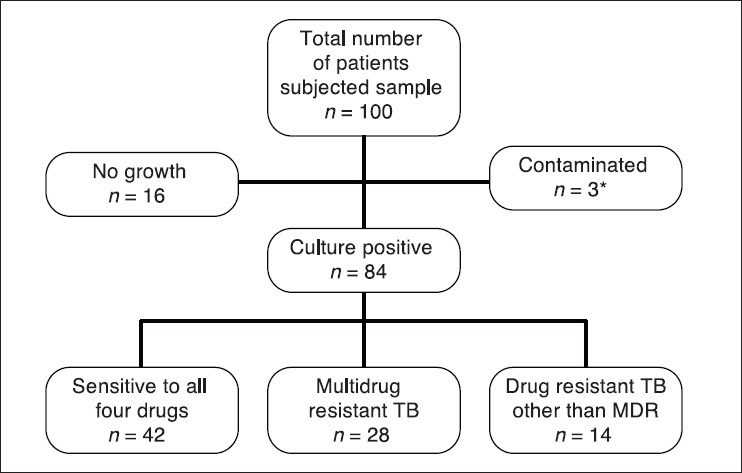
Prevalence drug resistance among retreatment pulmonary tuberculosis patients, inGovernment General and Chest Hospital, Hyderabad, India. *Contaminated samples are recollected within 1 week of the result
Among the samples showed drug resistance, nine patients were resistant to R, INH, SM and pyrazinamide (Z), seven patients were R, INH and SM resistant, 12 patients were R and INH resistant, 1 patient was resistant to only SM, 2 patients were resistant to only R and 11 patients were resistant to INH only.
The individual resistance to various anti-tubercular drugs is depicted in Figure 2.
Figure 2.
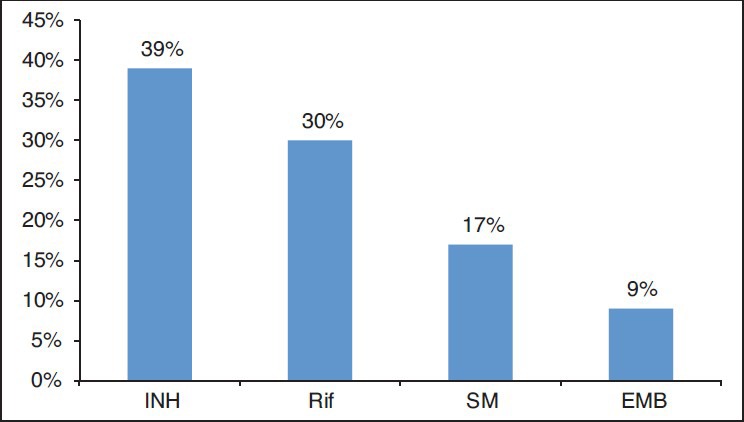
Individual drug resistance patterns includes individual and combined resistance in retreatment pulmonary tuberculosis patients
DISCUSSION
This study showed that every three patients among 10 retreatment cases are being declared as having MDR-TB bacilli, who need treatment with second line anti-TB drugs. According to the DOTS plus guidelines during the study period, these patients are delayed for 4 months in the initiation of the category IV (category four). As there is a chance of positive case to infect 10 or more persons in a year,[5] this study can indicate that this delay of 4 months for a patient, might infect 3 or more persons with MDR bacilli in this delayed period.
This study also revealed that INH resistance is seen in 39% cases in this geographical area. According to Burugina Nagaraja et al.,[6] treatment outcomes of patients who fail a first-line anti-TB therapy and who are not placed on an MDR-TB regimen are unacceptably poor.[6] Hence, there is a need to start MDR-TB regimen in cases of drug resistant TB other than MDR.
Hence, our study suggests following recommendations to the RNTCP:
First, subjecting every retreatment category patient to sputum AFB culture and DST at the initiation of the treatment, which will have a good impact on controlling the TB burden and preventing the MDR-TB in the community.
Second, as there was high prevalence of INH resistance, the program should give a serious thought to include ethambutol in the continuation phase of the treatment regimen of the new cases.
The strengths of this study are proper selection of the cases, proper collection and immediate transport of sputum samples to the RNTCP accredited IRL.
The limitation of our study includes the inability to report the treatment outcomes of these patients, because of the long duration of the treatment.
In conclusion, one-third of the retreatment pulmonary TB cases in a tertiary care institute will be MDR-TB at the initiation of the treatment and there is a need to include ethambutol in the continuation phase of the new TB case treatment in view of high prevalence INH resistance.
ACKNOWLEDGMENTS
I, acknowledge the support of State TB cell of Andhra Pradesh, microbiology staff of the state tuberculosis demonstration center, Hyderabad and staff of the DOTS center of the GG and CH.
Dr. Subhakar Kandi is professor of the pulmonary medicine, in Osmania Medical College, RNTCP zonal task force chairman, south India and state H1N1 coordinator Andhra Pradesh, India.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Geneva: World Health Organisation; 2010. World Health Organisation. WHO Report on global tuberculosis control: Epidemiology, strategy, financing. [Google Scholar]
- 2.World Health Organisation. [Last accessed on 2011 Sep 13]. Available from: http://www.who.int/tb/data .
- 3.Central Tuberculosis Division. Technical and operational guidelines for tuberculosis control, revised National Tuberculosis Control Programme. Government of India: Directorate General of Health Services, Ministry of Health and Family Welfare. 2005 [Google Scholar]
- 4.World Health Organization. [Last accessed on 2012 Jun 12]. Available from: http://www.whoindia.org/en/section3/section123.htm .
- 5.TBC India. [Last accessed on 2011 Sep 15]. Available from: http://www.tbcindia.org/rntcpasp .
- 6.Burugina Nagaraja S, Satyanarayana S, Chadha SS, Kalemane S, Jaju J, Achanta S, et al. How do patients who fail first-line TB treatment but who are not placed on an MDR-TB regimen fare in South India? PLoS One. 2011;6:e25698. doi: 10.1371/journal.pone.0025698. [DOI] [PMC free article] [PubMed] [Google Scholar]


